Abstract
Introduction
Clinical symptoms of rheumatic diseases can cause changes in the level of skin tactile sensitivity.
Aim
To determine the tactile threshold of the hands in female patients with rheumatic diseases. It also attempted to determine correlations between rheumatic patients’ tactile sensitivity and the degree of articular movement limitations, the Barthel Index (BI) and Edinburgh Handedness Inventory (EHI) results, the level of disability of the right hand and the left hand as well as age, education and eyesight.
Material and methods
Ninety-nine female rheumatic patients aged 19–87 years took part in the study. The control group comprised 45 healthy women aged 23–80 years. The measurement of the tactile threshold was performed using the Touch-Test™ Sensory Evaluators (Semmes-Weinstein Monofilaments Test). The tactile threshold was measured at three sites on the hand: the little finger, the index finger and the metacarpus.
Results
The patients’ tactile sensitivity ranges were classified as normal, diminished light touch and diminished protective touch. The degree of their disability was correlated with tactile sensitivity. The patients’ tactile sensitivity worsens with age, but it is not correlated with the level of education. The lateralization was similar to that of the control group and was not correlated with tactile sensitivity. The worsening eyesight, independent of rheumatic disease, corresponds, however, with decreasing tactile sensitivity.
Conclusions
The patients represented a group with a medium level of functional disability and lower tactile sensitivity.
Keywords: skin, tactile threshold, esthesiometer (Semmes-Weinstein Monofilaments), touch, rheumatic diseases
Introduction
Clinical symptoms of rheumatic diseases affecting a number of systems and organs in the human body have been widely described in the literature. Most rheumatic disease entities involve the damage of joints resulting in faulty alignments of sections of the organ of locomotion, and in deformities of the extremities.
The hand is a part of the organ of locomotion with prehensile and tactile functions. The most frequent clinical symptoms of rheumatic diseases of the hand can be observed in the wrist and finger joints whose ligaments become excessively extended. These symptoms lead to a decrease in the stability of the fingers and ultimately to their deformation. Also lesions on the skin of the fingers appear frequently.
Symptoms in the nervous system can be related to compression or inflammation of peripheral nerves. The deformities not only disturb the hand's mechanical ability to grasp, but they can also affect its tactile functions and tactile sensitivity. The relationship between tactile sensitivity and manual functions of the hand in rheumatic patients has been so far the subject of relatively few studies [1–4].
Tactile sensitivity is strictly connected with the functional abilities of the hand since on the basis of information from tactile units about the friction or pressure between the skin and an object, the grip strength of the hand is automatically adjusted to prevent the object from slipping or breaking. Furthermore, the information from the mechanoreceptors generally affects the sending of motor commands from the brain to the muscles of the metacarpus and fingers [5]. The digital pulps feature a special ability to distinguish physical characteristics of various surfaces.
The tactile sensitivity of the foot and the lower leg in rheumatic patients has been studied by a number of authors. The aim of their studies was to determine the causes of patients’ walking problems [6, 7].
Although few authors have studied the tactile sensitivity of the hand in patients, (including rheumatic patients) the significance of this problem was noted by Schady et al. [8]. During their examination of patients’ hands and feet they observed a decrease in tactile sensitivity in patients with scleroderma. Also Serup noted a deterioration of tactile sensitivity in patients with systemic sclerosis and its impact on their motor function [9]. Tactile sensitivity affects the course of cognitive processes and therefore the quality of life, physical fitness and general functioning in the physical environment [10, 11].
The motor function undergoes changes in patients with rheumatic diseases. The pain and movement constraints force these patients to choose different movement strategies than their healthy counterparts. The proper level of tactile sensitivity when touching different objects or performing self-care activities is very important for the patients’ physical fitness and performance of all sorts of activities.
Aim
The aim of the present study was to determine the tactile threshold of the hand in female patients suffering from rheumatic diseases.
Material and methods
The study was carried out in the State Clinical Hospital of the University of Medical Sciences and in the Municipal Hospital in Poznan, Poland. The study sample comprised 99 female rheumatic inpatients aged 19–87 years (mean age 48.9 years), who were admitted to the Rheumatology Departments of both hospitals due to recurring attacks of the disease. 44.4% of patients suffered from rheumatoid arthritis, 16.2% from systemic lupus erythematosus, and 3% from systemic sclerosis. 36.4% were patients with other rheumatic disease entities, with each disease occurring in one or two patients. The mean time from the appearance of first clinical symptoms of inflammatory lesions of the intercarpal, metacarpophalangeal and interphalangeal articulations of the hand as well as joints of the arm was 7.2 years. The patients were mature women as they, more often than men, are taken ill with rheumatoid arthritis and other rheumatic diseases. The control group comprised 45 healthy women aged 23–80 years (mean age 46.6 years).
The tactile threshold measurements were carried out using The Touch-Test™ Sensory Evaluators (Stoelting CO. 620 Wheat Lane, Wood Dale, IL 60191), which is a precision instrument providing a non-invasive evaluation of cutaneous sensation levels throughout the body with objective and repeatable results. The esthesiometer is individually calibrated to deliver its targeted force within a 5% standard deviation. It comprises 20 filaments with the target force between 0.008 g and 300 g, or expressed in the manufacturer's own measurement units (SWM) between 1.65 (log10F(mg)) and 6.65 (log10F(mg)). The filaments are divided into ranges corresponding to particular sensitivity thresholds of the hand (Table 1). The hand thresholds were measured according to the manufacturer's testing procedure in three areas of the skin of the right hand and the left hand: the palmar surface of the index finger, the little finger and hypothenar eminence, and the dorsum of the hand.
Table 1.
Ranges of sensory monofilaments according to pressure force expressed in the manufacturer's units (SWM) and in grams (target force) and respective hand tactile threshold ranges
| SWM | Target force | Hand thresholds |
|---|---|---|
| 1.65–2.83 | 0.008–0.07 | Normal |
| 3.22–3.61 | 0.16–0.4 | Diminished light Touch |
| 3.84–4.31 | 0.6–2 | Diminished protective Touch |
| 4.56–6.45 | 4–180 | Loss of protective Touch |
| 6.65 | 300 | Deep pressure Sensation only |
During the measurements patients remained in a sitting position with their eyes closed. The filaments were pressed consecutively against particular measurement sites on the skin until the patient responded to the stimulus. The measurement results, name of disease, time of the appearance of first clinical symptoms, patients’ age and education and eyesight self-assessment were recorded in a table.
A questionnaire was used to determine possible movement limitations in the axillary, elbow, wrist and hand joints on a scale as follows: no movement limitations, small limitations, great limitations. Also the patients made a self-assessment of the physical disability of the right hand and the left hand (from 0 to 5). The level of disability was also measured with the Barthel Index (BI) consisting of 10 variables describing activities of daily living (ADL) and mobility. The level and type of lateralization were assessed with the Edinburgh Handedness Inventory (EHI) containing 10 items describing the use of the hand to perform activities of daily living.
The study was approved by the Bioethical Committee of the Poznan University of Medical Sciences according to the Good Clinical Practice developed on the basis of the Declaration of Helsinki.
Statistical analysis
The results were entered into an Excel database. The statistical analysis was made with the use of the Statistica software package (ver. 10, StatSoft). The results of the Kolmogorov Smirnov test showed that the distributions of the dependent variable differed significantly from normal distribution. For inter-group analysis, the Mann-Whitney U test was used. The relations between the patients’ age and education and the sensitivity tactile threshold were determined with Spearman's rank correlation coefficient.
Results
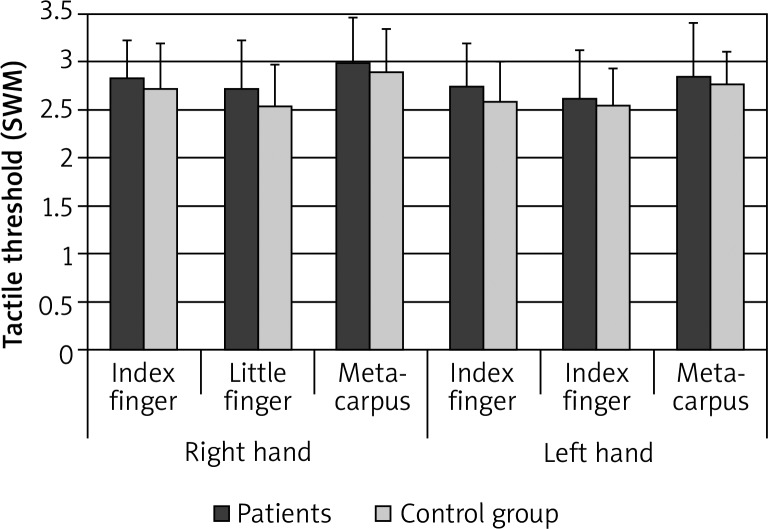
The comparison of mean tactile threshold values of the index finger, little finger and metacarpus of the right hand and the left hand in the patients and the controls revealed a higher tactile threshold (lower tactile sensitivity) in the patients at three measurement sites of both hands (Figure 1), with statistically significant differences on the little finger of the right hand (p = 0.014) and the index finger of the left hand (p = 0.013). The descriptive statistics of the dependent variable are presented in Table 2. The analysis of tactile sensitivity of three measurement sites on both hands in the patients and controls revealed the highest tactile sensitivity on the little finger, lower on the index finger and the lowest in the metacarpus (Figure 1). In patients, significant statistical differences were noted between the index finger and the metacarpus of the right hand (p = 0.018), the little finger and the metacarpus of the right hand (p < 0.001), and the little finger and the metacarpus of the left hand (p = 0.003). In the control group, statistically significant differences were found between the index finger and the little finger of the right hand (p = 0.003), the little finger and the metacarpus of the right hand (p = 0.0004), and the little finger and the metacarpus of the left hand (p = 0.01). The comparative analysis of the tactile threshold at the measurement sites on the right hand and the left hand did not reveal any significant differences in the patients or in the controls.
Figure 1.
Mean tactile threshold values with SD at three measurement sites on the right hand and the left hand in female rheumatic patients and women from the control group
Table 2.
Statistics of tactile sensitivity – patients and control group
| Measurement site | Patients | Control group | ||||||
|---|---|---|---|---|---|---|---|---|
| ME | Min. | Max. | SD | ME | Min. | Max. | SD | |
| Right hand: | ||||||||
| Index finger | 2.83 | 1.65 | 3.84 | 0.41 | 2.83 | 1.65 | 3.84 | 0.48 |
| Little finger | 2.83 | 1.65 | 3.84 | 0.51 | 2.44 | 1.65 | 3.61 | 0.44 |
| Metacarpus | 2.83 | 1.65 | 4.08 | 0.48 | 2.83 | 1.65 | 3.61 | 0.45 |
| Left hand: | ||||||||
| Index finger | 2.83 | 1.65 | 4.08 | 0.45 | 2.44 | 1.65 | 3.61 | 0.42 |
| Little finger | 2.44 | 1.65 | 3.61 | 0.51 | 2.44 | 1.65 | 3.61 | 0.40 |
| Metacarpus | 2.83 | 1.65 | 4.17 | 0.57 | 2.83 | 2.36 | 3.61 | 0.34 |
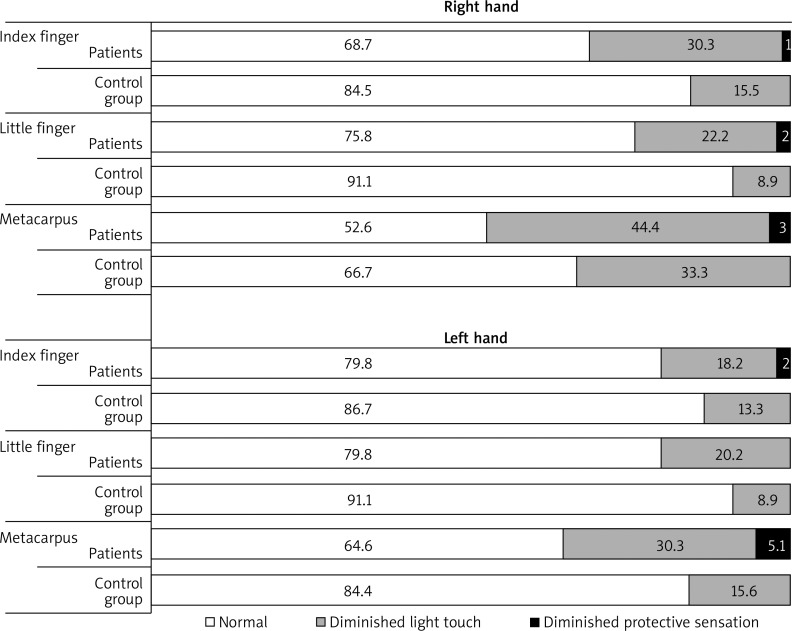
The evaluation of tactile sensitivity in the subjects is presented in Figure 2. The patients’ tactile sensitivity ranges measured with the Semmes-Weinstein Monofilaments were classified as normal, diminished light touch and diminished protective touch. The controls represented only two ranges: normal and diminished light touch. A much larger percentage of women from the control group represented the normal level at each measurement site of the right hand and the left hand in comparison with rheumatic patients. No woman from any group represented any of the last two ranges of tactile sensitivity: loss of protective touch and deep pressure sensation only.
Figure 2.
Assessment of tactile sensitivity of female rheumatic patients and women from the control group at three measurement sites of the right hand and the left hand (in percent)
On the basis of the EHI results, the subjects were divided into right-handed, ambidextrous and left-handed ones. The right-handed women amounted to 80.8% of all examined patients, ambidextrous patients – 8.1%, and left-handed – 11.1%. A similar distribution of EHI results was found in women from the control group (82.2% – right-handed, 13.3% – ambidextrous, and 8.9% – left-handed). Within the experimental and control groups, the newly formed groups with regard to handedness did not differ significantly from one another in terms of their tactile sensitivity threshold.
Beside the statistical significance of differences, the following observations were also made: in the patients: the hands were less sensitive at all measurement sites; in the controls: the right hands were less sensitive on the index finger and the metacarpus, and the left hands were less sensitive on the little finger.
The arithmetic means of tactile sensitivity in ambidextrous subjects were significantly lower, which indicates higher tactile sensitivity in this group. However, the low number of these subjects made statistical analysis impossible.
The articular movement limitations in the subjects are presented in Table 3. These limitations had no statistically significant effects on the women's tactile sensitivity.
Table 3.
Articular movement limitations (in percent)
| Limitation ofarticular movement | Wrist joint and fingers | Elbow joint | Axillary joint | |||
|---|---|---|---|---|---|---|
| R | L | R | L | R | L | |
| No limitation | 68.7 | 63.6 | 84.8 | 81.8 | 74.7 | 72.7 |
| Small | 24.2 | 30.3 | 12.1 | 16.2 | 18.2 | 21.2 |
| Large | 7.1 | 6.1 | 3.0 | 2.0 | 7.1 | 6.1 |
R – right hand, L – left hand
The results of self-assessment of the disability of the right hand and the left hand (from 0 to 5) are shown in Table 4. On the basis of these results, the patients were divided into groups with significant differences between their tactile threshold values. The degree of disability of the hand was correlated with tactile sensitivity: the fitter the hand was, the more sensitive it was (Table 5).
Table 4.
Degree of disability of the right hand and the left hand self-assessed by rheumatic patients (in percent)
| Disability level | Right hand | Left hand |
|---|---|---|
| 0 | 0 | 0 |
| 1 | 4.0 | 3.0 |
| 2 | 10.1 | 11.1 |
| 3 | 26.3 | 28.3 |
| 4 | 30.3 | 31.3 |
| 5 | 29.3 | 26.3 |
0 – fully disabled hand, 5 – fully dexterous hand
Table 5.
Correlations between tactile sensitivity and the functional fitness of the hand in patients’ subjective view
| Measurement site | R | Value of p |
|---|---|---|
| Right hand: | ||
| Index finger | −0.25 | 0.0036 |
| Little finger | −0.22 | 0.0115 |
| Metacarpus | No correlation | |
| Left hand: | ||
| Index finger | −0.27 | 0.0020 |
| Little finger | −0.27 | 0.0020 |
| Metacarpus | −0.28 | 0.0013 |
R – Spearman's rank correlation coefficient, p – statistical significance level
The Barthel Index value determining the patients’ independence in performing ADL was = 72.8 (min. 32, max. 80). The BI values for individual patients were not correlated with the threshold of their tactile sensitivity.
The obtained Spearman's rank correlation of coefficients showed a correlation between tactile sensitivity and age (Table 6). Older women were less tactilely sensitive both in the group of patients (non-significant differences only on the metacarpus of the right hand) and the control group.
Table 6.
Correlations between tactile sensitivity and age in patients and controls
| Measurement site | Patients | Control group | ||
|---|---|---|---|---|
| R | p | R | p | |
| Right hand: | ||||
| Index finger | 0.41 | < 0.0001 | 0.55 | 0.0001 |
| Little finger | 0.31 | 0.0018 | 0.32 | 0.0356 |
| Metacarpus | 0.19 | 0.0530 | 0.38 | 0.0138 |
| Left hand: | ||||
| Index finger | 0.23 | 0.0193 | 0.55 | 0.0001 |
| Little finger | 0.37 | 0.0001 | 0.50 | 0.0006 |
| Metacarpus | 0.24 | 0.0156 | 0.44 | 0.0029 |
R – Spearman's rank correlation coefficient, p – statistical significance level
The subjects differed in their education. In the rheumatic patients, their education was not correlated with tactile sensitivity. Women from the control group with a lower level of education displayed lower tactile sensitivity. Statistically significant correlations were found on the metacarpus of the right hand (p = 0.01), the index finger of the left hand (p = 0.04) and on the metacarpus of the left hand (p = 0.03).
Also statistically significant correlations were found between tactile sensitivity and eyesight self-assessment in the patients on the left and the right index fingers, little finger of the both hands, metacarpus of the left hand (p from 0.000 to 0.009); and in the control group on the little finger and the metacarpus of the right hand and the index finger and the little finger of the left hand (p from 0.008 to 0.038). Worse eyesight was correlated with lower tactile sensitivity.
Discussion
The hand is innervated with nerves branching from the brachial plexus: the median nerve, the ulnar nerve, and the radial nerve. The cutaneous branches of skin nerves reach the epidermis forming free nerve endings or end with encapsulated receptors. The tactile receptors which can be stimulated with the filaments include Merkel disc receptors responding to light sustained pressure, or Meissner's corpuscles, which are also responsible for light touch and point sensitivity [12, 13]. The tactile sensitivity of the hand is the highest on the fingertips, which are the preferred sites for tactile examination [14–16]. In the present study, the tactile thresholds were measured on the palmar surface of the index finger, and hypothenar eminence and the dorsum of the hand, i.e. two sites innervated with the median nerve branches, however, differing in the density of tactile receptors. The palmar areas of the fingers feature a high density of tactile receptors (about 100–140/cm 2), whose number decreases towards the wrist [12]. For comparison, also the palmar area of the little finger, innervated with the hand branch of the ulnar nerve was examined with the use of sensory filaments.
The majority of female patients in the present study suffered from rheumatoid arthritis. The course of this disease, more often than any other rheumatic diseases, involves the incidence of carpal tunnel syndrome (CTS). The inflammation of the tendons of finger flexors and the granulation under the flexor retinaculum of the hand cause a pressure on the median nerve. This leads to such characteristic symptoms as stiffening, paresthesia, finger pains, lack of sensation or hypersensitivity to tactile stimuli in the parts of the body innervated by the median nerve. The frequency of CTS in patients with rheumatic arthritis (RA) is estimated between 3.6% and 6% [17–20]. Another study revealed the CTS incidence in 25% of patients with RA, 87.5% of whom were at the active stage of the disease [21].
Other clinical symptoms of rheumatic diseases can also cause changes in the level of tactile sensitivity. They include finger deformations, skin lesions on the fingers (thinner, thicker, inelastic, shiny, wet skin) cicatricial lesions on the fingertips – characteristic of systemic sclerosis, decreasing mass of the fingers and their hardening. The function of tactile receptors depends on the physiological properties of the central and peripheral nervous systems and on physical properties of the skin. In comparison with the controls, female patients had lower tactile sensitivity (Figure 2). Their percentage within the “Normal” range of hand thresholds was higher than in the control group. Moreover, only few patients and no controls were in the range of “Diminished protective sensitivity”. However, significant differences between the two groups were found on the little finger of the right hand (p = 0.014) and the index finger of the left hand (p = 0.013). No patients or controls were found in the last two ranges of hand thresholds, i.e. “Loss of protective sensitivity” and “Deep pressure sensation only”, which could have otherwise indicated an eyesight dysfunction. According to our expectations, the highest tactile sensitivity was detected on the little finger followed by the index finger and the metacarpus in the patients and the control group (Figure 1). The little finger is innervated with the hand branch of the ulnar nerve, the index finger with the branches of the median nerve. The respectively higher density of tactile receptors on the fingertips as compared with the metacarpus is related to the higher tactile sensitivity [13]. Hodge et al. carried out an esthesiometric assessment of plantar pressure pain thresholds in patients with rheumatoid arthritis. He found no statistically significant differences in tactile sensitivity between the patients and the controls [6]. In a different study of patients with rheumatoid arthritis, plantar sensibility was significantly lower under all examined foot regions compared with the control group (p < 0.05) [17].
To determine the patients’ degree of independence in performing activities of daily living, the Barthel Index was used. It is a complementary instrument used to measure one's health status by determining one's physical fitness in daily life [22–24]. The total BI value in the present study amounted to = 72.8 (min. 32, max. 80), which shows that all the examined patients represented a group with a medium degree of functional disability, experiencing difficulties in performing some ADL.
The decreasing tactile sensitivity with age observed in the patients and controls corresponds to the results of earlier research [25–28]. Thornbury and Mistretta measured the tactile threshold on the palmar area of the index finger in individuals aged 19–88 years. They observed that the tactile thresholds increased significantly with age. A large proportion of elderly individuals had higher than average tactile thresholds of young adults, although older people varied widely in touch sensitivity. A population study also revealed that tactile sensitivity in both sexes decreases with age starting with pubescence [29].
Another factor which significantly differentiated tactile sensitivity between the patients and controls was the education level. Individuals with secondary or higher education are more sensitive than those with elementary or vocational education [30]. The present study revealed such a relationship in the control group but not in the patients at any measurement site. It can be asserted that a rheumatic disease introduces determinants of tactile sensitivity.
Conclusions
Female patients in the present study as compared with their counterparts from the control group, featured lower tactile sensitivity; however, none of them was classified in a range indicating a severe disturbance of the sense of touch. The articular movement limitations influence tactile sensitivity only non-significantly. The obtained Barthel Index values show that all the examined patients represented a group with a medium level of functional fitness. The level of hand disability determined by the patients’ self-assessment was correlated with tactile sensitivity: the more fit the hand was, the more sensitive it was. The lateralization in the observed patients was similar to that of the control group and was not correlated with tactile sensitivity. Rheumatic patients’ tactile sensitivity decreases with age but is not correlated with their level of education. The worsening eyesight, independent of rheumatic disease, corresponds, however, with decreasing tactile sensitivity.
Conflict of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.King PM. Sensory function assessment. J Hand Ther. 1997;10:24–8. [PubMed] [Google Scholar]
- 2.van Brakel WH, Kets CM, van Leerdam ME, et al. Functional sensibility of the hand in leprosy patients. Leprosy Rev. 1997;68:25–37. doi: 10.5935/0305-7518.19970005. [DOI] [PubMed] [Google Scholar]
- 3.Bjorkman A, Rosen B, Lundborg G. Acute improvement of hand sensibility after selective ipsilateral cutaneous forearm anaesthesia. Eur J Neurosci. 2004;20:2733–6. doi: 10.1111/j.1460-9568.2004.03742.x. [DOI] [PubMed] [Google Scholar]
- 4.Melchior H, Vatine JJ, Weiss PL. Is there a relationship between light touch-pressure sensation and functional hand ability. Disabil Rehabil. 2007;29:567–75. doi: 10.1080/09638280600902547. [DOI] [PubMed] [Google Scholar]
- 5.Marsden CD, Merton PA, Morton HB. The sensory mechanism of servo action in human muscle. J Physiol. 1977;265:521–35. doi: 10.1113/jphysiol.1977.sp011728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodge MC, Nathan D, Bach TM. Plantar pressure pain thresholds and touch sensitivity in rheumatoid arthritis. Foot Ankle Int. 2009;30:1–9. doi: 10.3113/FAI.2009.0001. [DOI] [PubMed] [Google Scholar]
- 7.Rosenbaum D, Schmiegel A, Meermeier M, Gaubitz M. Plantar sensitivity, foot loading and walking pain in rheumatoid arthritis. Rheumatology. 2006;45:212–4. doi: 10.1093/rheumatology/kei137. [DOI] [PubMed] [Google Scholar]
- 8.Schady W, Sheared A, Hassell A, et al. Peripheral nerve dysfunction in scleroderma. Q J Med Aug. 1991;80:661–75. [PubMed] [Google Scholar]
- 9.Serup J. Tactile sensitivity in systemic sclerosis. Assessment of two-point and circle discriminations of the digit. Dermatologica. 1984;168:279–82. [PubMed] [Google Scholar]
- 10.Keller PE, Ishihara M, Prinz W. Effects of feedback from active and passive body parts on spatial and temporal parameters in sensorimotor synchronization. Cogn Process. 2011;12:127–33. doi: 10.1007/s10339-010-0361-0. [DOI] [PubMed] [Google Scholar]
- 11.Cuypers K, Levin O, Thijs H, et al. Long-term TENS treatment improves tactile sensitivity in MS patients. Neurorehabil Neural Repair. 2010;24:420–7. doi: 10.1177/1545968309356301. [DOI] [PubMed] [Google Scholar]
- 12.Johannson RS. Tactile sensibility in the human hand: receptive field and absolute densities of four types of mechanoreceptive units in glabrous skin area. J Physiol. 1978;281:101–23. doi: 10.1113/jphysiol.1978.sp012411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johannson RS, Vallbo AB. Tactile sensibility in the human hand: relative and absolute densities of four types of mechanoreceptive units in glabrous skin. J Physiol. 1979;286:283–300. doi: 10.1113/jphysiol.1979.sp012619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellon ES, Keller K, Moratz V, Dellon AL. The relationships between skin hardness, pressure perception two-point discrimination in the fingertip. J Hand Surg. 1995;20:44–8. doi: 10.1016/s0266-7681(05)80015-4. [DOI] [PubMed] [Google Scholar]
- 15.Gescheider GA, Thorpe JM, Goodarz J, Bolanowski SJ. The effects of skin temperature on the detection and discrimination of tactile stimulation. Somatosens Mot Res. 1997;14:181–8. doi: 10.1080/08990229771042. [DOI] [PubMed] [Google Scholar]
- 16.Verrillo RT, Bolanowski SJ, Checkosky CM, Mcglone FP. Effects of hydration on tactile sensation. Somatosens Mot Res. 1998;15:93–109. doi: 10.1080/08990229870826. [DOI] [PubMed] [Google Scholar]
- 17.Lanzillo B, Pappone N, Crisci C, et al. Subclinical peripheral nerve involvement in patients with rheumatoid arthritis. Arthritis Rheum. 1998;41:1196–202. doi: 10.1002/1529-0131(199807)41:7<1196::AID-ART8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 18.Sivri A, Guler-Uysal F. The electroneurophysiological evaluation of rheumatoid arthritis patients. Clin Rheumatol. 1998;17:416–8. doi: 10.1007/BF01450907. [DOI] [PubMed] [Google Scholar]
- 19.Sivri A, Guler-Uysal F. The electroneurophysiological findings in rheumatoid arthritis patients. Electromyogr Clin Neurophysiol. 1999;39:387–91. [PubMed] [Google Scholar]
- 20.Shinoda J, Hashizume H, McCown C, et al. Carpal tunnel syndrome grading system in rheumatoid arthritis. J Orthop Sci. 2002;7:188–93. doi: 10.1007/s007760200032. [DOI] [PubMed] [Google Scholar]
- 21.Aluclu MU, Turhanoglu AD, Aluclu MA. The frequency of carpal tunnel syndrome in patients with rheumatoid arthritis. Internet J Neurol. 2006;5(2) [Google Scholar]
- 22.Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud. 1988;10:61–3. doi: 10.3109/09638288809164103. [DOI] [PubMed] [Google Scholar]
- 23.Wade DT, Collin C. The Barthel ADL index: a standard measure of physical disability? Int Disabil Stud. 1988;10:64–7. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 24.Bejer A, Kwolek A. Assessment of quality of life among elderly stroke patients – preliminary report. Physioterapy. 2008;16:52–63. [Google Scholar]
- 25.Kenshalo DR. Somesthetic sensitivity in young and elderly humans. J Gerontol. 1986;41:732–42. doi: 10.1093/geronj/41.6.732. [DOI] [PubMed] [Google Scholar]
- 26.Gescheider GA, Bolanowski SJ, Hall KL, et al. The effects of aging on information-processing channels in the sense of touch: I. Absolute sensitivity. Somatosens Mot Res. 1994;11:345–57. doi: 10.3109/08990229409028878. [DOI] [PubMed] [Google Scholar]
- 27.Verrillo RT, Bolanowski SJ, Gescheider GA. Effect of aging on the subjective magnitude of vibration. Somatosens Mot Res. 2002;19:238–45. doi: 10.1080/0899022021000009161. [DOI] [PubMed] [Google Scholar]
- 28.Desrosiers J, Hebert R, Bravo G, Dutil E. Hand sensibility of healthy older people. J Am Geriatr Soc. 1996;44:974–8. doi: 10.1111/j.1532-5415.1996.tb01871.x. [DOI] [PubMed] [Google Scholar]
- 29.Thornbury JM, Mistretta CM. Tactile sensitivity as function of age. J Gerontol. 1981;36:34–9. doi: 10.1093/geronj/36.1.34. [DOI] [PubMed] [Google Scholar]
- 30.Kozłowska A. Studying tactile sensitivity – population approach. Prz Antrop. 1998;61:3–30. [Google Scholar]