Abstract
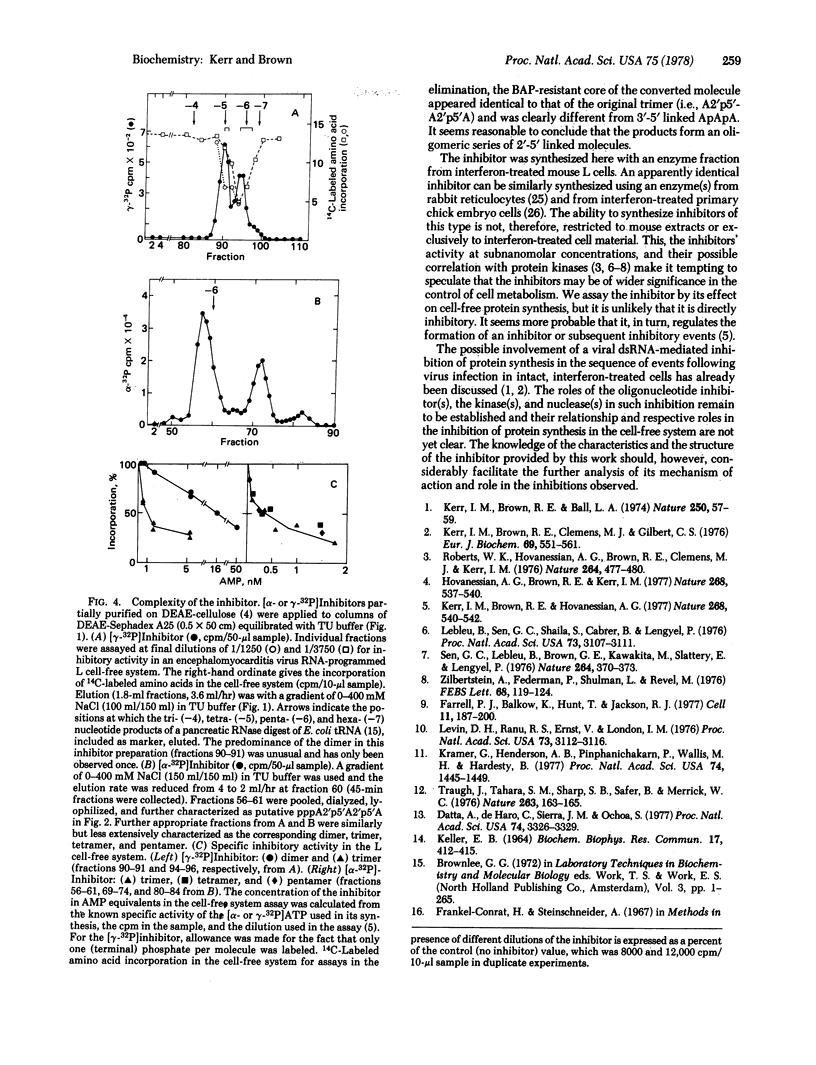
A low molecular weight inhibitor of cell-free protein synthesis effective at subnanomolar concentrations is formed on incubation of cytoplasmic extracts from interferon-treated cells with double-stranded RNA and ATP. It can be conveniently synthesized by incubating a poly(I).poly(C)-Sepharose-bound enzyme fraction from such cells with [3H]- or [alpha- or gamma-32P]ATP. The radioactive inhibitor has been characterized by its behavior on DEAE-Sephadex in the presence of urea and on the basis of the products obtained on enzymic, alkaline, and sequential degradation by periodate oxidation and beta elimination. Its structure appears to be pppA2'p5'A2'p5'A. We have found no evidence for any modification or abnormality other than the 2'-5' linkage. On occasion the inhibitor preparations have included what seems to be the corresponding dimer (pppA2'p5'A), tetramer [ppp(A2'p)3A], pentamer [ppp(A2'p)4A], and higher oligomers in decreasing amounts. The trimer, tetramer, and pentamer are similar in activity, but the dimer is less potent if active at all.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barzilay I., Sussman J. L., Lapidot Y. Further studies on the chromatographic behaviour of dinucleoside monophosphates. J Chromatogr. 1973 May 16;79:139–146. doi: 10.1016/s0021-9673(01)85282-1. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Datta A., de Haro C., Sierra J. M., Ochoa S. Mechanism of translational control by hemin in reticulocyte lysates. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3326–3329. doi: 10.1073/pnas.74.8.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Faust M., Millward S. Rapid analysis of the base-methylated and 2'-O- methylated ribonucleosides in messenger RNA. Anal Biochem. 1977 May 1;79(1-2):16–22. doi: 10.1016/0003-2697(77)90373-6. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Hovanessian A. G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977 Aug 11;268(5620):540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- Kramer G., Henderson A. B., Pinphanichakarn P., Wallis M. H., Hardesty B. Partial reaction of peptide initiation inhibited by phosphorylation of either initiation factor eIF-2 or 40S ribosomal proteins. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1445–1449. doi: 10.1073/pnas.74.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebleu B., Sen G. C., Shaila S., Cabrer B., Lengyel P. Interferon, double-stranded RNA, and protein phosphorylation. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3107–3111. doi: 10.1073/pnas.73.9.3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D., Ranu R. S., Ernst V., London I. M. Regulation of protein synthesis in reticulocyte lysates: phosphorylation of methionyl-tRNAf binding factor by protein kinase activity of translational inhibitor isolated from hemedeficient lysates. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3112–3116. doi: 10.1073/pnas.73.9.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Hovanessian A., Brown R. E., Clemens M. J., Kerr I. M. Interferon-mediated protein kinase and low-molecular-weight inhibitor of protein synthesis. Nature. 1976 Dec 2;264(5585):477–480. doi: 10.1038/264477a0. [DOI] [PubMed] [Google Scholar]
- Schibler U., Perry R. P. Characterization of the 5' termini of hn RNA in mouse L cells: implications for processing and cap formation. Cell. 1976 Sep;9(1):121–130. doi: 10.1016/0092-8674(76)90058-1. [DOI] [PubMed] [Google Scholar]
- Sen G. C., Lebleu B., Brown G. E., Kawakita M., Slattery E., Lengyel P. Interferon, double-stranded RNA and mRNA degradation. Nature. 1976 Nov 25;264(5584):370–373. doi: 10.1038/264370a0. [DOI] [PubMed] [Google Scholar]
- Traugh J. A., Tahara S. M., Sharp S. B., Safer B., Merrick W. C. Factors involved in initiation of haemoglobin synthesis can be phosphorylated in vitro. Nature. 1976 Sep 9;263(5573):163–165. doi: 10.1038/263163a0. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Federman P., Shulman L., Revel M. Specific phosphorylation in vitro of a protein associated with ribosomes of interferon-treated mouse L cells. FEBS Lett. 1976 Sep 15;68(1):119–124. doi: 10.1016/0014-5793(76)80418-8. [DOI] [PubMed] [Google Scholar]