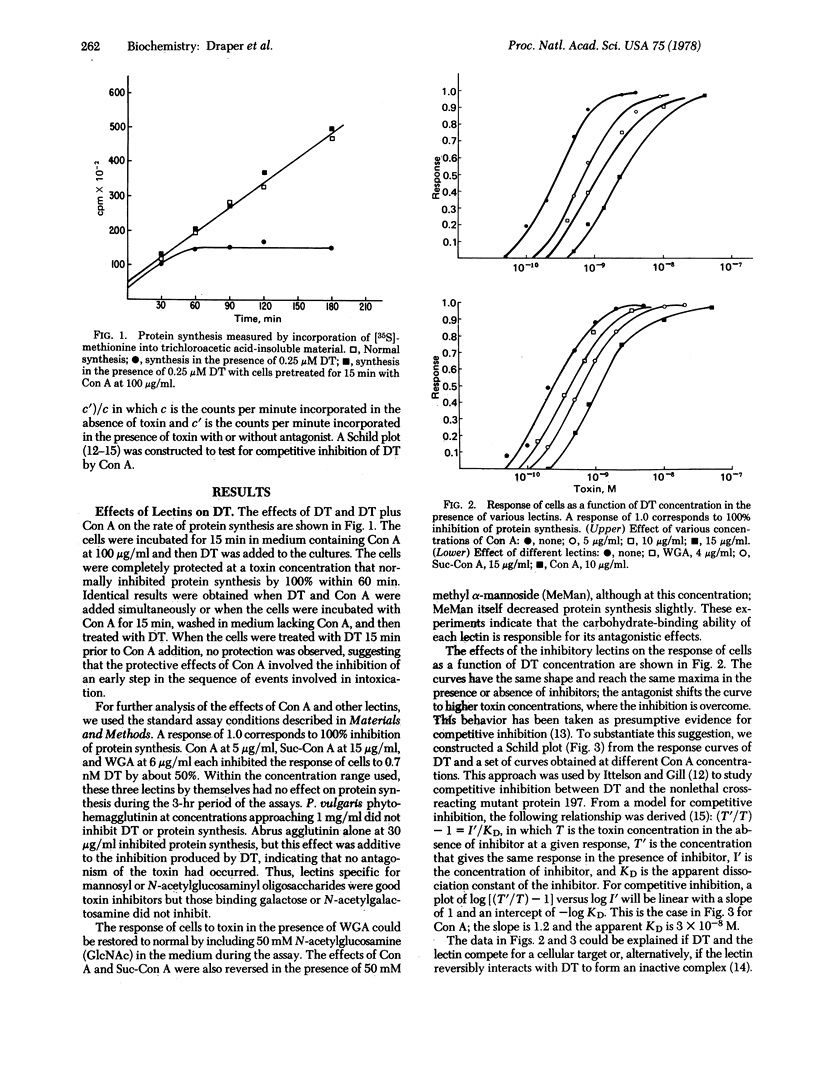
Abstract
The inhibition of protein synthesis in Chinese hamster V79 cells by diphtheria toxin is antagonized by the lectins concanavalin A, succinylated concanavalin A, and wheat germ agglutinin but not by Proteus vulgaris phytohemagglutinin or abrus agglutinin. The effects of concanavalin A and wheat germ agglutinin are reversed by methyl alpha-mannoside and N-acetylglucosamine, respectively. The inhibition of diphtheria toxin as a function of concanavalin A concentration fits a model of competitive inhibition with an apparent dissociation constant for concanavalin A of 3 X 10(-8) M. These results suggest that the diphtheria toxin receptor may be an oligosaccharide. To test this hypothesis, we screened several oligosaccharides for the ability to inhibit diphtheria toxin. The cell wall polysaccharide of Salmonella cholera suis and the ovalbumin glycopeptide were effective inhibitors. These studies suggest that diphtheria toxin may have the oligosaccharide binding properties of a lectin with specificity for N-acetylglucosamine and mannose.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VI. Isolation of concanavalin A by specific adsorption on cross-linked dextran gels. Biochim Biophys Acta. 1967 Oct 23;147(2):262–271. [PubMed] [Google Scholar]
- Agrawal B. B., Goldstein I. J. Protein-carbohydrate interaction. VII. Physical and chemical studies on concanavalin A, the hemagglutinin of the jack bean. Arch Biochem Biophys. 1968 Mar 20;124(1):218–229. doi: 10.1016/0003-9861(68)90322-6. [DOI] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arndt-Jovin D. J., Berg P. Quantitative binding of 125 I-concanavalin A to normal and transformed cells. J Virol. 1971 Nov;8(5):716–721. doi: 10.1128/jvi.8.5.716-721.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ash J. F., Singer S. J. Concanavalin-A-induced transmembrane linkage of concanavalin A surface receptors to intracellular myosin-containing filaments. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4575–4579. doi: 10.1073/pnas.73.12.4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou C. E., Raschke W. C. Polymorphism of the somatic antigen of yeast. Science. 1974 Apr 12;184(4133):127–134. doi: 10.1126/science.184.4133.127. [DOI] [PubMed] [Google Scholar]
- Boquet P., Pappenheimer A. M., Jr Interaction of diphtheria toxin with mammalian cell membranes. J Biol Chem. 1976 Sep 25;251(18):5770–5778. [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Fuller N. A., Staub A. M. Immunochemical studies on Salmonella. 13. Chemical changes appearing on the specific polysaccharide of S. cholerae suis (6-2,7) after its conversion by phage 14(6,7). Eur J Biochem. 1968 Apr;4(3):286–300. doi: 10.1111/j.1432-1033.1968.tb00207.x. [DOI] [PubMed] [Google Scholar]
- GADDUM J. H. Theories of drug antagonism. Pharmacol Rev. 1957 Jun;9(2):211–218. [PubMed] [Google Scholar]
- Gill D. M., King C. A. The mechanism of action of cholera toxin in pigeon erythrocyte lysates. J Biol Chem. 1975 Aug 25;250(16):6424–6432. [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollenberg M. D., Fishman P. H., Bennett V., Cuatrecasas P. Cholera toxin and cell growth: role of membrane gangliosides. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4224–4228. doi: 10.1073/pnas.71.10.4224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ittelson T. R., Gill D. M. Diphtheria toxin: specific competition for cell receptors. Nature. 1973 Mar 30;242(5396):330–332. doi: 10.1038/242330b0. [DOI] [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Comparative aspects of glycoprotein structure. Annu Rev Biochem. 1976;45:217–237. doi: 10.1146/annurev.bi.45.070176.001245. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Rogers J., Gregory W. The nature of the cell surface receptor site for Lens culinaris phytohemagglutinin. J Biol Chem. 1971 Nov;246(21):6581–6586. [PubMed] [Google Scholar]
- Ledley F. D., Lee G., Kohn L. D., Habig W. H., Hardegree M. C. Tetanus toxin interactions with thyroid plasma membranes. Implications for structure and function of tetanus toxin receptors and potential pathophysiological significance. J Biol Chem. 1977 Jun 25;252(12):4049–4055. [PubMed] [Google Scholar]
- Mullin B. R., Fishman P. H., Lee G., Aloj S. M., Ledley F. D., Winand R. J., Kohn L. D., Brady R. O. Thyrotropin-ganglioside interactions and their relationship to the structure and function of thyrotropin receptors. Proc Natl Acad Sci U S A. 1976 Mar;73(3):842–846. doi: 10.1073/pnas.73.3.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata Y., Burger M. M. Wheat germ agglutinin. Molecular characteristics and specificity for sugar binding. J Biol Chem. 1974 May 25;249(10):3116–3122. [PubMed] [Google Scholar]
- Olsnes S., Refsnes K., Pihl A. Mechanism of action of the toxic lectins abrin and ricin. Nature. 1974 Jun 14;249(458):627–631. doi: 10.1038/249627a0. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]
- SCHILD H. O. Drug antagonism and pAx. Pharmacol Rev. 1957 Jun;9(2):242–246. [PubMed] [Google Scholar]
- Waud D. R. Pharmacological receptors. Pharmacol Rev. 1968 Jun;20(2):49–88. [PubMed] [Google Scholar]
- Wei C. H., Koh C., Pfuderer P., Einstein J. R. Purification, properties, and crystallographic data for a principal nontoxic lectin from seeds of Abrus precatorius. J Biol Chem. 1975 Jun 25;250(12):4790–4795. [PubMed] [Google Scholar]


