Abstract
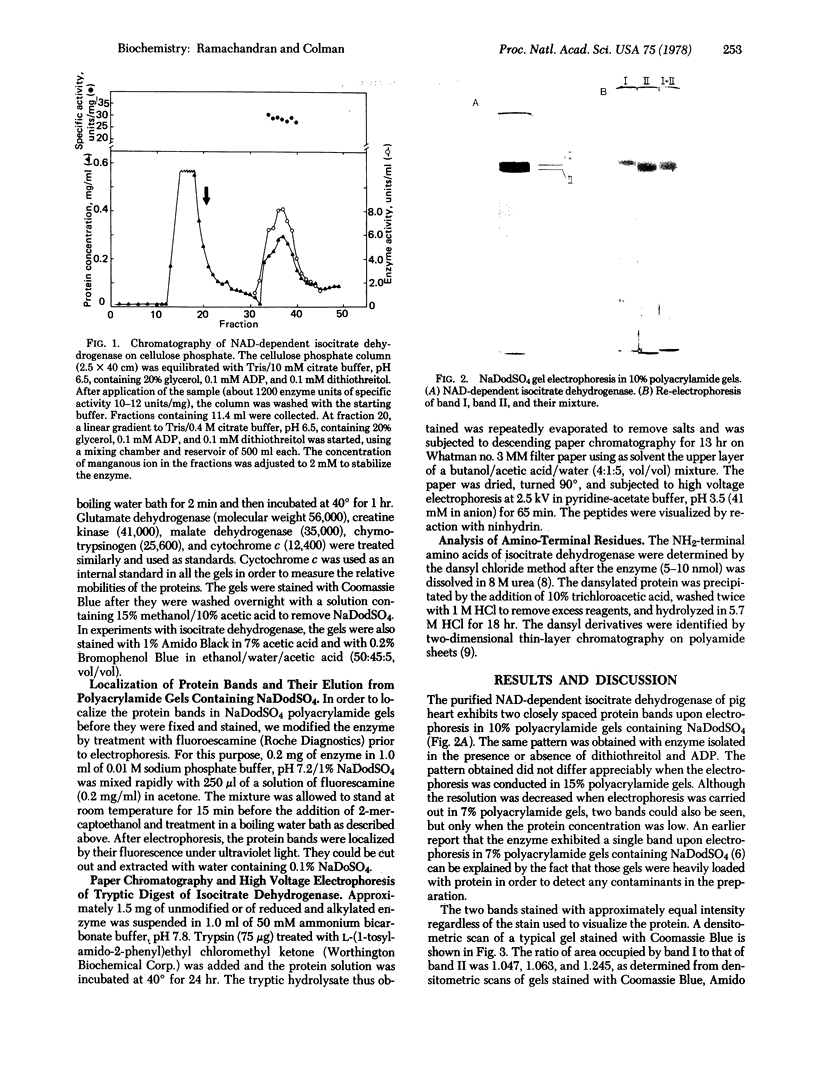
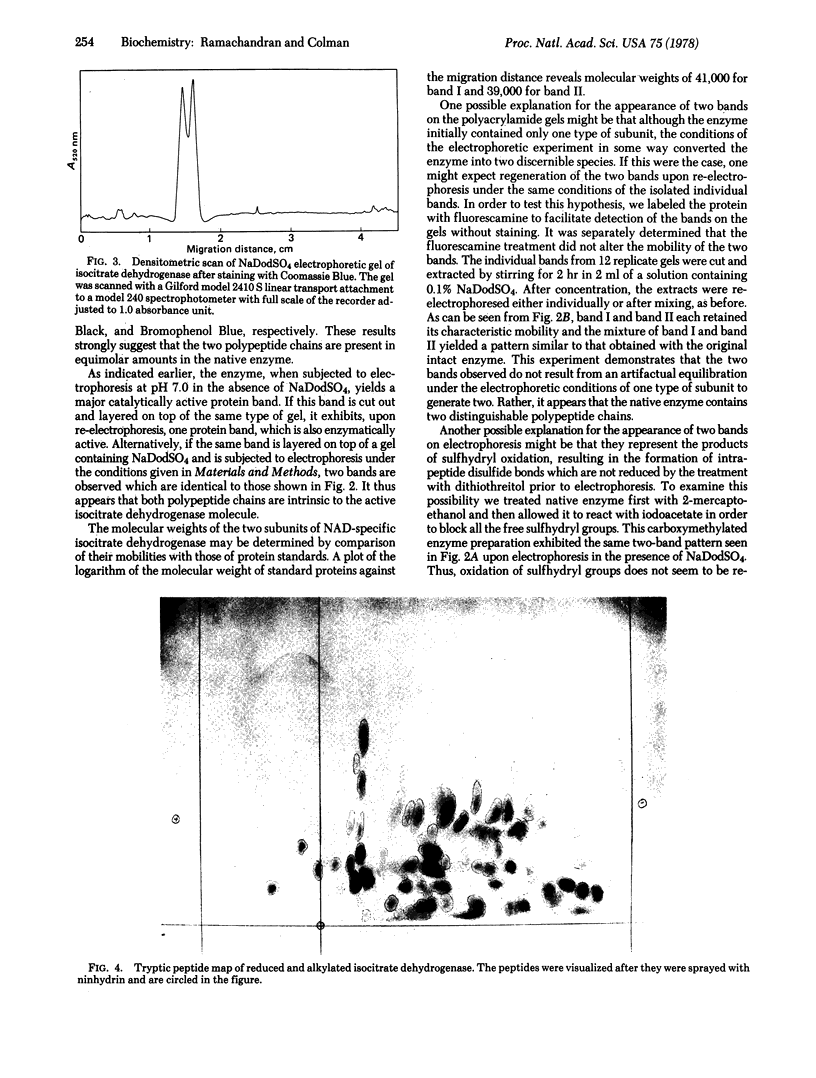
The NAD-dependent isocitrate dehydrogenase [threo-DS-isocitrate:NAD+ oxidoreductase (decarboxylating); EC 1.1.1.41] from pig heart is a multisubunit enzyme with a molecular weight of approximately 340,000. Electrophoresis of the enzyme in 10% polyacrylamide gels containing sodium dodecyl sulfate reveals two discrete bands with molecular weights of 41,000 and 39,000. The two bands exhibit approximately equal intensity when stained with Coomassie Blue, Amido Black, and Bromophenol Blue, suggesting that these polypeptide chains are present in equimolar quantities in the native enzyme. The same two-band pattern is observed when the sulfhydryl groups of the enzyme are blocked by alkylation with iodoacetate prior to electrophoresis, indicating that sulfhydryl oxidation is not responsible for the observed heterogeneity. Each of the subunits appears as a single band when eluted from the gel and again subjected to electrophoresis under the same conditions. Isocitrate dehydrogenase contains a total of 41 lysine and arginine residues per average subunit of 40,000 daltons. The observation of approximately 80 peptides upon paper chromatography and high voltage electrophoresis of tryptic digests of the enzyme is consistent with the existence of two distinct polypeptide chains. Dansylation yields two NH2-terminal amino acid derivatives: dansyl-phenylalanine and dansyl-alanine. It is concluded that the NAD-specific isocitrate dehydrogenase is composed of equal numbers of two nonidentical subunits.
Keywords: acrylamide gel electrophoresis, NH2-terminal amino acids, tryptic map
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes L. D., Kuehn G. D., Atkinson D. E. Yeast diphosphopyridine nucleotide specific isocitrate dehydrogenase. Purification and some properties. Biochemistry. 1971 Oct 12;10(21):3939–3944. doi: 10.1021/bi00797a022. [DOI] [PubMed] [Google Scholar]
- Cohen P. F., Colman R. F. Purification of NAD-specific isocitrate dehydrogenase from porcine heart. Biochim Biophys Acta. 1971 Aug 20;242(2):325–330. doi: 10.1016/0005-2744(71)90224-5. [DOI] [PubMed] [Google Scholar]
- Giorgio N. A., Jr, Yip A. T., Fleming J., Plaut G. W. Diphosphopyridine nucleotide-linked isocitrate dehydrogenase from bovine heart. Polymeric forms and subunits. J Biol Chem. 1970 Oct 25;245(20):5469–5477. [PubMed] [Google Scholar]
- Harvey R. A., Heron J. I., Plaut G. W. Regulation of diphosphopyridine nucleotide-linked isocitrate dehydrogenase from bovine heart. Binding of inhibitory nucleotides. J Biol Chem. 1972 Mar 25;247(6):1801–1808. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ramachandran N., Colman R. F. Evidence for a critical glutamyl and an aspartyl residue in the function of pig heart diphosphopyridine nucleotide dependent isocitrate dehydrogenase. Biochemistry. 1977 Apr 19;16(8):1564–1573. doi: 10.1021/bi00627a006. [DOI] [PubMed] [Google Scholar]
- Shen W. C., Mauck L., Colman R. F. Physicochemical properties of the diphosphopyridine nucleotide-specific isocitrate dehydrogenase of pig heart. J Biol Chem. 1974 Dec 25;249(24):7942–7949. [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]
- Zacharius R. M., Zell T. E., Morrison J. H., Woodlock J. J. Glycoprotein staining following electrophoresis on acrylamide gels. Anal Biochem. 1969 Jul;30(1):148–152. doi: 10.1016/0003-2697(69)90383-2. [DOI] [PubMed] [Google Scholar]