Summary
The spectral sensitivity of adult male Cupiennius salei Keys, a nocturnal hunting spider, was studied in a behavioural test. As known from earlier behavioural tests, C. salei walks towards a black target presented in front of a white background. In this study a black target (size 42 × 70 cm) was presented in a white arena illuminated by monochromatic light in the range of 365 to 695 nm using 19 monochromatic filters (HW in the range of 6 – 10 nm). In the first trial, the transmission of the optical filters was between 40 % and 80%. In a second trial the transmission was reduced to 5%, using a neutral density filter. At the high intensity the spiders showed a spectral sensivity in the range from 380 to 670 nm. In the second trial the animals only showed directed walks if the illumination was in the range of 449 to 599 nm, indicating a lower sensitivity at the margins of the spectral sensitivity. In previous intracellular recordings, the measured spectral sensitivity was between 320 and 620 nm. Interestingly, these results do not completely match the behaviourally tested spectral sensitivity of the photoreceptors, where the sensitivity range is shifted to longer wavelengths. In order to investigate the molecular background of spectral sensitivity, we searched for opsin genes in C. salei. We found three visual opsins that correspond to UV and middle to long wavelength sensitive opsins as described for jumping spiders.
Keywords: behavioural test, Cupiennius salei, eyes, spectral sensitivity, vision, opsins
Introduction
The hunting spider Cupiennius salei Keys is a nocturnal predator that has been in focus of interest for a long time because of its excellent mechano-sensory systems. The function and the use of the visual sense were unclear and believed to play no greater role in the behaviour of C. salei. For hunting and mating the spider depends mainly on its mechanosensory systems (Barth and Schmitt, 1991; Eckweiler and Seyfarth, 1988; Hergenröder and Barth 1983; Schuch and Barth, 1985; Schuch and Barth 1990; Seyfarth et al., 1985). Recent studies however, have shown that C. salei has also a well developed visual system (Fenk and Schmid, 2010; Grusch et al., 1997; Kaps and Schmid, 1996; Land and Barth, 1992). Fenk et al. (2010) showed that a visual stimulus alone can elicit attack behaviour. Behavioural experiments showed that the animals are able to select adequate dwelling plants for daytime hiding (Schmid, 1998).
Common to most of spiders, C. salei has eight eyes; a pair of principal eyes (anterior-median, AM) and three pairs of secondary eyes (anterior-lateral, AL; posterior-median, PM; posterior-lateral, PL). The retinae of the AM eyes are moved by a pair of muscles (Kaps, 1998). The rhabdomeres of the receptor cells of the AM-eyes are orientated towards the light. The secondary eyes possesss a tapetum in the back of the eyes and have inverted photoreceptor cells (Land, 1985). The receptive fields of the eyes nearly cover the whole surrounding area of the spider, except a small section behind it. The visual fields of the AM- and the PM-eyes overlap almost completely. Overlapping visual fields and the different anatomy of the principal and the secondary eyes presume that these two pairs of eyes might have separate functions (Land, 1985). Only the AM-eyes have muscles with spontaneous activity, which leads to the conclusion that they allow the discrimination of stationary targets, while the secondary eyes are tuned to detect movable objects (Land, 1985; Schmid, 1998; Neuhofer et al., 2009).
Behavioural studies with the nocturnal, desert-living jumping spider called Dancing White Lady spider (Leucorchestris arenicola), which is known to cover some distance to find mating partners or food, have shown that its ability to find its way back home is diminished when the eyes are covered. Gravity, odour marks or vibrations of the ground seem to be of no importance (Nørgaard et al., 2008). In an investigation on the spectral sensitivity of the jumping spider Maevia inclemens it was shown that wavelengths between 330 to 700 nm could be detected (Peaslee and Wilson, 1989). The home of C. salei offers a lot more landmarks for orientation, so there might be the possibility that the eyes do play a role in orientation behaviour, for example to find bromeliads, the preferred mating place of C. salei.
ERG-recordings revealed a possible spectral sensitivity from 300 to 700 nm and a sensitivity threshold for white light below 0.01 l× (Barth et al., 1993). Using intracellular electrophysiology recordings, Walla et al. (1996) demonstrated the existence of three photoreceptors with sensitivity maxima at 340 nm (UV-receptor), 480 nm (blue receptor) and 520 nm (green receptor). The blue and the green receptors both have a second peak in the range of the λmax of the UV-receptor. The UV-receptors could only be found in the secondary eyes, and only once in each of them. The existence of UV-cells, however, does not prove that these animals use the information from this part of the spectrum in visually guided behaviour.
In previous behavioural experiments it was shown that C. salei runs towards a presented black target if there are no other visual stimuli. In a twofold choice experiment, different shapes of cardboards were tested. C. salei showed a preference for black oblongs that were presented upright (Schmid, 1998). Why the spiders are heading for a black target at all, may be because it provides a good hiding place for the bay-coloured spider, which is nearly invisible on a dark background such as the bark of a tree. That makes it difficult for predators and also for potential prey to detect it. This crypsis through background matching is a strategy well investigated for the crab spiders Misumena vatia and Thomismus spectabilis. These spiders can even change the colour of their bodies from white to yellow depending on the colour of the flower they are sitting on and waiting for prey (Defrize et al., 2010; Heiling et al., 2005a; Insausti and Casas, 2008; Théry, 2007; Weigel, 1941).
In the present study, we use a behavioural test to assess if C. salei uses the complete spectral sensitivity range that was detected in electrophysiological recordings. Specifically, we assayed the ability of C. salei to detect a black target on a background of monochromatic light at different wavelengths in the 365 to 695 nm range. In order for an eye to detect light of different wavelengths, it needs multiple opsins that respond to light of different wavelengths. In a study of two species of jumping spiders that have colour vision, three opsins were found (Koyanagi et al., 2008). Phylogenetic analysis indicated that one of these opsins (Rh3) was UV sensitive, and the other two (Rh1 and Rh2) grouped with middle and long wavelength sensitive opsins of other arthropods.
To determine if C. salei has the molecular equipment to detect light of different wavelengths, we searched for the presence of opsin RNA in the different eye types.
Material and Methods
Animals
Adult males of C. salei raised in our laboratory in Vienna were used for this experiment. The spiders were kept individually in glass jars (25 cm high, 14.5 cm diameter) and fed once per week on flies. The temperature was 22°C and the relative humidity above 60%.
Male spiders have much longer active phases than females and therefore are used in running experiments (Schmitt et al., 1990).
Experimental apparatus
The animals used in the experiments were kept under an artificial photoperiod (12:12 LD). The experiments took place one hour after the night phase started. Experiments were performed in a room without natural light. The size of the quadratic arena was 210 cm × 210 cm. The walls were painted white and the ground was covered with a white polythene sheet. A black cardboard (42 × 70 cm) was used as target. The arena was illuminated by a light projector (Xenotar, Götschmann, Munich, Germany), used in combination with monochromatic filters between 365 and 695 nm (Tab.1) and a neutral density filter NG -9 (Schott, Mainz, Germany).
Tab. 1. Statistical evaluation of the data from the experiment with monochromatic filters.
The length of the mean vector (r) gives an indication of one-sidedness. r can have a minimum value of 0 and a maximum of 1. If r is sufficiently large, the hypothesis of randomness can be rejected in favour of one-sidedness. mvd: mean vector direction, r: length of the mean vector, s: angular deviation. Numbers in bold show the mean vectors which point within sector 0°, e.g. to the black bar (n=15, N=30). Asterisk marks significance.
| Filter (nm) | number of positive runs | mvd | r | s | Rayleigh-Test |
|---|---|---|---|---|---|
| 365 | 11 | 353.6 ° | 0.580 | 52.2° | p ≤0.01* |
| 389.9 | 22 | 357.8 ° | 0.815 | 34.8° | p ≤0.01* |
| 403.2 | 21 | 2.3 ° | 0.827 | 33.7° | p ≤0.01* |
| 420.2 | 26 | 359.0 ° | 0.847 | 31.7° | p ≤0.01* |
| 434.5 | 24 | 357.0 ° | 0.788 | 37.3° | p ≤0.01* |
| 448.5 | 27 | 4.6 ° | 0.945 | 19.0° | p ≤0.01* |
| 478 | 24 | 0.7 ° | 0.846 | 31.8° | p ≤0.01* |
| 499.3 | 23 | 5.5 ° | 0.861 | 30.2 | p ≤0.01* |
| 513.9 | 28 | 0.0 ° | 0.954 | 17.4° | p ≤0.01* |
| 519.6 | 25 | 357.5 ° | 0.879 | 28.2° | p ≤0.01* |
| 538.2 | 27 | 2.0 ° | 0.929 | 21.5° | p ≤0.01* |
| 547.2 | 25 | 359.3 ° | 0.802 | 36.1° | p ≤0.01* |
| 575.1 | 27 | 356.2 ° | 0.961 | 16.1° | p ≤0.01* |
| 588.3 | 27 | 358.7 ° | 0.894 | 26.4° | p ≤0.01* |
| 598.6 | 27 | 5.7 ° | 0.906 | 24.9° | p ≤0.01* |
| 614.6 | 27 | 358.0 ° | 0.890 | 26.8° | p ≤0.01* |
| 654 | 25 | 353.4 ° | 0.839 | 32.5° | p ≤0.01* |
| 670.1 | 15 | 2.3 ° | 0.675 | 46.2° | p ≤0.01* |
| 695 | 4 | 42.2° | 0.360 | 64.8° | p >0.01 |
In the first experiment only the 19 different monochromatic filters were used (transmission between 40 and 80% and a half-width in the range of 6 – 10 nm). In a second experiment a neutral density filter NG-9 was used in combination with each monochromatic filter to reduce the transmission to 5%.
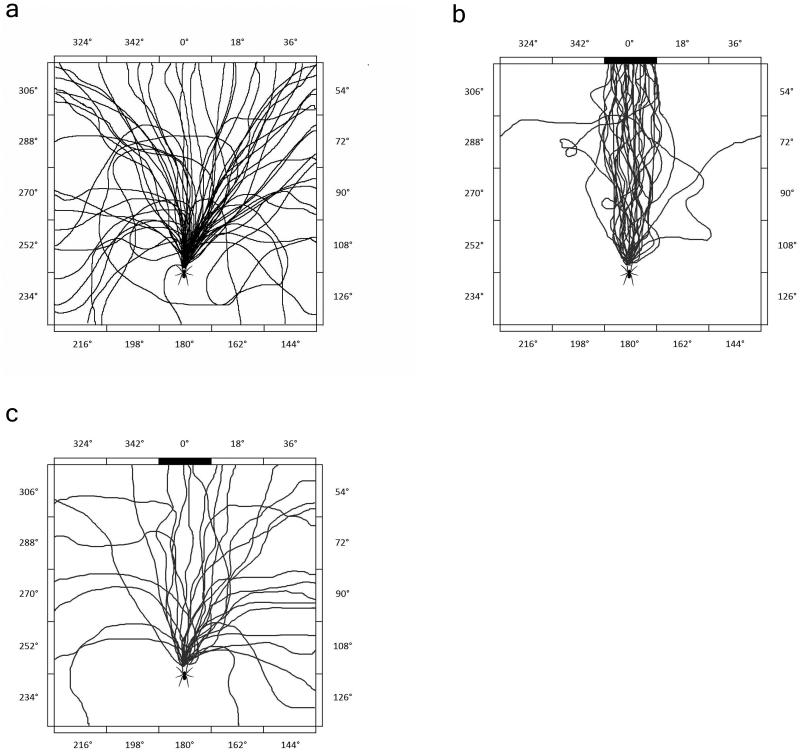
For statistical analysis, the arena was divided into 20 sectors, each sector corresponding to a specific orientation angle. Sector 0° corresponds to an angle from 351° to 8° (which is exactly the position of the black bar), sector 18° from 9° to 26° and sector 36° from 27° to 44° and so on (Fig.1).
Fig. 1. (a) Pathways of 60 runs of 15 spiders at the control experiment, without a target and at white light illumination.
(b) Pathways of 30 runs of 15 spiders at 513.9 nm, displaying 28 positive runs, one spider touching the wall at sector 288°, and another at 72°. (c) Pathways at 695 nm. Only 4 runs ended at the black bar, and other runs were distributed over a wide range.
The spiders were put into the arena at a distance of 2 meters from the black target in a plastic box that was removed when the spiders were in the correct position, i.e. oriented towards the black target. If the spider walked erratically and touched the wall or showed a fright-posture, the experiment was stopped and repeated the next day. If the spider did not move but was in a ready-posture, we gently nudged its hind legs using a cotton-coated stick to activate it. The walking path of the spider was observed by the eye and plotted by hand.
In total, 15 animals were tested in two runs each, and the number of runs within each sector was counted. A run to sector 0°, e.g. the black target was rated as a positive run. The other runs were regarded as negative. All runs were measured and used for statistical analysis.
The mean vector and vector length were calculated with the program Rayleigh & Co. (Oxalis GmbH, Germany), and the statistical support for directedness tested using circular statistics (Batschelet, 1981). We used 95% confidence limits for the mean, and the level of significance was p ≤ 0.01.
A control experiment with no target was performed to see whether the spiders showed any preferences for one side of the arena even without a visual stimulus.
Screening for opsin genes
Total RNA was isolated with the Trizol method (Invitrogen/Life technologies) from mixed embryonic tissue, CNS from adults and adult retinas. The extracted RNA was sent to Genecore (EMBL, Heidelberg) for sequencing (Illumina hi-seq, paired-end 100 bp). Following de novo assembly, we searched the resulting transcript database for matches to a diverse set of opsin proteins downloaded from NCBI. BLAST searches and sequence analysis were done with the computer program Geneious version 5.6.6 created by Biomatters (http://www.geneious.com/). Opsin sequence orthology was established by aligning the identified C. salei sequences to arthropod visual opsin sequences, with an onychophoran rhodopsin sequence included as an outgroup, followed by calculation of a Bayesian tree using MrBayes with a Poisson distribution model of amino acid substitutions (Hulsenbeck and Ronquist, 2001). Opsin sequences were aligned with clustalW and regions outside of the 7 transmembrane domains were excluded.
Reverse transcript PCR
Total RNA was isolated from dissected retinas from each of the four eye types; AM, PM, AL and PL. RNA was extracted with the Trizol method (Invitrogen/Life technologies) and used for reverse transcription using Thermoscript (Invitrogen/Life technologies). The resulting cDNA was used as template in subsequent polymerase chain reactions (PCR). Primers were constructed from opsin sequences found in the C. salei transcriptome database using the software primer3 (Rozen and Skaletsky, 2000). The following primers were used: Cs-Rh1 forward 5′ TTTTCGGACCCATACGAGAG 3′ Cs-Rh1 reverse 5′ GGTTTACCCAGGCATTTGAA3′, Cs-Rh2 forward 5′ GTGGTCCTGTTGGCTAGCAT 3′ Cs-Rh2 reverse 5′ ATGACACTCGTTTCGGACCT 3′, Cs-Rh3 forward 5′ GGCATTCCTGGACGAGATAA 3′ Cs-Rh3 reverse 5′ ATTCATTTTGCGAGCCTGTT 3′. A PCR reaction was done with primers from each of the three visual opsins on cDNA from each of the four eye types using GoTaq Flexi DNAPolymerase (Promega). The opsin sequences have been submitted to EMBL and have the following accession numbers: Cs-Rh1 HF549177, Cs-Rh2 HF549178, Cs-Rh3 HF549179
Results
Behaviour during the experiment
The animals were positioned at a release point in the arena, and after the release, typically they turned slightly to the left and to the right before they started walking. While running towards the bar, they showed a characteristic zigzag mode, which indicates a target-oriented behaviour. At a distance of about 50 cm to the target the spiders accelerated and ran straight to the bar. In most cases, they didn’t head for the middle of the bar, but for an edge. If the spiders did not run towards the bar, their paths showed various curves terminating at the wall.
Control experiment
In the control experiment performed under white light illumination but without a target the spiders showed no preference for any side of the arena. The mean vector points towards 12.7°. The runs were distributed randomly as it is shown by the length of the mean vector amounts 0.299 and the angular deviation was considerably high with 67.8° (Fig.1).
Experiment with monochromatic filters
Between the wavelengths 389.9 and 654 nm the spiders showed very good spectral sensitivity. On average 82% of the spiders showed positive runs (directed walk to the black bar) and the mean vector is within sector 0° (between 351° and 9). The vector length minimum amounts 0.6 and the angular deviation is below 40°. The best result with 28 out of 30 positive runs was observed at 513.9 nm. The pathways of the spiders at this wavelength are shown in Fig. 1b.
The mean vector at a wavelength of 365 nm points within sector 0°, but the brief length of the vector (0.58) and the big angular deviation (52.2°) indicate a less distinctive result, despite the statistical significance of p ≤0.01*. The results for the 365 nm and 670.1 nm filters show a significant result with angular deviations of 52.2° and 46.2° respectively and mean vector lengths of, 0.580 and 0.675 respectively; however, these results are lower than the results for the intervening wavelengths, suggesting that the wavelengths at 365 and 670.1 nm are close to the limit of the spectral sensitivity. At 695 nm the result shows clearly that C. salei is not able to detect the black bar at this light condition, for the mean vector points to 42.2°, the vector length is very short with 0.36 and the angular deviation very big with 64.8°. The pathways of the spiders at 695 nm are shown in Fig. 1c.
Monochromatic filters in combination with neutral density filter
From 389.9 to 420.2 nm the runs are distributed randomly. At 434.5 nm the mean vector only approximate to sector 0°, but point to the neighbouring sector. Not until 448.5 nm the mean vector is within sector 0° and the results are significant. Between 448.5 and 598.6 nm on average 72% of the spiders showed positive runs. The mean vectors at the wavelengths 513.9 and 538.2 nm are slightly shifted to the left. There, most of the negative runs are directed to the left wall. From 614.6 to 670.1 nm the runs are again distributed randomly. Therefore, the spectral sensitivity is limited to a range between 448.5 and 589.6 nm.
Opsin genes in C. salei
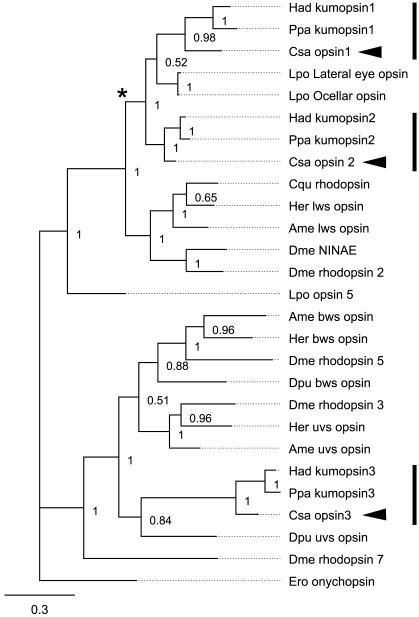
We found six opsin genes in our screening of the transcriptomes. Two were only detected in the transcriptome based on CNS specific RNA that excluded eye tissue, and therefore not further considered in this paper. Phylogenetic analysis (Fig. 2) showed that three of the remaining four opsin sequences clearly grouped with arthropod visual opsins. The Cs-Rh1 and Cs-Rh2 grouped together with jumping spider opsin Rh1 and Rh2 with the long to middle wavelength sensitive opsins and Cs-Rh3 grouped together with jumping spider Rh3 with UV with short wave length sensitive opsins. The Cs-Rh3 sequence also contains the lysine residue in transmembrane region 2 that has been shown to be responsible for UV sensitivity (Salcedo et al., 2003). The fourth opsin sequence detected in the retina showed greatest similarity to peropsin of the jumping spider Hasarius adansoni and is not further discussed here. The three opsins were expressed in all of the eyes (Fig. 3). The quantity of transcripts of the three different visual opsins was very different with Cs-Rh2 representing 31% of all transcripts (reads per kilobase per million reads (RPKM) of 243860), Cs-Rh1 0.6 % of transcripts (6400 RPKM) and Cs-Rh3 with 41 PPM (45 RPKM).
Fig. 2. Phylogenetic reconstruction of arthropod and onychophoran visual opsins.
The three is from Bayesian likelihood analysis using MrBayes on protein sequences: half compatibility consensus from 451,000 replicates, burn-in of 100,000 replicates. One onychophoran rhodopsin was used as out-group (Ero onychopsin). Numbers at nodes represent posterior probabilities of Bayesian likelihood analysis. Star marks chelicerate opsins except for UV opsins. Arrowheads mark opsins of Cupiennius salei. Vertical bars mark spider opsins. Scale bar show 0.3 substitutions per site. Species included in the analysis are: Ame = Apis mellifera, Cqu = Culex quinquefasciatus, Csa = Cupiennius salei, Dme = Drosophila melanogaster, Dpu = Daphnia pulex, Her = Heliconius erato, Ero = Euperipatoides rowelli, Had = Hasarius adansoni, Lpo = Limulus polyphemus, Ppa = Plexippus paykulli.
Fig. 3. Images of gel-electrophoresis run with reverse transcriptase PCR products from different tissue.
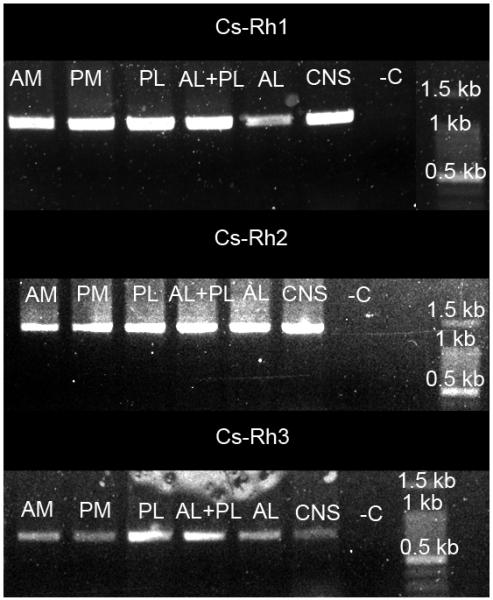
The image show products of: Cs-Rh1 (1164 base pairs), Ca-Rh2 (1285 base pairs) and Cs-Rh3 (748 base pairs). AM = anterior median eye (retina), PM = posterior median eye (retina), AL = anterior lateral eye (retina), PL = posterior lateral eye (retina), CNS = central nervous system, -C = negative control.
Discussion
The results of the control experiment showed that when no target is present, C. salei is more likely to turn left, right, or wander aimlessly, than to walk straight ahead. At wavelengths outside of their spectral sensitivity range, the spiders walked very slowly and used the first pair of legs as guide sticks, which indicates that they cannot see (Schmid, 1997). During the behavioural experiments with monochromatic light C. salei showed an overall sensitivity from 389.9 to 654 nm at the higher intensity. This range is narrow compared to the results of the ERG-recordings (300-700 nm) (Barth et al., 1993), and differs from the spectral sensitivity range of the intracellular recordings of single photoreceptors (Walla et al., 1996). The spectral sensitivity shown by the intracellular recordings ceased at 620 nm, while in our experiment at least half of the tested spiders could detect the black target up to 670 nm at the bright illumination. Although the ERG-recordings indicate the spectral sensitivity to start at 300 nm and the intracellular recordings demonstrate the existence of an UV-receptor with λmax at 340 nm, the spiders were clearly able to see the target down to wavelengths of 389.9 nm and less pronounced to a wavelength of 365 nm, this may be due to a very low amount of UV-receptors or possibly that they have other functions e.g. navigation. On the other hand, the perception in the red-colour range of the spectrum is better than expected and might be due to the large spectral sensitivity range of the green receptor.
In the second experiment at the lower intensity, the range of spectral sensitivity was reduced to 448.5 to 598.6 nm (blue to green). This fits with our expectation, since the light reflected by the leaves of the dwelling plants dominates at these wavelengths (Menzel, 1979; Chittka et al., 1994). The spectral reflectance of Aechmea bractea, one of the preferred mating places of C. salei, ranges from 300 to 500 nm, with a greater peak from 400 to 500 nm (measured in Vienna, October 2010 at midday). De Omena and Romero (2010) suggested that the colour of bromeliads could play a role in microhabitat selection for jumping spiders.
The green and blue receptors show a second peak in the UV-range in the electrophysiological recordings (Walla et al. 1996), which is likely to be the result of β-band peaks of visual pigments (Dyer, 1998, 1999). The UV-receptor in the PL-eyes has a second peak at the λmax of the blue receptor too. This could be an indication of the existence of a sensitizing pigment, like in fly photoreceptors (Minke and Kirschfeld, 1979; Stavenga, 2004). In that case, the UV-receptor transfers the energy to the blue receptors to enlarge its sensitivity. Blue receptors are usually of great importance for nocturnal animals. Barth (2001) showed a ten-fold increase of the sensitivity of the blue receptors in the PM-eyes at night.
The reason why the spiders couldn’t see the target at very short wavelenghts could be the different function of the principal and secondary eyes. If the AM-eyes really lack UV-receptors, as indicated by Walla et al. (1996) the perception of UV-light could be used only for detecting movable objects using the PM-eyes. Alternatively, the number of UV-receptors is simply too small to be sufficient. The amount of the UV-receptors in the electrophysiology recordings is very small. From 57 intracellular-recordings, only three UV-receptors could be found. No recordings of UV-receptors could be gained from the AM-eyes. A reason for this can be that the AM-eyes possesss eye muscles to move the retina, a circumstance that hardens the work of an electrophysiologist considerably. But they could still be useful to discriminate between different shades of grey.
The reverse transcript PCR experiments showed that all three visual opsins were present in all eyes and therefore at least the molecular prerequisite for the detection of light in the UV part of the spectrum up to longer wavelengths is there. However, since according to quantity of expression the Rh 2 gene is by far the most abundant, and the fact that it groups with the middle to long wavelength opsin might indicate that the UV spectrum is of less importance to the spider. The appreciation of UV perception of C. salei has been discussed earlier (Barth, 2001). We know from jumping spiders that they use UV-reflecting marks on their body for intraspecific communication (Lim and Li, 2006; Lim et al., 2008). But in C. salei both sexes lack such UV-cues. It might be possible that the UV-receptors are positioned at the bottom of the retina and point upwards as described for several species of lycosid spiders (Kovoor et al., 1993; Dacke et al., 2001). In that case, they could be used for orientation at night, since moonlight reaches the UV-range. The black bar presented in our experiments would be outside of the sensitivity range of the UV-receptors. But this explanation seems to be unlikely, as not even the desert living wandering spider Leucorchestris arenicola uses the moon or polarized light for orientation (Nørgaard et al., 2008).
In summary, we found that the behaviour of C. salei is guided by visual input from only a fraction of the spectrum indicated by ERG experiments. Particularly surprising was its inability to utilize short wavelengths. Although C. salei possesss UV-receptors, their function still remains unclear.
Tab. 2. Statistical evaluation of the data from the experiment with monochromatic filters in combination with a neutral density filter.
mvd: mean vector direction, r: length of the mean vector, s: angular deviation. Numbers in bold show the mean vectors which point within sector 0°, e.g. to the black bar (n=15, N=30). Asterisk marks significance.
| Filter (nm) | number of positive runs | mvd | r | s | Rayleigh-Test |
|---|---|---|---|---|---|
| 389.9 | 4 | 308.2° | 0.218 | 71.6° | p>0.01 |
| 403.2 | 4 | 70.0° | 0.200 | 72.5° | p>0.01 |
| 420.2 | 6 | 13.9° | 0.345 | 65.6° | p>0.01 |
| 434.5 | 12 | 15.3° | 0.627 | 49.5° | p ≤0.01* |
| 448.5 | 21 | 354.8 ° | 0.877 | 28.4° | p ≤0.01* |
| 478 | 25 | 1.1 ° | 0.912 | 24.1° | p ≤0.01* |
| 499.3 | 23 | 358.4 ° | 0.977 | 12.3° | p ≤0.01* |
| 513.9 | 22 | 349.8° | 0.765 | 39.3° | p ≤0.01* |
| 519.6 | 19 | 358.3 ° | 0.703 | 44.2° | p ≤0.01* |
| 538.2 | 21 | 345.7° | 0.775 | 38.4° | p ≤0.01* |
| 547.2 | 21 | 3.6 ° | 0.766 | 39.2 | p ≤0.01* |
| 575.1 | 22 | 355.3 ° | 0.841 | 32.3° | p ≤0.01* |
| 588.3 | 24 | 354.8 ° | 0.887 | 27.3° | p ≤0.01* |
| 598.6 | 17 | 354.6 ° | 0.665 | 46.9° | p ≤0.01* |
| 614.6 | 10 | 342.4° | 0.407 | 62.4° | p >0.01 |
| 654 | 2 | 20.7° | 0.168 | 73.9° | p >0.01 |
| 670.1 | 2 | 20.5° | 0.160 | 74.3° | p >0.01 |
Acknowledgements
We thank two anonymous reviewers for giving comments and suggestions that improved the manuscript.
Funding: This work was funded by the Austrian Science Fund (FWF) [Grant number M1296-B17] to BJE.
List of symbols and abbreviations
- AM
Anterior median eye
- AL
Anterior lateral eye
- PL
Posterior lateral eye
- PM
Posterior median eye
- RPKM
reads per kilobase pare per million reads
Footnotes
Author competing interests;: The authors declare that they have no competing interests.
References
- Barth FG. Sinne und Verhalten: aus dem Leben einer Spinne. Springer; Berlin, Heidelberg, New York: 2001. [Google Scholar]
- Barth FG, Schmitt A. Species recognition and species isolation in wandering spiders (Cupiennius Spp.; Ctenidae) Behav. Ecol. Sociobiol. 1991;29:333–339. [Google Scholar]
- Barth FG, Nakagawa T, Eguchi E. Vision in the ctenid spider Cupiennius salei: Spectral range and absolute sensitivity. J. Exp. Biol. 1993;181:63–79. [Google Scholar]
- Batschelet E. Circular statistics in biology. Academic Press; London: 1981. [Google Scholar]
- Chittka L, Shmida A, Troje N, Menzel R. Ultraviolet as a component of flower reflections, and the colour perception of hymenoptera. Vision res. 1994;34:1489–1508. doi: 10.1016/0042-6989(94)90151-1. [DOI] [PubMed] [Google Scholar]
- Dacke M, Doan TA, ÓCarrol DC. Polarized light detection in spiders. J. Exp. Biol. 2001;204:2481–2490. doi: 10.1242/jeb.204.14.2481. [DOI] [PubMed] [Google Scholar]
- de Omena PM, Romero GQ. Using visual cues of microhabitat traits to find home: the case study of a bromeliad-living jumping spider (Salticidae) Behav. Ecol. 2010;21:690–695. [Google Scholar]
- Defrize J, Thery M, Casas J. Background colour matching by a crab spider in the field: a community sensory ecology perspective. J. Exp. Biol. 2010;213:1425–1435. doi: 10.1242/jeb.039743. [DOI] [PubMed] [Google Scholar]
- Dyer AG. The colour of flowers in spectrally variable illumination and insect pollinator vision. J. Comp. Physiol. A. 1998;183:203–212. [Google Scholar]
- Dyer AG. Broad spectral sensitivities in the honeybee’s photoreceptors limit colour constancy. J. Comp. Physiol. A. 1999;185:445–453. [Google Scholar]
- Eckweiler W, Seyfarth EA. Tactile Hairs And The Adjustment Of Body Height In Wandering Spiders - Behavior, Leg Reflexes, And Afferent-Projections In The Leg Ganglia. J. Comp. Physiol. A. 1988;162:611–621. [Google Scholar]
- Fenk LM, Hoinkes T, Schmid A. Vision as a third sensory modality to elicit attack behavior in a nocturnal spider. J. Comp. Physiol. A. 2010;196:957–961. doi: 10.1007/s00359-010-0575-8. [DOI] [PubMed] [Google Scholar]
- Fenk LM, Schmid A. The orientation-dependent visual spatial cut-off frequency in a spider. J. Exp. Biol. 2010;213:3111–3117. doi: 10.1242/jeb.041939. [DOI] [PubMed] [Google Scholar]
- Grusch M, Barth FG, Eguchi E. Fine structural correlates of sensitivity in the eyes of the ctenid spider, Cupiennius salei keys. Tissue & Cell. 1997;29:421–430. doi: 10.1016/s0040-8166(97)80028-6. [DOI] [PubMed] [Google Scholar]
- Heiling AM, Cheng K, Chittka L, Goeth A, Herberstein ME. The role of UV in crab spider signals: effects on perception by prey and predators. J. Exp. Biol. 2005a;208:3925–3931. doi: 10.1242/jeb.01861. [DOI] [PubMed] [Google Scholar]
- Hergenroder R, Barth FG. The Release Of Attack And Escape Behavior By Vibratory Stimuli In A Wandering Spider (Cupiennius-Salei Keys) J. Comp. Physiol. 1983;152:347–358. [Google Scholar]
- Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17:754–755. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- Insausti TC, Casas J. The functional morphology of color changing in a spider: development of ommochrome pigment granules. J. Exp. Biol. 2008;211:780–789. doi: 10.1242/jeb.014043. [DOI] [PubMed] [Google Scholar]
- Kaps F, Schmid A. Mechanism and possible behavioural relevance of retinal movements in the ctenid spider Cupiennius salei. J. Exp. Biol. 1996;199:2451–2458. doi: 10.1242/jeb.199.11.2451. [DOI] [PubMed] [Google Scholar]
- Kaps F. Anatomische und physiologische Untersuchungen zur Funktion der Retinabewegungen bei Cupiennius salei (Araneae, Ctenidae). Dissertation. Universität Wien; 1998. [Google Scholar]
- Kovoor J, Muñoz-Cuevas A, Ortega-Escobar J. Microanatomy of the anterior median eye and its possible relation to polarized light reception in Lycosa tarantula (Araneae, Lycosidae) Boll. Zool. 1993;60:367–375. [Google Scholar]
- Koyanagi M, Nagata T, Katoh K, Yamashita S, Tokunaga F. Molecular evolution of arthropod color vision deduced from multiple opsin genes of jumping spiders. J. Mol. Evol. 2008;66:130–137. doi: 10.1007/s00239-008-9065-9. [DOI] [PubMed] [Google Scholar]
- Land MF. The Morphology and optics of spider eyes. In: Barth FG, editor. Neurobiology of Arachnids. Springer; Berlin, Heidelberg, New York: 1985. pp. 53–78. [Google Scholar]
- Land MF, Barth FG. The quality of vision in the ctenid spider Cupiennius salei. J. Exp. Biol. 1992;164:227–242. [Google Scholar]
- Lim MLM, Li DQ. Behavioural evidence of UV sensitivity in jumping spiders (Araneae: Salticidae) J. Comp. Physiol. A. 2006;192:871–878. doi: 10.1007/s00359-006-0126-5. [DOI] [PubMed] [Google Scholar]
- Lim MLM, Li JJ, Li D. Effect of UV-reflecting markings on female mate-choice decisions in Cosmophasis umbratica, a jumping spider from Singapore. Behav. Ecol. 2008;19:61–66. [Google Scholar]
- Menzel R. Spectral sensitivity and color vision in invertebrates. In: Autrum H, editor. Handbook of Sensory Physiology. VII. Springer; Berlin, Heidelberg New York, Tokyo: 1979. pp. 6pp. 503–580. [Google Scholar]
- Minke B, Kirschfeld K. Contribution of a Sensitizing Pigment to the Photosensitivity Spectra of Fly Rhodopsin and Metarhodopsin. J. Gen. Physiol. 1979;73:517–540. doi: 10.1085/jgp.73.5.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhofer D, Machan R, Schmid A. Visual perception of motion in a hunting spider. J. Exp. Biol. 2009;212:2819–2823. doi: 10.1242/jeb.027136. [DOI] [PubMed] [Google Scholar]
- Norgaard T, Nilsson DE, Henschel JR, Garm A, Wehner R. Vision in the nocturnal wandering spider Leucorchestris arenicola (Araneae: Sparassidae) J. Exp. Biol. 2008;211:816–823. doi: 10.1242/jeb.010546. [DOI] [PubMed] [Google Scholar]
- Peaslee AG, Wilson G. Spectral sensitivity in jumping spiders (Araneae, Salticidae) J. Comp Physiol. 1989;164:359–363. doi: 10.1007/BF00612995. [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, editors. Bioinformatics Methods and Protocols: Methods in Molecular Biology. Humana Press; Totowa: 2000. pp. 365–386. [DOI] [PubMed] [Google Scholar]
- Salcedo E, Zheng L, Phistry M, Bagg EE, Britt SG. Molecular basis for ultraviolet vision in invertebrates. J. Neurosci. 2003;23:10873–10878. doi: 10.1523/JNEUROSCI.23-34-10873.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid A. A visually induced switch in mode of locomotion of a spider. Z. Nat.forsch. 1997;52:124–128. [Google Scholar]
- Schmid A. Different functions of different eye types in the spider Cupiennius salei. J. Exp. Biol. 1998;201:221–225. doi: 10.1242/jeb.201.2.221. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Schuster M, Barth FG. Daily locomotor activity patterns in three species of Cupiennius araneae ctenidae. The males are the wandering spiders. J. Arach. 1990;18:249–256. [Google Scholar]
- Schuch W, Barth FG. Temporal Patterns in the vibratory courtship signals of the wandering spider Cupiennius salei Keys. Behav. Ecol. Sociobiol. 1985;16:263–271. [Google Scholar]
- Schuch W, Barth FG. Vibratory communication in a spider-Female responses to synthetic male vibrations. J. Comp. Physiol. A. 1990;166:817–826. [Google Scholar]
- Seyfarth E-A, Eckweiler W, Hammer K. Proprioceptors and sensory nerves in the legs of a spider, Cupiennius salei (Arachnida, Araneida) Zoomorphology. 1985;105:190–196. [Google Scholar]
- Stavenga DG. Visual acuity of fly photoreceptors in natural conditions - dependence on UV sensitizing pigment and light-controlling pupil. J. Exp. Biol. 2004;207:1703–1713. doi: 10.1242/jeb.00949. [DOI] [PubMed] [Google Scholar]
- Théry M. Colours of background reflected light and of the prey’s eye affect adaptive coloration in female crab spiders. Anim. Behav. 2007;73:797–804. [Google Scholar]
- Walla P, Barth FG, Eguchi E. Spectral sensitivity of single photoreceptor cells in the eyes of the ctenid spider Cupiennius salei Keys. Zool. Sci. (Tokyo) 1996;13:199–202. [Google Scholar]
- Weigel G. Färbung und Farbwechsel der Krabbenspinne Misumena vatia (L.) Z. Vergl. Physiol. 1941;29:195–248. [Google Scholar]