Abstract
Our knowledge of the binding sites for neutralizing antibodies (NAbs) that recognize a broad range of HIV-1 strains (bNAb) has substantially increased in recent years. However, gaps remain in our understanding of how to focus B-cell responses to vulnerable conserved sites within the HIV-1 envelope glycoprotein (Env). Here we report an immunization strategy composed of a trivalent HIV-1 (clade B envs) DNA prime, followed by a SIVmac239 gp140 Env protein boost that aimed to focus the immune response to structurally conserved parts of the HIV-1 and SIV Envs. Heterologous NAb titres, primarily to tier 1 HIV-1 isolates, elicited during the trivalent HIV-1 env prime, were significantly increased by the SIVmac239 gp140 protein boost in rabbits. Epitope mapping of antibody binding reactivity revealed preferential recognition of the C1, C2, V2, V3 and V5 regions. These results provide a proof of concept that a distally related retroviral SIV Env protein boost can increase pre-existing NAb responses against HIV-1.
Keywords: Other animals, AIDS, antibodies, vaccination
Introduction
The high antigenic variability of HIV-1 is one of the major problems in the development of an effective HIV-1 vaccine. However, even HIV-1 has its Achilles’ heel, since segments of the virus cannot be changed without deleterious consequences for viral replication (1). Accordingly, studies have revealed that 10–30% of HIV-1 infected individuals develop broad serum NAb activity and that a small fraction (approximately 1%) develop very potent responses (2–9), clearly demonstrating that the immune system can generate NAbs that recognize a wide variety of HIV-1 strains.
Successfully licensed antiviral vaccines, such as the yellow fever and measles vaccines, elicit NAbs as correlates of protection (10,11) and passive transfer of bNAbs can protect macaques against virus infection with chimeric SIVs encoding the HIV-1 Env (SHIV) (12–19). Taken together with the recent finding that antibody reactivity against Env was required for the observed vaccine-induced protection of macaques (20) this strongly suggests that the induction of bNAbs is a pivotal property of a protective HIV-1 vaccine (8). The recent identification of epitopes targeted by new sets of bNAbs from HIV-1 infected individuals represented a major achievement for our understanding of the vulnerable sites on the HIV-1 Env (21). Target sites include the membrane-proximal epitope region in gp41 (MPER), the CD4 binding site (CD4bs) and some of the conserved elements in the variable regions V1/V2 and V3 including the integrin (α4β7) and CCR5 binding sites (2, 22).
Eliciting responses against the conserved regions in Env may be critical for a vaccine to achieve protection against a broad spectrum of viral species, overcome viral immune escape and circumvent individual host variation. However, despite the increased understanding of the different target sites for effective neutralizing activity, we still lack knowledge on how to design immunogens and immunization regimes with the capacity to elicit antibody responses focused towards such structurally conserved sites that are crucial for viral function. During natural HIV-1 infection the NAb responses generally fail to neutralize concurrent viral isolates (23–26) and multiple rounds of antibody selection and viral escape occur over several years before bNAb responses are induced (4, 21, 25, 27). Therefore, the eventual expansion of B-cells with high specificity for subdominant constant regions of viral proteins appears to be required but lag behind viral evolution and generally occurs too late in order to be of substantial benefit for the infected individual (21, 28, 29).
The immunological principle put forward here is therefore based on recurrent host exposure to divergent Envs and the novel use of the highly divergent gp140 Env from SIVmac239 as protein boost. We repeatedly immunized rabbits with a trivalent DNA vaccination (clade B envs gp140) followed by boosting with the heterologous recombinant gp140 Env protein (30–32). The novelty of our concept was to use a highly divergent gp140 Env from SIVmac239 for the protein boost. SIVmac239 is a highly pathogenic virus in macaques, that causes rapid depletion of CD4+ T-cells and destruction of the immune system, a similar picture to human AIDS (33). Hence, natural infection with SIVmac239 generally does not induce bNAbs (34). However, we previously noticed the development of NAbs to several SIVs in an attenuated SIVmac239 infection model when animals were treated with daily tenofovir between ten days and four months following inoculation (35). The SIVmac239 virus is very resistant to NAbs (36) and the macaques displayed potent neutralization to sensitive heterologous SIVs before the appearance of neutralization to homologous SIVmac239 (35). This attenuated SIVmac239 infection study, additionally revealed neutralization of HIV-1 in sera from the macaques (35) even though the HIV-1 and SIVmac239 gp140 proteins have only about 30% sequence identity and divergent antigenicity. We therefore here hypothesize that the neutralization resistant SIVmac239 Env may have immunogenic features suitable for the induction of NAbs of which some appear cross-reactive between HIV-1 and SIVmac239. Accordingly, they both bind human CD4 and display significantly conserved topological architectures (37). Additionally, the higher stability of SIVmac239 trimers when compared to those generally produced from HIV-1 Env (38–40) is likely to provide additional advantages during immunization. In conclusion, the vaccination strategy designed in this study made use of repetitive DNA priming using HIV-1 gp140 and a highly heterologous SIVmac239 gp140 boost and resulted in high titre heterologous NAbs against clade B viruses and activity against CRF01 AE and clade C viruses, including HIV-1 Env-specific responses to conserved epitopes primarily in the C1, C2, V2, V3 and V5 regions.
Materials and methods
Animals
New Zealand White (male and female) rabbits (10–12 weeks of age at start of experiment, approximately 3 kg) were housed at the animal facility of the Swedish Institute for Infectious Control according to directives and guidelines of the Swedish Board of Agriculture and the Swedish Animal Protection Agency. The study was performed under approval of the Stockholm North Ethical Committee on Animal Experiments.
Expression and purification of recombinant gp140
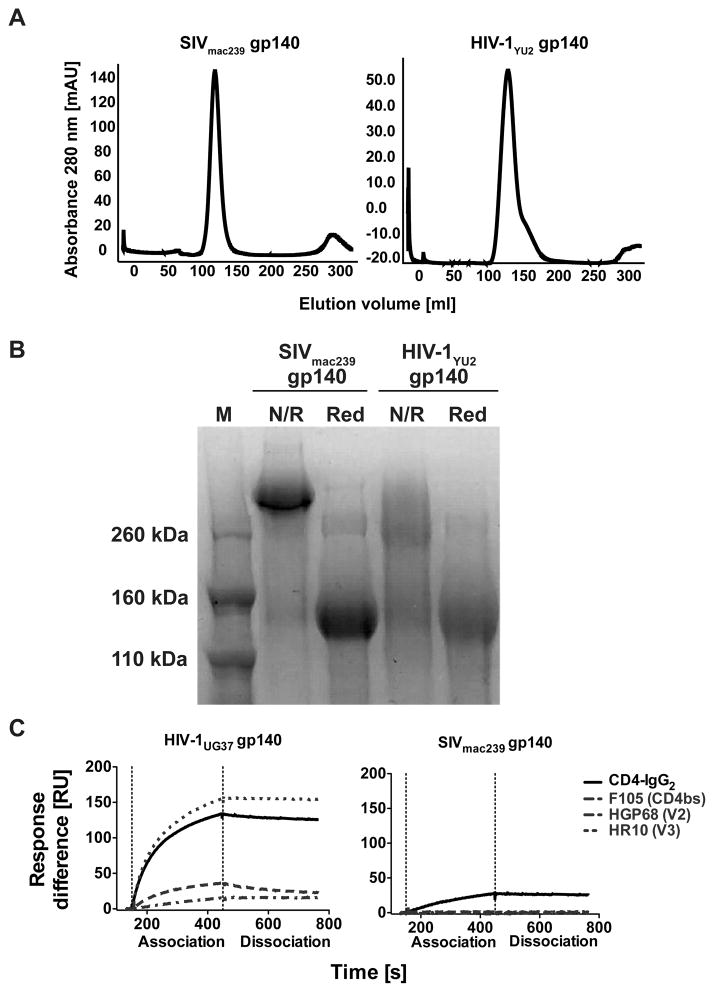
SIVmac239, HIV-1UG37, YU2, ITM1_4, NIBSC40-9 and HIV-2 (accession numbers, UG37: AY494974; YU2: M93258; ITM1_4: FM165626;NIBSC 40-9: KJ579955; HIV-2 is JN863894) (41–45) gp140 were produced following transient transfection of 293T cells cultured in multilayer Cell Bind Hyperflasks (Corning) in high glucose DMEM (Sigma) supplemented with 10% FCS (Sigma) and Penicillin-Streptomycin solution (Sigma). Two mg plasmid DNA was incubated with 3.6 mg PEI in media without FCS for 30 minutes to allow complex formation. This was added to cells and brought to 500 ml with DMEM containing 2% FCS. Supernatants were collected after 48 hours and fresh media, containing 10% FCS, was added to the cells for a further 48 hours at which point the media was exchanged once again. All supernatant was centrifuged at 7000 × g for 4 hours to remove cell debris, and passed through a 0.22 μm filter. After adjusting to pH 8 using 1 M Tris HCl (Sigma), media was passed over a cobalt chloride metal-affinity column made of Talon superflow resin (Clontech). After washing with 2 column volumes of 0.015 M Tris Buffered Saline (Sigma), protein was eluted with 250 mM imidazole. The eluted gp140 was concentrated and separated by gel filtration chromatography using a Superdex200 26/60 size-exclusion column (GE Healthcare). Fractions corresponding to the trimer were identified and further purified using GNA-lectin (Vectorlabs) to specifically bind the glycoprotein. A further SEC fractionation allowed separation of pure gp140 (Fig. 1).
FIGURE 1. Trimerization of SIVmac239 gp140 and binding to human CD4.
A) Gelfiltration chromatography analysis of the soluble gp140 trimers from SIVmac239 and HIV-1YU2. Both complexes elute as a single symmetric peak at the expected elution volume for the trimeric species demonstrating that the gp140 trimers produced were pure and homogenous. The chromatography trace for HIVYU2 is representative for the different HIV-1 gp140 used in this study. B) Non-reducing (N/R) and reducing (Red) SDS PAGE analysis of SIVmac239 and HIV-1YU2 gp140. The SIVmac239 gp140 protein runs as a high molecular weight band under non-reducing conditions, indicating that the three polypeptides of the trimer are covalently linked by disulfide bonds between the three gp140 chains. HIV-1YU2 gp140 runs as a smear under non-reducing conditions suggesting that the three polypeptides of the trimer are not covalently linked. C) Surface plasmon resonance analysis of SIVmac239 gp140 and HIV-1 UG37 gp140 binding to human CD4 and anti-HIV-1 NAbs. SIVmac239 (right panel) or HIV-1UG37 (left panel) were immobilized and their binding to tetrameric soluble human CD4 (CD4-IgG2) as well as mAb F105, HGP68 and HR10 was measured.
Surface plasmon resonance
Surface plasmon resonance experiments were performed on a Biacore 3000 (Biacore Inc., Sweden) in HBS-EP buffer (Biacore Inc.) at 37°C. 700 response units (RUs) of gp140 were immobilized on a CM5 sensor chip using standard amine coupling at pH 4.5. Binding responses were measured by injecting mAbs or CD4-IgG2 over the surfaces for 5 min at 50 μl/min followed by a 5 min dissociation phase. For kinetic analysis a 2-fold dilution of sCD4 starting at 20 nM was used under similar conditions. Between cycles, the sensor surface was regenerated by two 30-second injections of 10 mM glycine, pH 2.0. Data were analyzed in the BIA evaluation software (V 4.0.1). Double referencing was performed using a blank control flow cell as well as a buffer injection and kinetic data were fitted to a 1:1 Langmuir binding model.
DNA vaccine plasmids
The construction of BX08 gp140 (GenBank JX473289) plasmid used codons from highly expressed human genes as described earlier (46–48) and two other primary envs from Danish patients (ctl21 (JX473290) and ctl27 (JX473291) (49) were similarly codon optimized and cloned in the same expression plasmid with the key elements CMV IE promotor-enhancer, tPA secretion signal, and a bovine growth hormone poly A signal. Clones were verified by sequencing.
Immunizations
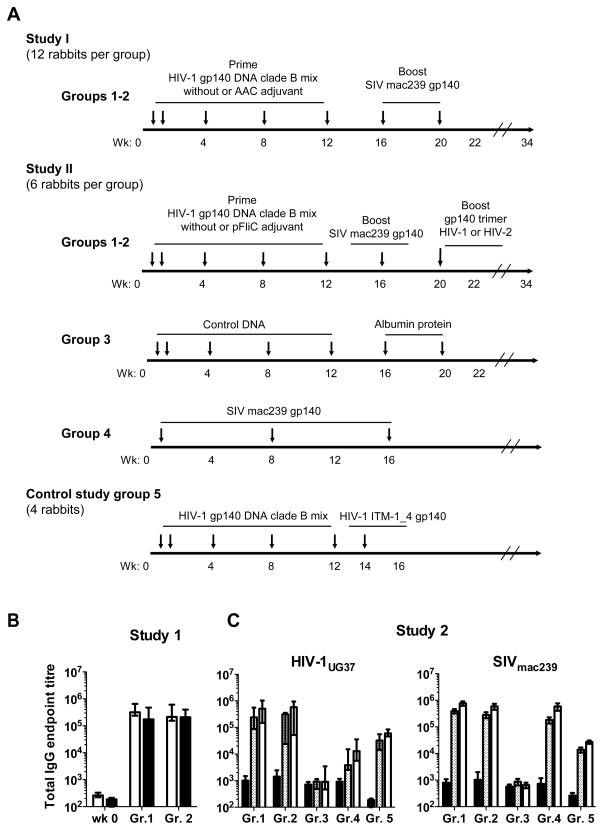
Groups of rabbits (52 animals in total), 10–12 weeks old, were immunized as shown in Fig. 2A. Animals were immunized at weeks 0 (twice first week), 4, 8 and 12 with a mix of the three clade B env-DNA plasmids encoding HIV-1 env gp140 i.d. with the Derma Vax EP device (CytoPulse Sciences/Cellectis, Romainville, France) (Fig. 2A). A total concentration of 200 μg DNA was injected i.d. at two injection sites, as described in (50). Blood for collection of sera and PBMC was taken from the ear vein before immunizations (time point 0 (wk 0)) and four weeks after the last DNA immunization (wk 16). DNA immunizations were administered in 12 animals (group 1 (Gr.1)) without adjuvant and in 12 animals (group 2) with a cellular adjuvant consisting of allogeneic activated cells (AAC) in the first study. The AAC was prepared as previously described (51–55) using allogeneic rabbit splenocytes activated in vitro with Con A (2,5μg/ml) (Sigma) for 48 hours before apoptosis induction. The gamma-irradiated cells were diluted in PBS and mixed with the DNA before administration. In total each rabbit received 10×106 AAC per injection time point. At week 16, all DNA primed animals received a heterologous protein trimer SIVmac239 (50 μg) by i.d. injection without adjuvant and sera were collected 4 weeks later (wk 20). A second identical boost was performed 4 weeks after the first protein immunization and blood was collected 2 weeks later (wk 22). Three months later blood samples were analyzed for memory responses (wk 34). A repeat study with 24 animals divided into 4 groups was performed: the first group (group 1 study II; n=6) received the DNA-env clade B mix priming at weeks 0 (twice first week), 4, 8 and 12 delivered by electroporation; a second group (group 2 study II; n=6) received the addition of a TLR5 agonist encoding flagellin (pFliC) as previously described (56). These two groups subsequently received a heterologous protein trimer SIVmac239 (50 μg) by i.d. injection (no adjuvant) and sera was collected 4 weeks later (wk 20). A cross-over experiment with three animals from groups 1 and 2 (study II) was then performed 4 weeks after the first protein immunization (wk 20) and blood was collected 2 weeks later (wk 22). Three animals from group 1 and three from group 2 received a second gp140 boost from HIV-1 NIBSC 40-9 clade A at week 20. The other three animals in groups 1 and 2 received gp140 from HIV-2 (primary isolate CA65330.5) as the second protein boost at week 20.
FIGURE 2. Schematic representation of the immunization regimes and binding antibodies elicited.
A) Schematic immunization schedules of two independent studies (n= 48) and a comparative group 5 (n=4) with arrows depicting immunizations. Sera were collected before immunization (wk 0). Animals (New Zealand white rabbits) received a mix of three clade B env-DNA plasmids at weeks 0 (twice first week), 4, 8 and 12 (200 μg DNA by i.d. electroporation). Sera were collected 4 weeks after last DNA immunization (wk 16). Animals then received a heterologous protein SIVmac239 gp140 by i.d. injection and sera were collected 4 weeks after first protein boost (50 μg)(wk 20). Animals received a second protein boost with SIVmac239 gp140 4 weeks after the first protein immunization and sera were collected 2 weeks after second protein boost (50 μg) (wk 22). Three months later sera were analyzed for memory responses (wk 34). In the first study, twelve animals were included in Gr. 1 and the second study entailed six animals in this group. In the first study, Gr. 2 (n=12) received AAC adjuvant during the DNA priming phase and in the second study Gr. 2 (n=6) received a TLR5 agonist pFliC. Gr. 3 (n=6) received control DNA and albumin protein and Gr. 4 received three immunizations with SIVmac239 gp140 protein by i.d. injection (n=6). Gr.5 received the mix of three clade B env DNA immunizations followed by one i.d. injection with HIV-1ITM-1_4 gp140 (n=4). (B) Endpoint titres (log10) of IgG binding to SIVmac239 gp140 (white bars) or heterologous HIV-1UG37 gp140 (black bars) as measured by ELISA analyzing sera obtained before immunizations (wk 0) and after immunizations at wk 22 from the first study. Data are depicted as median and range. (C) Endpoint IgG titres from the second study are shown for binding to heterologous HIV-1UG37 gp140 and SIVmac239 gp140, as marked, in sera obtained at wk 0 (black bars), after the DNA priming (grey bars), and after the first gp140 boost (white bars) from the second study. Statistically significant lower endpoint IgG titres against SIVmac239 were found in Gr. 3 and against HIV-1UG37 in Groups 3 and 4 when compared with Groups 1 and 2 (One-way ANOVA using Bonferroni’s Multiple Comparison Test p<0.05).
The third group (group 3; n=6) received empty vector plasmids and albumin as protein boost. The fourth group (group 4; n=6) received only protein trimer SIVmac239 (50 μg) by i.d. injection at weeks 0, 8 and 16. A comparative group (group 5; n=4) received the DNA-env clade B mix at weeks 0, 4, 8 and 12 delivered by electroporation followed by a gp140 from HIV-1 ITM-1_4 in CAF01 adjuvant (57) by i.d. injection at week 14 and sera was collected 2 weeks later (wk 16). Sera were separated and stored at −20°C until IgG purification or direct analysis.
Enzyme-linked immunosorbent assay (ELISA)
Endpoint binding titres were determined as described previously (58). Briefly, serially diluted serum samples were added to microtitre plates coated with 0.5 μg/ml gp140 (HIV-1UG37 or SIVmac239) or HIV-1CN54 C1 (74-CVPADPNPQEMVLEN, HXB2 numbering), HIV-1CN54 V2 (175-LFYRLDIVPLTKKNY), HIV-1CN54 V3 (300-NNTRKSIRIGPGQTF), HIV-1CN54V5 (461-EPNDTETFRPGGGDM), SIVmac239 V2 (169-KFTMTGLKRDKTKEYNETWY) or SIVmac239 V3 (319-VLPVTIMSGLVFHSQPINDR) peptides and blocked with 2% bovine serum albumin in washing buffer (0.05% Tween-20 in PBS). Bound antibodies were detected with anti-rabbit IgG-HRP (Sigma-Aldrich), developed with 1-STEP Ultra TMB-ELISA substrate (Thermo Fisher Scientific) and OD values were read at 450 nm. Data were analyzed by subtracting the background followed by fitting of a sigmoidal dose-response curve to each dataset. Endpoint titres were defined as the concentration at which the curve reached the threshold (0.01), which was greater than two standard deviations above background. HIV peptides were obtained from the EU Eurovac consortium.
Peptide Scanning
Overlapping linear 15-mer and circularized 15-mer peptides based on gp140 of HIV-1CN54 were tested for reactivity against heat-inactivated rabbit sera by Pepscan Therapeutics (Netherlands). Positive responses were defined as higher than two times the median of all peptides tested. The effect of boosting was determined by dividing the ELISA value after boosting by the ELISA value after DNA prime for each peptide. Peptide binding breadth for each animal was calculated as the percentage of peptides that showed positive responses. For graphical representation, average ELISA values of 9aa windows were calculated for each position, as described previously (59).
Neutralization assays
IgG were purified from serum using Protein G HP SpinTrap columns (GE Healthcare) according to the manufacturer’s instructions. Eluted IgG were quantified spectrophotometrically with yields ranging from 2 to 4 mg/ml. The purified IgG or sera were assayed in pseudovirus neutralization assays using TZM-bl cells, expressing CD4 as well as both CCR5 and CXCR4, conducted in triplicates as described previously (60) (http://www.europrise.org/neutnetsops.html; SOP10). A panel of pseudoviruses was used and prepared as previously described (60, 61). Infection levels were determined after 48 hours by measuring firefly luciferase activity in TZM-bl. Neutralizing antibody activity was also assessed using A3R5.7 cells (62). A3R5.7 cells (63) were obtained from Drs. Jerome Kim and Robert McLinden at the US Medical HIV Research Program (MHRP). This is a human CD4+ lymphoblastoid cell line (CEM/A3.01) (64) that was engineered at the US MHRP to express CCR5. Infectious molecular clones of HIV-1 carrying the entire ectodomain of the viral env of choice and a Tat-regulated LucR reporter gene (65) were obtained from Drs. Christina Ochsenbauer and John Kappes at the University of Alabama, Birmingham. Briefly, a dose of virus that generates approximately 50,000 relative luminescence units (RLU) after 4 days of infection was incubated with serial 3-fold dilutions of test sample in duplicate in a total volume of 150 μl for 1 hour at 37°C in 96-well flat-bottom culture plates. Exponentially dividing A3R5.7 cells (90,000 cells in 100 μl of growth medium containing 25 μg/ml DEAE dextran) were added to each well. One set of control wells received cells + virus (virus control) and another set received cells only (background control). Infection levels were determined after four days by measuring Renilla luciferase activity in A3R5.7 cells. The ID50 was calculated as the reciprocal serum dilution causing 50% reduction of relative light units compared to the virus alone (without test sample). Magnitude-breadth curves were prepared as previously described (66). A Mantel-Cox log-rank test was used to compare the different curves.
Peptide competition neutralization assays
Peptide inhibition of IgG neutralization was measured using a modified pseudovirus neutralization assay (43) in which the purified IgGs were pre-incubated for 30 min with peptide dissolved in DMSO at a final concentration of 16 μg/ml prior to addition of the pseudovirus and a final DMSO concentration of 0.3%. The HIV-1 MN.3 V3 peptide (CTRPNYNKRKRIHIGPGRAFYTTKNIIGTIRQAHC, EVA7019), the GPGR HIV-1 SF2 clade B V3 peptide (TRKSIYIGPGRAFHTT, ARP797), the GPGQ HIV-1 consensus clade A peptide (KSVHIGPGQAFYAT, ARP7012.1) and the scrambled control (ARP7099) were obtained from the EVA Centre for AIDS Reagents, NIBSC, UK.
Statistical analyses
Figures as well as statistical analyses were prepared using Graph Pad Prism statistical software Version 4.03 (GraphPad Software, La Jolla, CA) according to the statistical tests described in the figure legends.
Results
Production of stable covalent SIVmac239 gp140 for heterologous boost
All gp140 trimers used in the present study were produced in HEK 293T cells using transient expression plasmid DNA transfection. The SIVmac239 and the HIV-1 gp140 trimers were highly pure and uniformly trimeric in solution as analyzed by gelfiltration chromatography (Fig. 1A). However, whereas the SIVmac239 gp140 trimer migrated as a tight high molecular weight band under non-reducing SDS PAGE, the HIV-1 gp140 trimers demonstrated at least partial dissociation into monomeric species (Fig. 1B). The analyzed proteins migrated as single monomeric species under reducing conditions (Fig. 1B). Together this indicates that both HIV-1 and SIVmac239 yielded uniformly trimeric gp140 and that the SIVmac239 trimer is stabilized by covalent disulfide bonds between the monomers. Surface plasmon resonance binding analysis revealed that HIV-1 and SIVmac239 gp140s bind to soluble recombinant human CD4. However, no binding to SIVmac239 gp140 was detected using a panel of mAb against HIV-1 gp160 including F105, VRC01, HGP68 and HR10 which recognize the CD4 binding site, the V2 and the V3 regions, respectively (Fig. 1C and Suppl. Fig. 1 A and B). Hence, these findings suggest poor HIV-1 antigenicity of SIVmac239 gp140 and that SIVmac239 binds with a lower affinity to human CD4 compared with HIV-1UG37.
Induction of binding antibodies to clade A HIV-1UG37 and SIVmac239 Envs after trivalent clade B env immunization
In a previous study we optimized a DNA prime regimen in which clade B gp140 env was delivered as a mixture composed of three synthetic codon optimized envs (from the clinical isolates HIV-1 Bx08, ctl21 and ctl27) with the aim to increase the proportional concentration of epitopes derived from shared regions (49). In the present study we used the same DNA prime followed by a highly heterologous trimeric SIVmac239 gp140 protein boost, hypothesizing that such a regime might focus the response to more conserved structures. Sequence alignments comparing env from Bx08, ctl21, ctl27 and SIVmac239 are shown in Suppl. Fig. 1 C. Although the overall sequence identity between HIV-1 env and SIVmac239 env is only in the order of 30%, certain parts of env display shared motifs including the V3 region. To mimic the long-term antigenic exposure required for the development of bNAbs in HIV-1-infected individuals, an intense immunization regimen was adopted, which consisted of repeated DNA immunizations of rabbits over a four month period prior to boosting with SIVmac239 or HIV-1 (clade A) gp140 Env. Plasmid DNA was delivered by intradermal (i.d.) injections in the first week (day 0 and 2) as well as at weeks 4, 8 and 12 (Fig. 2A). Each i.d. injection was followed by electroporation, which previously has been demonstrated to substantially increase immunogenicity in both animal and human studies (67). Two SIVmac239 gp140 boosts were delivered i.d. at weeks 16 and 20 (50 μg/animal/time-point) without adjuvant in both groups 1 and 2. Additionally, we also evaluated if an adjuvant, consisting of activated apoptotic cells (AAC) that engage TLR4 (52–54), would increase DNA priming efficiency (Study I Gr. 2 n=12) (Fig. 2A). Group 1 (Study I n=12) did not receive any adjuvant. Subsequently, a second independent study with six animals in each group was performed in which group 2 received a TLR5 agonist that was delivered as a DNA plasmid (pFliC) together with the DNA prime (Study II Gr. 2 n=6) (Fig. 2A) (56), while group 1 (Study II n=6) did not receive any adjuvant. All animals in groups 1 and 2 from the second study also received an i.d. SIVmac239 gp140 boost at week 16. Additionally, a cross-over experiment with three animals from each of these two groups was then performed at week 20 to evaluate whether a recombinant gp140 trimer from HIV-1 NIBSC 40-9 clade A (n=6) or HIV-2 (n=6) could be used as a second protein boost. Furthermore, the second study included a negative control group receiving empty plasmid and albumin (Gr. 3 n=6) as well as a group which received only the recombinant SIVmac239 trimer (Gr. 4 n=6). Finally a comparative group (Gr.5) was included in order to compare the boosting capacity of a gp140 HIV-1 clade A ITM-1_4 trimer with the results obtained by the SIVmac239 boost.
We first assessed the prevalence of Env-specific binding IgGs in serum against both SIVmac239 gp140 and heterologous clade A HIV-1UG37 gp140 by ELISA. High titres of binding antibodies against both antigens were detected in all animals following the protein boost (wk 22), without any demonstrable effect of the AAC adjuvant (Fig. 2B). These high IgG titres were confirmed in the second study as high ELISA titres were already detected after DNA immunizations (wk 16) and found to be generally increased by the protein boost (Fig. 2C). Similarly to the results obtained with AAC, no difference in binding antibody titres was detected between the groups that did or did not receive the pFliC adjuvant during the DNA priming phase (Fig. 2C Gr. 1 and 2). The empty vector and albumin protein control group did not mount any SIVmac239 or HIV-1 UG37 gp140-binding antibodies (Fig. 2C, Gr. 3). Importantly, repeated administration of SIVmac239 trimer without HIV-1 DNA priming did not elicit high titre binding antibodies to HIV-1UG37 gp140 even though responses to SIVmac239 were induced (Fig. 2C, Gr. 4). Conversely, binding IgG responses towards SIVmac239 were induced by the HIV-1 DNA prime prior to the SIVmac239 Env boost (Fig. 2C, Gr. 1 and 2). The comparative group receiving a gp140 HIV-1 boost after priming (Gr. 5) mounted similar binding titres to HIV-1UG37 and SIVmac239 but tended to be lower when compared to those of groups 1 and 2 (Fig. 2C). Conclusively, these findings show that the trivalent clade B env DNA prime was able to induce antibodies capable of binding to both clade A HIV-1UG37 and to SIVmac239.
Trimeric SIV Env protein boost significantly increases NAbs induced by HIV-1 env DNA priming
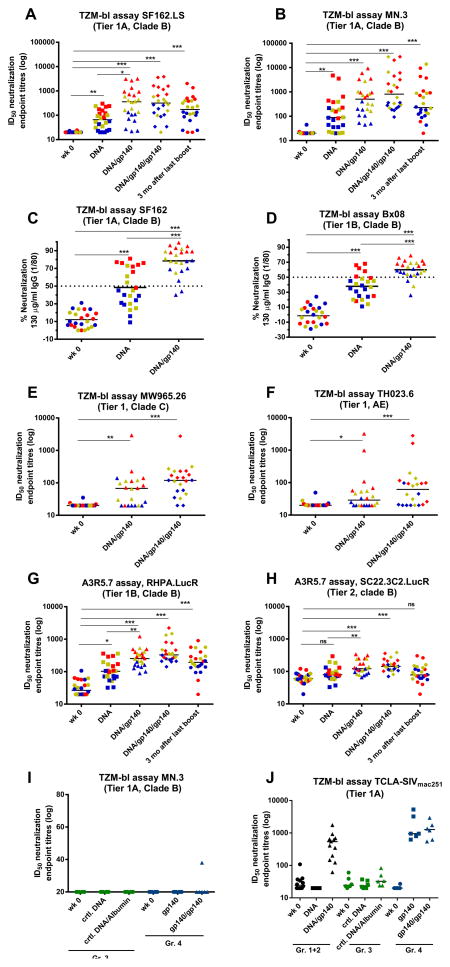
Sera were analyzed for neutralizing activity against a panel of tier 1 viruses in the TZM-bl assay, as previously defined by Seaman et al. (68), and responses are displayed as 50% inhibitory dose (ID50) titres (Fig. 3). Serum neutralization responses in groups 1 and 2 of the first study are displayed together as no significant differences in NAb activity were found in the group receiving the AAC adjuvant (Suppl. Table 1). A significant induction of neutralizing activity was measured against heterologous HIV-1SF162.LS and MN.3 viruses following the DNA priming phase (wk 16, DNA) with strongly increased neutralization titres after the first SIVmac239 gp140 boost (wk 20, DNA/gp140, Fig. 3). Measurements with purified serum IgG confirmed the induction of neutralization activity (Fig. 3C–D). Furthermore, neutralizing activity was also detected against tier 1 viruses from clade C (MW965.26) and CRF01 AE (TH023.6) (Fig. 3E–F) after boosting with SIVmac239 gp140. To further assess the breadth of the responses after boosting with SIVmac239 gp140, neutralization titres were measured against tier 1 and tier 2 viruses using the A3R5.7 assay as well as against HIV-2 (Fig. 3G–H and Suppl. Table1). Significant neutralization was induced against RHPA.LucR (tier 1B clade B) and SC22.3C2. LucR (tier 2 clade B) using the A3R5.7 assay and also against HIV-2 in the TZM-bl assay. The sera did not, however, show neutralization to viruses such as JR-FL, Q23.17, 6535.3, QH0692.42 (data not shown). An arbitrary color code was used in Fig. 3 where the top eight neutralization responders against SF162.LS are depicted in red and the eight animals with the lowest neutralization titres to SF162.LS in blue and the intermediary responders in yellow (n=8). The lowest responders to SF162.LS neutralization (blue) seemed to also be among the low responders against the other viruses, while the best SF162.LS neutralizers (red) tended to also be high responders against the other viruses. Interestingly, the second SIVmac239 protein boost delivered four weeks after the first boost did not always significantly increase the neutralization titres. Nevertheless, when long-term persistence of neutralization was evaluated three months after the final SIVmac239 protein boost (wk 34), responses were still detected against both tier 1A viruses (Fig. 3A and B) and the tier 1B clade B virus RHPA.LucR (Fig. 3G). In line with the measured antibody binding titres (Fig. 2C), SIVmac239 gp140 boosting without prior HIV-1 priming (Gr.4) did not induce NAbs against HIV-1 viruses (Fig. 3I), whereas significant responses against a sensitive TCLA-SIVmac251 were observed (Fig. 3J). Although the trivalent clade B env DNA immunization induced binding antibodies to SIVmac239, it was not sufficient to induce a neutralization response to TCLA-SIVmac251 while boosting with SIVmac239 resulted in a detectable SIVmac251 neutralization (Gr.1+2 Fig. 3J). Hence, the SIVmac239 gp140 was able to induce significant neutralization to a neutralization sensitive clone of TCLA-SIVmac251.
FIGURE 3. Induction of high-titre neutralizing antibodies against clade B viruses through the highly heterologous DNA-prime protein-boost regime.
Data are shown for individual animals from groups 1 and 2 combined (n=24) (A–H). Lines represent median. Sera were collected before immunization (wk 0), 4 weeks after last DNA immunization (DNA), 4 weeks after first SIVmac239gp140 boost (DNA/gp140) and 2 weeks after the second SIVmac239gp140 boost (DNA/gp140/gp140). Three months later sera were analyzed for the longevity of responses (3 mo after last boost). High ID50 neutralizing titres were detected in a TZM-bl assay against tier 1 isolates from clade B SF162.LS (A) and MN.3 (B). Purified IgGs were tested in a TZM-bl assay against HIV-1 clade B SF162 and BX08 (C and D). Percent neutralization is shown using 130 μg/ml IgG final concentration approximately corresponding to a 1/80 serum dilution and dotted line indicates ID50. Neutralizing titres in sera were detected in a TZM-bl assay against tier 1 isolates from clade C (MW965.26, E) and CRF01 AE (TH023.6, F). An arbitrary color code indicate the animals with the highest titres against SF162.LS in red (n=8), lowest in blue (n=8) and those in between in yellow (n=8). These colors are kept throughout the figure to be able to track the animals and their respective response to other viruses. ID50 titres in sera from groups 1 and 2 were tested in the A3R5.7 assay against clade B viruses RHPA.LucR (G) and SC22.3C2.LucR (H). Neutralization titres obtained in the TZM-bl assay for the control groups that received control DNA and albumin (Gr.3, displayed in green) or only SIVmac239 protein without a DNA prime (Gr.4, displayed in blue) are shown for neutralization of HIV-1 MN.3 (I) and the lab-adapted sensitive TCLA-SIVmac251 isolate (J). Significant values are indicated by *** p<0.001, **p<0.01 and *p<0.05 using One-Way ANOVA with Multiple Comparison Test.
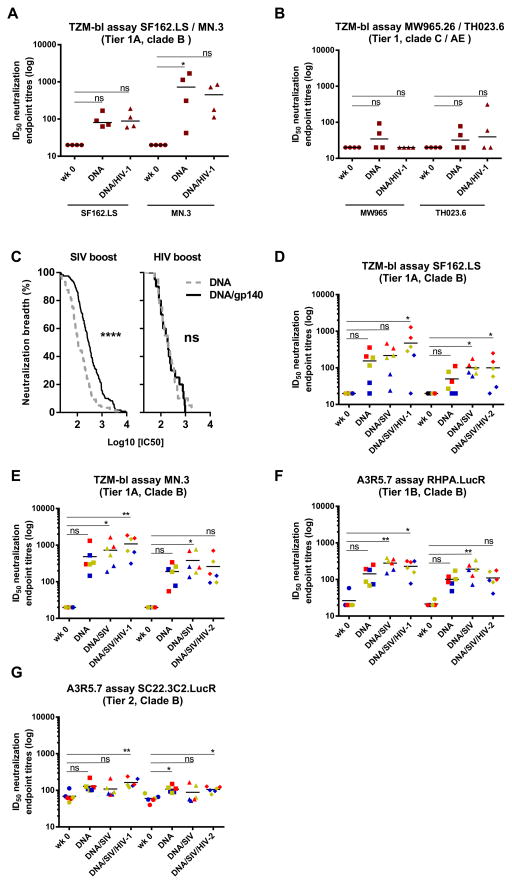
To further analyze and compare the boosting effect of the highly heterologous gp140 SIVmac239, a comparative group, in which the animals (n=4) received clade B env DNA priming followed by a boost with HIV-1ITM-1_4 gp140 (clade A) was included (Fig. 4A). HIV-1ITM-1_4 gp140 was selected based on comparative analyses of a large set of HIV-1 gp140s and was found to be one of the most potent for induction of neutralizing activity when given in an adjuvant without prior priming (45). However, in contrast to SIVmac239 gp140, HIV-1 ITM-1_4 gp140 was not able to boost the response against the clade B viruses HIV-1SF162.LS and MN.3. When tested against clade C (MW965) and CRF01 AE (TH023.6) only two out of four animals had detectably increased neutralization titres against the CRF01 AE virus after boosting with HIV-1ITM-1_4 gp140 (Fig. 4B). Visualization of the magnitude-breadth curves of data pooled from both TZM-bl and A3R5.7 assays for the viruses that were tested on all animals, revealed a significant increase of neutralizing activity after the SIVmac239 gp140 boost but not after the HIV-1ITM-1_4 gp140 boost (Fig. 4C). We then asked the question whether HIV-1NIBSC40-9 (clade A) and/or HIV-2 gp140 trimer would be able to further boost neutralization titres induced by the DNA (clade B envs) prime and SIVmac239 gp140 boost (Fig 4D–G). As seen in the primary study, boosting with the SIVmac239 gp140 timer increased neutralization titres induced by the DNA. Interestingly, there was a clear trend for increased neutralization titres against HIV-1 induced by the HIV-1 gp140 trimer that was not observed with HIV-2 gp140.
FIGURE 4. SIVmac239 can boost HIV-1 neutralization better than HIV-1 clade A ITM-1_4 while HIV-1 NIBSC40-9 clade A can support SIVmac239 responses.
(A, B). A comparative arm that received DNA clade B priming followed by the HIV-1 clade A ITM-1_4 gp140 boost (Gr.5), was tested against SF162.LS, MN.3, MW965 and TH023.6 with ID50 titres displayed in brown. (C) Magnitude-Breadth curves show pooled data from both TZM-bl and A3R5.7 assays for the viruses that were tested on all animals (HIV-1 clade B isolates MN.3, SF162.LS, 9020.A13.LucR., RHPA.LucR., SC22.3C2.LucR.). Data obtained after DNA prime and after one protein boost are shown for sera from groups 1 and 2 (left panel) as well as the HIV-1 comparative arm (group5) (right panel). A Mantel-Cox log-rank test was used for statistical comparison. **** p<0.0001; ns= not significant. (D–G) Sera were collected in another comparative study before immunization (wk 0), 4 weeks after last DNA immunization (DNA), 4 weeks after first SIVmac239gp140 boost (DNA/SIV) and 2 weeks after the second gp140 protein boost ((DNA/SIV/HIV-1) or (DNA/SIV/HIV-2)). An arbitrary color code indicate the animals with the highest titres against SF162.LS in red (n=2), lowest in blue (n=2) and those in between in yellow (n=2). These colors are kept throughout the figure to be able to track the animals and their respective response to other viruses. Significant values are indicated by *** p<0.001, **p<0.01 and *p<0.05 as well as non-significant (ns) using One-Way ANOVA, Friedman’s test with Dunns’s Multiple Comparison Test.
Taken together these data demonstrate that the highly heterologous, stable SIVmac239 gp140 trimer was a potent and important component in the induction of high titres of NAb activity against heterologous clade B viruses with evidence of cross-clade NAb activity. Furthermore, the DNA prime – protein boost protocol induced durable antibody responses that lasted for at least three months after final immunization with SIVmac239.
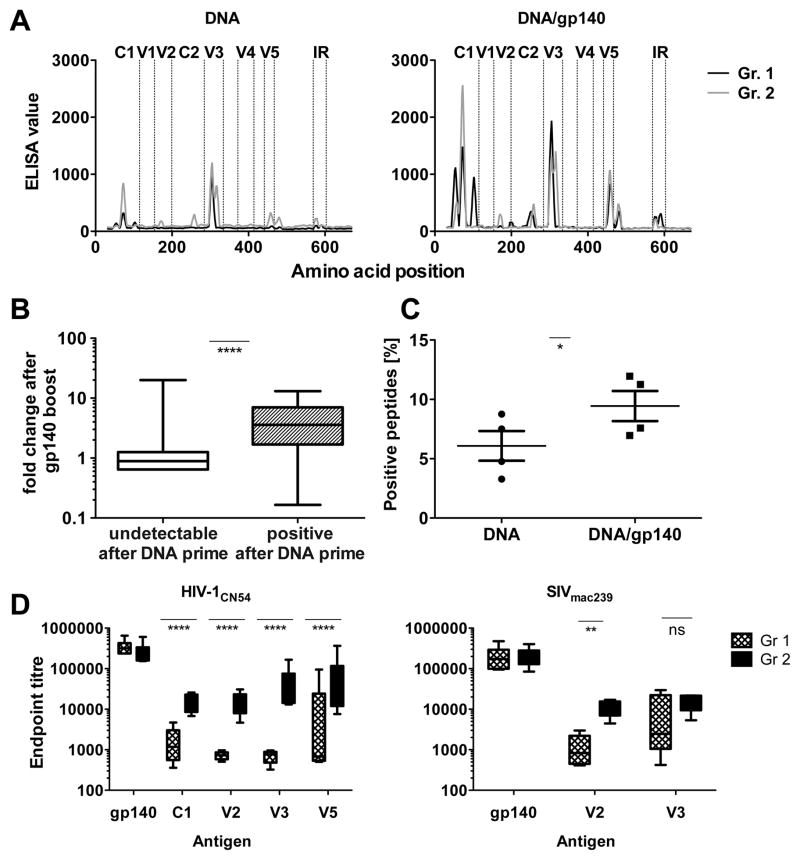
Epitope mapping reveals preferential antibody binding to C1, C2, V2, V3 and V5
Epitope mapping using linear and circularized 15-mer HIV-1CN54 peptides was performed to obtain additional insights into the specificities of the antibody binding response. Sera obtained after the DNA priming schedule (wk 16, DNA) primarily recognized epitopes in the C1 and V3 regions, and to a lesser extent in the V2, C2 and V5 regions (Fig. 5A). Notably, almost all the observed antibody responses increased following the SIVmac239 gp140 boost (wk 20, DNA/gp140) (Fig. 5A–C). Indeed, antibody-binding responses that were already positive after priming displayed on average a significantly stronger increase after the boost (Fig. 5B). In addition, antibody binding to some peptides that were not recognized after DNA priming was detected after the protein boost, demonstrating an increased breadth following the SIVmac239 boost (Fig. 5B and C).
FIGURE 5. Epitope mapping reveals preferential binding to C1, C2, V2, V3 and V5.
(A) Selected sera (n=2 per group from the first study) from time points after HIV-1 DNA-prime (wk 16, DNA) and after the first SIVmac239 protein boost (wk 20, DNA/gp140) were subjected to epitope mapping by pepscan peptide arrays using heterologous HIV-1CN54 peptides. (B) The change in response intensity after SIVmac239 protein boosting is shown for peptides that were undetectable after DNA immunizations (white) or showed positive responses after DNA priming (grey) (C) Percentage of positive peptides detected before (wk 16, DNA) and after the boost (wk20, DNA/gp140) in the peptide array. (D) ELISA binding titres to indicated peptides from HIV-1 or SIVmac239 were determined after the second protein boost (wk 22) in animals immunized without (Gr. 1; n=6) or with a cellular adjuvant AAC during the priming phase (Gr. 2; n=6) (mean+SEM). Significant values are indicated by *p<0.05, **p<0.01 and ****p<0.0001 as well as ns=not significant using a Mann-Whitney test (B), a paired t test (C), or a one-way ANOVA of log-transformed data with a Bonferroni’s Multiple Comparison Test (D).
Peptides from regions in C1, V2, V3 and V5 were used in ELISA in order to assess the impact of the AAC adjuvant on binding titres against individual epitopes. Although the AAC adjuvant did not improve binding antibody titres against the complete gp140 trimer as measured by the ELISA, it significantly increased responses against all the HIV-1 peptides tested (Fig. 5D). Altogether, these results indicate that the SIVmac239 protein strongly boosted primed HIV-1 responses, and led to broadening of the recognition, while the addition of AAC during the priming phase induced higher antibody binding titres to C1, V2, V3, and V5.
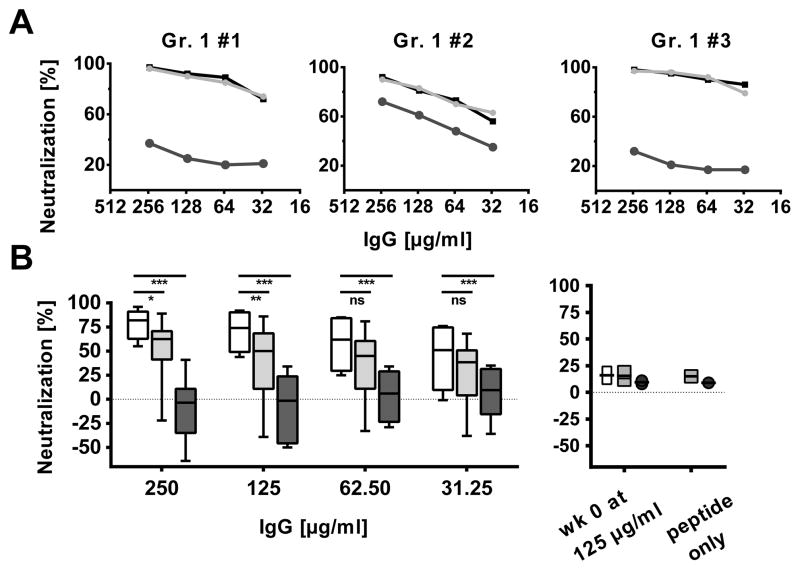
The neutralization of HIV-1SF162 was assessed in the presence of a cyclic peptide that comprises the entire V3 region of MN HIV-1 gp120 (residues 266 – 301) in sera obtained following two protein boosts (wk 22) (Fig. 6A, Suppl. Fig. 2). The neutralization inhibition assay demonstrated recognition of the peptide in sera from all of the animals tested and blocking of a major part of the NAb responses in eight out of the twelve animals tested. Further neutralization assays were performed with shorter peptides that were localized centrally on the V3-crown motifs of clade B (TRKSIYIGPGRAFHTT) or clade A (KSVHIGPGQAFYAT) (Fig. 6B). This revealed a limited but significant heterologous inhibition of the NAb responses by the clade A peptide in addition to the potent inhibition by the clade B peptide with the GPGR motif (Fig. 6B).
FIGURE 6. Neutralization against the SF162 virus is targeted to the V3 region in majority of animals.
IgG from animals from the first study were assessed for their capacity to neutralize HIV-1SF162 in the TZM-bl assay in the presence of blocking peptides and representative individual results are displayed. IgG were purified from sera two weeks after the second SIVmac239 gp140 boost. Percent neutralization is depicted on the y-axis and concentration of purified IgG on the x-axis.
(A) Neutralization is displayed for three representative individual animals in the absence (black) or presence of a peptide covering the V3 region from HIV-1 clade B virus MN.3 (dark grey) or a scrambled control peptide (light grey). Peptide controls without IgG did not result in significant enhancement or neutralization of the virus (Suppl. Fig. 2)
(B) Pooled analysis of the neutralization (no peptide, white) and inhibition by a short V3 peptide from clade A containing the GPGQ motif (light grey) or clade B containing the GPGR motif (dark grey), respectively. Results from peptide inhibition without IgG as well as control neutralization using pre-immunization IgG in combination with the peptides (wk 0) did not show significant enhancement or neutralization of the virus. Significant values are indicated by *** p<0.001, **p<0.01 and *p<0.05 as well as ns=not significant using a two-way repeated measures ANOVA with a Bonferroni’s Multiple Comparison Test.
Discussion
This study reports the induction of HIV-1 neutralization through repeated HIV-1 DNA env mixture priming followed by a highly heterologous boost based on a SIVmac239 gp140 trimer. These results provide a proof of concept that a distally related retroviral SIV Env protein boost can increase pre-existing NAb responses against HIV-1. The HIV-1 DNA immunization schedule was based on a previously published optimization (49) and its effectiveness in this study is highlighted by the fact that strong binding titres as well as significant neutralization were already observed after the DNA immunization phase (Figs.2C and 3). It is conceivable that the use of attenuated vectors, or more effective DNA delivery systems/adjuvants, may be required for the induction of potent antibody priming in primates (69). The pivotal role of effective priming, for induction of the neutralizing antibodies, is indicated by the observation that the top responders after DNA priming were the animals with a tendency of responding with the highest neutralizing activity against HIV-1 after the SIVmac239 boost (Fig. 3). These results also suggest a genetic impact on the ability of the animals to generate high titre NAbs.
It should be noted that the mechanistic basis for this induction of heterologous neutralization remains unresolved. The HIV-1 antigenicity profile of SIVmac239 gp140 was poor as defined by lack of binding using a panel of mAb against HIV-1 gp160 including F105, HGP68 and HR10 which recognize the CD4 binding site, the V2 and the V3 regions, respectively. Nevertheless, HIV-1 Env has been repeatedly shown to possess different antigenicity compared with immunogenicity, as recently reviewed in (70). Therefore, it appears possible that the SIVmac239 trimer used in this study possessed favourable immunogenic features for the boosting of anti-HIV-1 antibodiey responses irrespectively of the lack of conserved antigenicity with HIV-1. Furthermore, the immunogenic profile of SIVmac239 was recently shown to confer selective protection against repetitive intra-rectal challenge with SIVsmE660 (71). A recent study suggested that SIVmac239 displays increased gp160 trimer stability in vivo and specifically highlighted a twin-cysteine motif located in V2 to be responsible for disulfide bonding between the protomers and the higher stability (38–40). It is therefore interesting to note that the recombinant SIVmac239 gp140 used in this study also appeared to be uniformly bound by covalent disulfide bonds between the protomers. This is likely to confer higher stability to the gp140 trimer, which has been demonstrated to be of pivotal importance for other protein-based recombinant vaccines, such as the human papilloma virus vaccine (72, 73). Further support for the importance of stable trimeric immunogens comes from the observation that trimeric HIV-1 gp140 induced more potent NAb responses compared to monomeric gp120 (74). Beyond the scope of this study it would be highly interesting to evaluate if the favourable properties of the SIVmac239 trimer result in the induction or boosting of cross-reactive T helper cell responses and thus strengthening the primed B-cell response.
Even though the mechanism underlying how the SIVmac239 trimer boost provided such robust and broad increases of B-cell responses against HIV-1 remains unclear, the results presented here highlight the ability of this highly heterologous SIVmac239 gp140 protein boost to increase HIV-1 NAb titres. A recent study using a gp120 HIV-1 DNA vaccine prime followed by an adjuvanted formulation of a heterologous gp120 protein (BG505 Env) elicited tier 1 NAbs in rabbits (75). Our study confirms and extends the observation that heterologous prime-boost regiments do not only elicit high level but may also improve quality of antigen-specific antibody responses and thus offer a new platforms for eliciting bNAbs to HIV-1 (76–78).
Further boosting of the neutralization responses was observed with HIV-1NIBSC40-9 clade A gp140 but not with a gp140 trimer from HIV-2 gp140. Hence, more work is required to understand how to optimally expose the immune system with repeated exposures to different Envs to reach the goal to induce high titre bNAbs. Sequential immunization using Envs isolated during the course of a natural infection that resulted in high titre bNAbs is one principle put forward (79). Here we adopted repeated immunizations but used highly divergent gp140 Env of SIVmac239 as one component in a recurrent immunization schedule. Our data indicate that the SIVmac239 protein largely boosted primed responses and alone was not able to induce either HIV-1 binding or HIV-1 NAbs. We have attempted to reveal epitopes induced by the vaccination regime by cross-competition ELISA. Using this assay, we were unable to detect antibodies against the CD4bs and a range of other epitopes tested (data not shown). We were, however, able to demonstrate inhibition of neutralization using peptides against V3.
In our opinion, this study provides a proof of principle for an immunization strategy that induces immune responses, including the induction of antibody recognition to conserved elements in HIV-1 Env and in particular to the C1, V2, V3, and V5. A recent study by Luo et al used a similar approach in which the use of SIV-HIV-1 cross-immunization induced reactivity against C1, V3, and V5 as well as broad neutralizing activity with low magnitude (80). However, a fundamental difference from our study is the use by Luo et al. (80) of SIVmac239 for DNA priming and HIV-1CN54 gp140 for the boost. The use of reversed order of HIV-1-SIV cross-immunization could be beneficial as the NAb response reported here comprised high magnitude responses (in the order of serum titres of 1/10,000) to tier 1 viruses. It should be noted that we could detect significant neutralization even three months after the final SIVmac239 protein boost. While the neutralizing activity against HIV-1SF162 was largely mapped to the V3 region as expected (22), it is interesting to note that a significant portion of the neutralization activity was not inhibited by the V3 peptide in at least three of the twelve animals tested, indicating that other neutralization specificities were present in the sera. In addition, it is conceivable that the neutralizing activity against tier 2 viruses may be governed by other regions such as conserved glycan epitopes that may be present on both HIV-1 and SIVmac239 gp140 proteins.
In conclusion, the present study provides a framework for the induction of HIV-1 Env specific NAbs against clade B viruses as well as reactivity against sensitive CRF01 AE and clade C viruses by repeated DNA env mixture priming followed by boosting with distally related and relatively stable trimeric SIVmac239.
Supplementary Material
Acknowledgments
This work was supported by European Commission; EC-FP7-201433 NGIN, EC-FP6-037611 EUROPRISE, Sida-Sarec, SLL, Swedish Foundation for Strategic Research and the National Institute of Health contract HHSN27201100016C. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Reagents and peptides were kindly provided by the EVA Centre for AIDS Reagents, NIBSC, UK. The authors would like to thank Professor Quentin Sattentau, University of Oxford for critically reading the manuscript and Professor Sarah Rowland-Jones, University of Oxford for the HIV-2 expression construct.
Abbreviations
- NAbs
neutralizing antibodies
- bNAbs
broad NAbs
- Env
Envelope glycoprotein
- MPER
membrane-proximal epitope region in gp41
- CD4bs
CD4 binding site
- AAC
allogeneic activated cells
- RLU
relative luminescence units
- ID50
50% inhibitory dose
- RUs
response units
- Gr
group
References
- 1.Pietzsch J, Scheid JF, Mouquet H, Klein F, Seaman MS, Jankovic M, Corti D, Lanzavecchia A, Nussenzweig MC. Human anti-HIV-neutralizing antibodies frequently target a conserved epitope essential for viral fitness. J Exp Med. 2010;207:1995–2002. doi: 10.1084/jem.20101176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burton DR, Weiss RA. AIDS/HIV. A boost for HIV vaccine design. Science. 2010;329:770–773. doi: 10.1126/science.1194693. [DOI] [PubMed] [Google Scholar]
- 3.Trkola A, Pomales AB, Yuan H, Korber B, Maddon PJ, Allaway GP, Katinger H, Barbas CF, 3rd, Burton DR, Ho DD, et al. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J Virol. 1995;69:6609–6617. doi: 10.1128/jvi.69.11.6609-6617.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sather DN, Armann J, Ching LK, Mavrantoni A, Sellhorn G, Caldwell Z, Yu X, Wood B, Self S, Kalams S, Stamatatos L. Factors associated with the development of cross-reactive neutralizing antibodies during human immunodeficiency virus type 1 infection. J Virol. 2009;83:757–769. doi: 10.1128/JVI.02036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doria-Rose NA, Klein RM, Daniels MG, O’Dell S, Nason M, Lapedes A, Bhattacharya T, Migueles SA, Wyatt RT, Korber BT, Mascola JR, Connors M. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol. 2010;84:1631–1636. doi: 10.1128/JVI.01482-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, Zhou T, Schmidt SD, Wu L, Xu L, Longo NS, McKee K, O’Dell S, Louder MK, Wycuff DL, Feng Y, Nason M, Doria-Rose N, Connors M, Kwong PD, Roederer M, Wyatt RT, Nabel GJ, Mascola JR. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010;329:856–861. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science. 2009;326:285–289. doi: 10.1126/science.1178746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamatatos L, Morris L, Burton DR, Mascola JR. Neutralizing antibodies generated during natural HIV-1 infection: good news for an HIV-1 vaccine? Nat Med. 2009;15:866–870. doi: 10.1038/nm.1949. [DOI] [PubMed] [Google Scholar]
- 9.Simek MD, Rida W, Priddy FH, Pung P, Carrow E, Laufer DS, Lehrman JK, Boaz M, Tarragona-Fiol T, Miiro G, Birungi J, Pozniak A, McPhee DA, Manigart O, Karita E, Inwoley A, Jaoko W, Dehovitz J, Bekker LG, Pitisuttithum P, Paris R, Walker LM, Poignard P, Wrin T, Fast PE, Burton DR, Koff WC. Human immunodeficiency virus type 1 elite neutralizers: individuals with broad and potent neutralizing activity identified by using a high-throughput neutralization assay together with an analytical selection algorithm. J Virol. 2009;83:7337–7348. doi: 10.1128/JVI.00110-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amanna IJ, Messaoudi I, Slifka MK. Protective immunity following vaccination: how is it defined? Hum Vaccin. 2008;4:316–319. doi: 10.4161/hv.4.4.5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Plotkin SA. Immunologic correlates of protection induced by vaccination. Pediatr Infect Dis J. 2001;20:63–75. doi: 10.1097/00006454-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 12.Shibata R, Igarashi T, Haigwood N, Buckler-White A, Ogert R, Ross W, Willey R, Cho MW, Martin MA. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat Med. 1999;5:204–210. doi: 10.1038/5568. [DOI] [PubMed] [Google Scholar]
- 13.Baba TW, Liska V, Hofmann-Lehmann R, Vlasak J, Xu W, Ayehunie S, Cavacini LA, Posner MR, Katinger H, Stiegler G, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Lu Y, Wright JE, Chou TC, Ruprecht RM. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat Med. 2000;6:200–206. doi: 10.1038/72309. [DOI] [PubMed] [Google Scholar]
- 14.Mascola JR, Stiegler G, VanCott TC, Katinger H, Carpenter CB, Hanson CE, Beary H, Hayes D, Frankel SS, Birx DL, Lewis MG. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat Med. 2000;6:207–210. doi: 10.1038/72318. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann-Lehmann R, Vlasak J, Rasmussen RA, Smith BA, Baba TW, Liska V, Ferrantelli F, Montefiori DC, McClure HM, Anderson DC, Bernacky BJ, Rizvi TA, Schmidt R, Hill LR, Keeling ME, Katinger H, Stiegler G, Cavacini LA, Posner MR, Chou TC, Andersen J, Ruprecht RM. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J Virol. 2001;75:7470–7480. doi: 10.1128/JVI.75.16.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parren PW, Marx PA, Hessell AJ, Luckay A, Harouse J, Cheng-Mayer C, Moore JP, Burton DR. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J Virol. 2001;75:8340–8347. doi: 10.1128/JVI.75.17.8340-8347.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishimura Y, Igarashi T, Haigwood N, Sadjadpour R, Plishka RJ, Buckler-White A, Shibata R, Martin MA. Determination of a statistically valid neutralization titer in plasma that confers protection against simian-human immunodeficiency virus challenge following passive transfer of high-titered neutralizing antibodies. J Virol. 2002;76:2123–2130. doi: 10.1128/jvi.76.5.2123-2130.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hessell AJ, Poignard P, Hunter M, Hangartner L, Tehrani DM, Bleeker WK, Parren PW, Marx PA, Burton DR. Effective, low-titer antibody protection against low-dose repeated mucosal SHIV challenge in macaques. Nat Med. 2009;15:951–954. doi: 10.1038/nm.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willey R, Nason MC, Nishimura Y, Follmann DA, Martin MA. Neutralizing antibody titers conferring protection to macaques from a simian/human immunodeficiency virus challenge using the TZM-bl assay. AIDS Res Hum Retroviruses. 2010;26:89–98. doi: 10.1089/aid.2009.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barouch DH, Liu J, Li H, Maxfield LF, Abbink P, Lynch DM, Iampietro MJ, SanMiguel A, Seaman MS, Ferrari G, Forthal DN, Ourmanov I, Hirsch VM, Carville A, Mansfield KG, Stablein D, Pau MG, Schuitemaker H, Sadoff JC, Billings EA, Rao M, Robb ML, Kim JH, Marovich MA, Goudsmit J, Michael NL. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moir S, Malaspina A, Fauci AS. Prospects for an HIV vaccine: leading B cells down the right path. Nat Struct Mol Biol. 2011;18:1317–1321. doi: 10.1038/nsmb.2194. [DOI] [PubMed] [Google Scholar]
- 22.Zolla-Pazner S, Cardozo T. Structure-function relationships of HIV-1 envelope sequence-variable regions refocus vaccine design. Nat Rev Immunol. 2010;10:527–535. doi: 10.1038/nri2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albert J, Abrahamsson B, Nagy K, Aurelius E, Gaines H, Nystrom G, Fenyo EM. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. Aids. 1990;4:107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Moog C, Fleury HJ, Pellegrin I, Kirn A, Aubertin AM. Autologous and heterologous neutralizing antibody responses following initial seroconversion in human immunodeficiency virus type 1-infected individuals. J Virol. 1997;71:3734–3741. doi: 10.1128/jvi.71.5.3734-3741.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A. 2003;100:4144–4149. doi: 10.1073/pnas.0630530100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei X, Decker JM, Wang S, Hui H, Kappes JC, Wu X, Salazar-Gonzalez JF, Salazar MG, Kilby JM, Saag MS, Komarova NL, Nowak MA, Hahn BH, Kwong PD, Shaw GM. Antibody neutralization and escape by HIV-1. Nature. 2003;422:307–312. doi: 10.1038/nature01470. [DOI] [PubMed] [Google Scholar]
- 27.Frost SD, Wrin T, Smith DM, Kosakovsky Pond SL, Liu Y, Paxinos E, Chappey C, Galovich J, Beauchaine J, Petropoulos CJ, Little SJ, Richman DD. Neutralizing antibody responses drive the evolution of human immunodeficiency virus type 1 envelope during recent HIV infection. Proc Natl Acad Sci U S A. 2005;102:18514–18519. doi: 10.1073/pnas.0504658102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bunnik EM, Pisas L, van Nuenen AC, Schuitemaker H. Autologous neutralizing humoral immunity and evolution of the viral envelope in the course of subtype B human immunodeficiency virus type 1 infection. J Virol. 2008;82:7932–7941. doi: 10.1128/JVI.00757-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doria-Rose NA, Klein RM, Manion MM, O’Dell S, Phogat A, Chakrabarti B, Hallahan CW, Migueles SA, Wrammert J, Ahmed R, Nason M, Wyatt RT, Mascola JR, Connors M. Frequency and phenotype of human immunodeficiency virus envelope-specific B cells from patients with broadly cross-neutralizing antibodies. J Virol. 2009;83:188–199. doi: 10.1128/JVI.01583-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang S, Arthos J, Lawrence JM, Van Ryk D, Mboudjeka I, Shen S, Chou TH, Montefiori DC, Lu S. Enhanced immunogenicity of gp120 protein when combined with recombinant DNA priming to generate antibodies that neutralize the JR-FL primary isolate of human immunodeficiency virus type 1. J Virol. 2005;79:7933–7937. doi: 10.1128/JVI.79.12.7933-7937.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vaine M, Wang S, Crooks ET, Jiang P, Montefiori DC, Binley J, Lu S. Improved induction of antibodies against key neutralizing epitopes by human immunodeficiency virus type 1 gp120 DNA prime-protein boost vaccination compared to gp120 protein-only vaccination. J Virol. 2008;82:7369–7378. doi: 10.1128/JVI.00562-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu S. Heterologous prime-boost vaccination. Curr Opin Immunol. 2009;21:346–351. doi: 10.1016/j.coi.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 34.Sato S, Yuste E, Lauer WA, Chang EH, Morgan JS, Bixby JG, Lifson JD, Desrosiers RC, Johnson WE. Potent antibody-mediated neutralization and evolution of antigenic escape variants of simian immunodeficiency virus strain SIVmac239 in vivo. J Virol. 2008;82:9739–9752. doi: 10.1128/JVI.00871-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ozkaya Sahin G, Bowles EJ, Parker J, Uchtenhagen H, Sheik-Khalil E, Taylor S, Pybus OG, Makitalo B, Walther-Jallow L, Spangberg M, Thorstensson R, Achour A, Fenyo EM, Stewart-Jones GB, Spetz AL. Generation of neutralizing antibodies and divergence of SIVmac239 in cynomolgus macaques following short-term early antiretroviral therapy. PLoS Pathog. 2010;6:e1001084. doi: 10.1371/journal.ppat.1001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson J, Sanford WEH, Schwall L, Burton DR, Parren PW, Robinson JE, Desrosiers RC. Assorted mutations in the envelope gene of simian immunodeficiency virus lead to loss of neutralization resistance against antibodies representing a broad spectrum of specificities. J Virol. 2003;77:9993–10003. doi: 10.1128/JVI.77.18.9993-10003.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.White TA, Bartesaghi A, Borgnia MJ, Meyerson JR, de la Cruz MJ, Bess JW, Nandwani R, Hoxie JA, Lifson JD, Milne JL, Subramaniam S. Molecular architectures of trimeric SIV and HIV-1 envelope glycoproteins on intact viruses: strain-dependent variation in quaternary structure. PLoS Pathog. 2010;6:e1001249. doi: 10.1371/journal.ppat.1001249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chertova E, Bess JW, Jr, Crise BJ, Sowder IR, Schaden TM, Hilburn JM, Hoxie JA, Benveniste RE, Lifson JD, Henderson LE, Arthur LO. Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus. J Virol. 2002;76:5315–5325. doi: 10.1128/JVI.76.11.5315-5325.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zanetti G, Briggs JA, Grunewald K, Sattentau QJ, Fuller SD. Cryo-electron tomographic structure of an immunodeficiency virus envelope complex in situ. PLoS Pathog. 2006;2:e83. doi: 10.1371/journal.ppat.0020083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bohl C, Bowder D, Thompson J, Abrahamyan L, Gonzalez-Ramirez S, Mao Y, Sodroski J, Wood C, Xiang SH. A twin-cysteine motif in the V2 region of gp120 is associated with SIV envelope trimer stabilization. PLoS One. 2013;8:e69406. doi: 10.1371/journal.pone.0069406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gao F, Morrison SG, Robertson DL, Thornton CL, Craig S, Karlsson G, Sodroski J, Morgado M, Galvao-Castro B, von Briesen H, Beddows S, Weber J, Sharp PM, Shaw GM, Hahn BH. Molecular cloning and analysis of functional envelope genes from human immunodeficiency virus type 1 sequence subtypes A through G. The WHO and NIAID Networks for HIV Isolation and Characterization. J Virol. 1996;70:1651–1667. doi: 10.1128/jvi.70.3.1651-1667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dhillon AK, Donners H, Pantophlet R, Johnson WE, Decker JM, Shaw GM, Lee FH, Richman DD, Doms RW, Vanham G, Burton DR. Dissecting the neutralizing antibody specificities of broadly neutralizing sera from human immunodeficiency virus type 1-infected donors. J Virol. 2007;81:6548–6562. doi: 10.1128/JVI.02749-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dieltjens T, Willems B, Coppens S, Van Nieuwenhove L, Humbert M, Dietrich U, Heyndrickx L, Vanham G, Janssens W. Unravelling the antigenic landscape of the HIV-1 subtype A envelope of an individual with broad cross-neutralizing antibodies using phage display peptide libraries. J Virol Methods. 2010;169:95–102. doi: 10.1016/j.jviromet.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 44.de Silva TI, Aasa-Chapman M, Cotten M, Hue S, Robinson J, Bibollet-Ruche F, Sarge-Njie R, Berry N, Jaye A, Aaby P, Whittle H, Rowland-Jones S, Weiss R. Potent autologous and heterologous neutralizing antibody responses occur in HIV-2 infection across a broad range of infection outcomes. J Virol. 2012;86:930–946. doi: 10.1128/JVI.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Heyndrickx L, Stewart-Jones G, Jansson M, Schuitemaker H, Bowles E, Buonaguro L, Grevstad B, Vinner L, Vereecken K, Parker J, Ramaswamy M, Biswas P, Vanham G, Scarlatti G, Fomsgaard A. Selected HIV-1 Env trimeric formulations act as potent immunogens in a rabbit vaccination model. PLoS One. 2013;8:e74552. doi: 10.1371/journal.pone.0074552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Corbet S, Vinner L, Hougaard DM, Bryder K, Nielsen HV, Nielsen C, Fomsgaard A. Construction, biological activity, and immunogenicity of synthetic envelope DNA vaccines based on a primary, CCR5-tropic, early HIV type 1 isolate (BX08) with human codons. AIDS Res Hum Retroviruses. 2000;16:1997–2008. doi: 10.1089/088922200750054738. [DOI] [PubMed] [Google Scholar]
- 47.Vinner L, Wee EG, Patel S, Corbet S, Gao GP, Nielsen C, Wilson JM, Ertl HC, Hanke T, Fomsgaard A. Immunogenicity in Mamu-A*01 rhesus macaques of a CCR5-tropic human immunodeficiency virus type 1 envelope from the primary isolate (Bx08) after synthetic DNA prime and recombinant adenovirus 5 boost. J Gen Virol. 2003;84:203–213. doi: 10.1099/vir.0.18589-0. [DOI] [PubMed] [Google Scholar]
- 48.Vinner L, Therrien D, Wee E, Laursen I, Hanke T, Corbet SL, Fomsgaard A. Immune response in rhesus macaques after mixed modality immunisations with DNA, recombinant adenovirus and recombinant gp120 from human immunodeficiency virus type 1. APMIS. 2006;114:690–699. doi: 10.1111/j.1600-0463.2006.apm_395.x. [DOI] [PubMed] [Google Scholar]
- 49.Borggren M, Vinner L, Skovgaard Andresen B, Grevstad B, Repits J, Melchers M, Elvang TL, Sanders RW, Martinon F, Dereuddre-Bosquet N, Bowles EJ, Stewart-Jones G, Biswas P, Scarlatti G, Jansson M, Heyndrickx L, Le Grand R, Fomsgaard A. Optimization of HIV-1 Envelope DNA Vaccine Candidates within Three Different Animal Models, Guinea Pigs, Rabbits and Cynomolgus Macaques. Vaccines. 2013;1:305–327. doi: 10.3390/vaccines1030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Roos AK, Eriksson F, Timmons JA, Gerhardt J, Nyman U, Gudmundsdotter L, Brave A, Wahren B, Pisa P. Skin electroporation: effects on transgene expression, DNA persistence and local tissue environment. PLoS One. 2009;4:e7226. doi: 10.1371/journal.pone.0007226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spetz AL, Sorensen AS, Walther-Jallow L, Wahren B, Andersson J, Holmgren L, Hinkula J. Induction of HIV-1-specific immunity after vaccination with apoptotic HIV-1/murine leukemia virus-infected cells. J Immunol. 2002;169:5771–5779. doi: 10.4049/jimmunol.169.10.5771. [DOI] [PubMed] [Google Scholar]
- 52.Johansson U, Walther-Jallow L, Smed-Sorensen A, Spetz AL. Triggering of dendritic cell responses after exposure to activated, but not resting, apoptotic PBMCs. J Immunol. 2007;179:1711–1720. doi: 10.4049/jimmunol.179.3.1711. [DOI] [PubMed] [Google Scholar]
- 53.Brave A, Johansson U, Hallengard D, Heidari S, Gullberg H, Wahren B, Hinkula J, Spetz AL. Induction of HIV-1-specific cellular and humoral immune responses following immunization with HIV-DNA adjuvanted with activated apoptotic lymphocytes. Vaccine. 2010;28:2080–2087. doi: 10.1016/j.vaccine.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 54.Pathak SK, Skold AE, Mohanram V, Persson C, Johansson U, Spetz AL. Activated Apoptotic Cells Induce Dendritic Cell Maturation via Engagement of Toll-like Receptor 4 (TLR4), Dendritic Cell-specific Intercellular Adhesion Molecule 3 (ICAM-3)-grabbing Nonintegrin (DC-SIGN), and beta2 Integrins. J Biol Chem. 2012;287:13731–13742. doi: 10.1074/jbc.M111.336545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergsmedh A, Szeles A, Henriksson M, Bratt A, Folkman MJ, Spetz AL, Holmgren L. Horizontal transfer of oncogenes by uptake of apoptotic bodies. Proc Natl Acad Sci U S A. 2001;98:6407–6411. doi: 10.1073/pnas.101129998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Applequist SE, Rollman E, Wareing MD, Liden M, Rozell B, Hinkula J, Ljunggren HG. Activation of innate immunity, inflammation, and potentiation of DNA vaccination through mammalian expression of the TLR5 agonist flagellin. J Immunol. 2005;175:3882–3891. doi: 10.4049/jimmunol.175.6.3882. [DOI] [PubMed] [Google Scholar]
- 57.Rosenkrands I, Vingsbo-Lundberg C, Bundgaard TJ, Lindenstrom T, Enouf V, van der Werf S, Andersen P, Agger EM. Enhanced humoral and cell-mediated immune responses after immunization with trivalent influenza vaccine adjuvanted with cationic liposomes. Vaccine. 2011;29:6283–6291. doi: 10.1016/j.vaccine.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 58.Wegmann F, Krashias G, Luhn K, Laamanen K, Vieira S, Jeffs SA, Shattock RJ, Sattentau QJ. A novel strategy for inducing enhanced mucosal HIV-1 antibody responses in an anti-inflammatory environment. PLoS One. 2011;6:e15861. doi: 10.1371/journal.pone.0015861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, Evans DT, Montefiori DC, Karnasuta C, Sutthent R, Liao HX, DeVico AL, Lewis GK, Williams C, Pinter A, Fong Y, Janes H, DeCamp A, Huang Y, Rao M, Billings E, Karasavvas N, Robb ML, Ngauy V, de Souza MS, Paris R, Ferrari G, Bailer RT, Soderberg KA, Andrews C, Berman PW, Frahm N, De Rosa SC, Alpert MD, Yates NL, Shen X, Koup RA, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Michael NL, Kim JH. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–1286. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Montefiori DC. Measuring HIV neutralization in a luciferase reporter gene assay. Methods Mol Biol. 2009;485:395–405. doi: 10.1007/978-1-59745-170-3_26. [DOI] [PubMed] [Google Scholar]
- 61.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV, and SHIV in lu Montefiori, D.C. 2005 Evaluating neutralizing antibodies against HIV, SIV, and SHIV in luciferase reporter gene assays. Curr Protoc Immunol Chapter. 2005;12(Unit 12):11. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 62.Montefiori DC, Karnasuta C, Huang Y, Ahmed H, Gilbert P, de Souza MS, McLinden R, Tovanabutra S, Laurence-Chenine A, Sanders-Buell E, Moody MA, Bonsignori M, Ochsenbauer C, Kappes J, Tang H, Greene K, Gao H, LaBranche CC, Andrews C, Polonis VR, Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Self SG, Berman PW, Francis D, Sinangil F, Lee C, Tartaglia J, Robb ML, Haynes BF, Michael NL, Kim JH. Magnitude and breadth of the neutralizing antibody response in the RV144 and Vax003 HIV-1 vaccine efficacy trials. J InfectDis. 2012;206:431–441. doi: 10.1093/infdis/jis367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pitisuttithum P, Nitayaphan S, Thongcharoen P, Khamboonruang C, Kim J, de Souza M, Chuenchitra T, Garner RP, Thapinta D, Polonis V, Ratto-Kim S, Chanbancherd P, Chiu J, Birx DL, Duliege AM, McNeil JG, Brown AE. Safety and immunogenicity of combinations of recombinant subtype E and B human immunodeficiency virus type 1 envelope glycoprotein 120 vaccines in healthy Thai adults. J Infect Dis. 2003;188:219–227. doi: 10.1086/376506. [DOI] [PubMed] [Google Scholar]
- 64.Folks T, Benn S, Rabson A, Theodore T, Hoggan MD, Martin M, Lightfoote M, Sell K. Characterization of a continuous T-cell line susceptible to the cytopathic effects of the acquired immunodeficiency syndrome (AIDS)-associated retrovirus. Proc Natl Acad Sci U S A. 1985;82:4539–4543. doi: 10.1073/pnas.82.13.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang Y, Gilbert PB, Montefiori DC, Self SG. Simultaneous Evaluation of the Magnitude and Breadth of a Left and Right Censored Multivariate Response, with Application to HIV Vaccine Development. Stat Biopharm Res. 2009;1:81–91. doi: 10.1198/sbr.2009.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: current progress. Clin Infect Dis. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Seaman MS, Janes H, Hawkins N, Grandpre LE, Devoy C, Giri A, Coffey RT, Harris L, Wood B, Daniels MG, Bhattacharya T, Lapedes A, Polonis VR, McCutchan FE, Gilbert PB, Self SG, Korber BT, Montefiori DC, Mascola JR. Tiered categorization of a diverse panel of HIV-1 Env pseudoviruses for assessment of neutralizing antibodies. J Virol. 2010;84:1439–1452. doi: 10.1128/JVI.02108-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu MA. DNA vaccines: an historical perspective and view to the future. Immunol Rev. 2011;239:62–84. doi: 10.1111/j.1600-065X.2010.00980.x. [DOI] [PubMed] [Google Scholar]
- 70.Kong L, Sattentau QJ. Antigenicity and Immunogenicity in HIV-1 Antibody-Based Vaccine Design. J AIDS Clin Res. 2012;S8:3. doi: 10.4172/2155-6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Roederer M, Keele BF, Schmidt SD, Mason RD, Welles HC, Fischer W, Labranche C, Foulds KE, Louder MK, Yang ZY, Todd JP, Buzby AP, Mach LV, Shen L, Seaton KE, Ward BM, Bailer RT, Gottardo R, Gu W, Ferrari G, Alam SM, Denny TN, Montefiori DC, Tomaras GD, Korber BT, Nason MC, Seder RA, Koup RA, Letvin NL, Rao SS, Nabel GJ, Mascola JR. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505:502–508. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jarrett WF, O’Neil BW, Gaukroger JM, Laird HM, Smith KT, Campo MS. Studies on vaccination against papillomaviruses: a comparison of purified virus, tumour extract and transformed cells in prophylactic vaccination. Vet Rec. 1990;126:449–452. [PubMed] [Google Scholar]
- 73.Roldao A, Mellado MC, Castilho LR, Carrondo MJ, Alves PM. Virus-like particles in vaccine development. Expert Rev Vaccines. 2010;9:1149–1176. doi: 10.1586/erv.10.115. [DOI] [PubMed] [Google Scholar]
- 74.Kovacs JM, Nkolola JP, Peng H, Cheung A, Perry J, Miller CA, Seaman MS, Barouch DH, Chen B. HIV-1 envelope trimer elicits more potent neutralizing antibody responses than monomeric gp120. Proc Natl Acad Sci U S A. 2012;109:12111–12116. doi: 10.1073/pnas.1204533109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hoffenberg S, Powell R, Carpov A, Wagner D, Wilson A, Kosakovsky Pond S, Lindsay R, Arendt H, Destefano J, Phogat S, Poignard P, Fling SP, Simek M, Labranche C, Montefiori D, Wrin T, Phung P, Burton D, Koff W, King CR, Parks CL, Caulfield MJ. Identification of an HIV-1 clade A envelope that exhibits broad antigenicity and neutralization sensitivity and elicits antibodies targeting three distinct epitopes. J Virol. 2013;87:5372–5383. doi: 10.1128/JVI.02827-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Vaine M, Wang S, Hackett A, Arthos J, Lu S. Antibody responses elicited through homologous or heterologous prime-boost DNA and protein vaccinations differ in functional activity and avidity. Vaccine. 2010;28:2999–3007. doi: 10.1016/j.vaccine.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Muthumani K, Wise MC, Broderick KE, Hutnick N, Goodman J, Flingai S, Yan J, Bian CB, Mendoza J, Tingey C, Wilson C, Wojtak K, Sardesai NY, Weiner DB. HIV-1 Env DNA Vaccine plus Protein Boost Delivered by EP Expands B- and T-Cell Responses and Neutralizing Phenotype In Vivo. PLoS One. 2013;8:e84234. doi: 10.1371/journal.pone.0084234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pissani F, Malherbe DC, Schuman JT, Robins H, Park BS, Krebs SJ, Barnett SW, Haigwood NL. Improvement of antibody responses by HIV envelope DNA and protein co-immunization. Vaccine. 2014;32:507–513. doi: 10.1016/j.vaccine.2013.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Malherbe DC, Doria-Rose NA, Misher L, Beckett T, Puryear WB, Schuman JT, Kraft Z, O’Malley J, Mori M, Srivastava I, Barnett S, Stamatatos L, Haigwood NL. Sequential immunization with a subtype B HIV-1 envelope quasispecies partially mimics the in vivo development of neutralizing antibodies. J Virol. 2011;85:5262–5274. doi: 10.1128/JVI.02419-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Luo Z, Ren L, Zheng Y, Qi Z, Liang H, Liu Y, Hong K, Shao Y. Eliciting broad neutralizing antibody to HIV-1: Envelopes of different lentivirus cross immunization by prime-boost vaccination. Vaccine. 2012;30:5316–5323. doi: 10.1016/j.vaccine.2012.06.053. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.