Abstract
Background: Low-level laser is being evaluated for treating rheumatoid arthritis (RA). Recently, the linear polarized infrared light (Super Lizer, SL) irradiation may also be useful for RA treatment. However, the molecular mechanism underlying the effectiveness of SL on RA is unclear. It has been IL-20 may involved in RA disease progression.
Aim: To understand how SL action, we constructed the experimental model in vitro using human rheumatoid fibroblast-like synoviocyte (MH7A) and collagen induced (CIA) RA rat in vivo. We examined the effect of SL irradiation on IL-20 gene expression in MH7A and IL-20 protein production in CIA) rat joints.
Materials and methods: MH7A was cultured and challenged with IL-1ß, then examined IL-20 and IL-20R mRNA level by DNA microarray. IL-20 protein expression was examined by immunohistochemistry using a specific antibody against rat IL-20.
Result: Scatter plot analysis demonstrated that an increase in IL-20 gene expression by IL-1ß was reduced by SL irradiation, but IL-20R did not show a significant change. The Immunohistochemical analysis demonstrated a strong IL-20 staining in synovial membrane tissue of CIA rat joint, and SL irradiation significantly reduced the staining.
Discussion: Since IL-20 has been identified as an important cytokine in the pathogenesis of RA, the reduction of IL-20 expression by SL irradiation may be one of mechanisms in reduction of inflammation in RA joints by SL irradiation suggesting that SL irradiation may be useful for RA therapy.
Keywords: Rheumatoid arthritis, linear polarized infrared light, IL-20
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by pain and inflammation, progressive joint destruction 1). This disease is characterized by the infiltration of leukocytes into the synovial tissue and synovial fluid of joints, ultimately leading to destruction of cartilage and bone 2). Elevated levels of IL-1ß have been reported in RA 3). IL-1ß is known to up-regulate the expression of cell adhesion molecules on endothelial cells, thereby directing the migration of blood cells from the circulation into the synovium 4, 5).
Several treatments are available to relieve RA-associated pain. Non-steroidal anti-inflammatory drugs, disease-modifying anti-rheumatic drugs and newer biologics are currently used, but have side effects when used over a prolonged period and are not effective in all patients. A non-invasive physiological therapy such as low level light therapy could be important and expected for managing pain, and it has been used for many years for relief of the chronic pain in RA 6). One of such instruments, the linear polarized light instrument, SuperLizer (SL) has been developed. SL irradiation suppressed the super oxide anion and hypochlorite production of human neutrophils, and suggesting an inhibitory effect against chronic pain via the reduction of reactive oxygen species production and opsonic activity. 7) It has also been reported that RA-affected temporomandibular joint pain was reduced by SL irradiation. 8) Furthermore, SL was used in orthopedics and anesthesiology to treat arthralgia and neuralgia, and suggested SL is a useful tool for the treatment of alopecia areata 9). More recently, Ide 10) reported that the significant analgesic effects of SL to RA patients by double blind test. However, the molecular mechanism of anti-inflammatory effect of SL to RA is unknown.
Human rheumatoid fibroblast-like synoviocyte line, MH7A, has been established by immortalization with SV40 T antigen from RA patient, and retains the morphological and functional characteristics of primary synovial cells. Thus the cell was often used in RA research. 11) On the other hand, type II collagen-induced arthritis (CIA) has been widely used in the study to analyze the pathogenesis of RA in vivo, because its pathological features are similar to RA with proliferative synovitis with infiltration of polymorphonuclear and mononuclear cells, pannus formation, cartilage degradation and erosion of bone 12). IL-20, a member of IL-10 family is a proinflammatory cytokine that provokes potent inflammation, and is involved in the pathogenesis of RA. 13)
In the present study, we examined the effect of SL irradiation on the IL-20 gene expression in the MH7A and IL-20 protein production in knee joints of CIA RA rat by immunohistochemisty.
Materials and methods
1. Synovial cell culture experiment
SL irradiation of MH7A cells was previously described in the literature 17). SuperLizer (HA2200, Tokyo Iken Co., Ltd, Tokyo, Japan) was used to irradiate MH7A cells. An output spectrum from SuperLiser is at wavelengths ranging from 600 nm to 1600 nm. The transmitted light irradiated to dishes thorough the polarizing plate in CO2 incubator. Irradiation occupancy was “0.5”, because the turn on/off of light was each a second. The energy density was calculated as follows: (Energy density) = (average power of density) × (exposure time). Irradiation area of laser was 45 cm2 (6.7 cm × 6.7 cm). Energy density = 0.0056 W (average power of density) × 685 s (exposure time) = 3.8 J/cm2 [Average power = 0.5 W × 0.5 (occupancy; lamp lighting time/exposure time) = 0.25 W, Average power of density = 0.25 W (average power)/45 cm2 (irradiation area) = 0.0056 W]. Cultured dishes containing IL-1ß-stimulated MH7A cells were irradiated for 685 s in the CO2 incubator (5% CO2 in 95% air at 37 °C). The depth and intensity of the irradiation were regulated by, respectively, varying their distance from the laser source and the duration of the irradiation. For experimentation, we used pulsed irradiation at a frequency of 1 Hz, which produced an energy density of 3.8 J/cm2. After 3 hrs, the cells were harvested, and the RNA was extracted.
2. Animal experiment
Female Lewis (LEW/CrlCrlj) rats, 6 weeks old, were obtained from Charles River Japan Inc. (Kanagawa, Japan), and injected with type II collagen (Sigma, Tokyo, Japan) in 250 µl of 0.1 M acetic acid emulsified in an equal volume of complete Freund's adjuvant (Difco Labs, Michigan, USA) containing 2 mg/ml muramyl dipeptide (Wako, Tokyo, Japan) by multiple intradermal injections. Rats were anaesthetized with an intraperitoneal injection of sodium pentobarbital (Somnopentyl, Kyoritsu Seiyaku, Tokyo, Japan) at 25 mg/kg prior to the injection. All animals were maintained and used in accordance with the guide the Care and Use of Laboratory Animals of Nihon University, School of Dentistry at Matsudo (No. 04-008). SL (SuperLizer, HA2200, Tokyo Iken Co., Ltd, Tokyo, Japan) was used to irradiate RA rats according to the previous report. The irradiation time was 20 min, which equaled an incident energy density of 7.64 J/cm2.
3. Gene expression analysis by DNA microarray
Total cellular RNA was isolated using Trizol (GIBCO BRL, Life Technologies, Rockville, MD, USA), and cDNA was synthesized using a Superscript II RNaseH(−) reverse transcriptase system (Invitrogen) with oligo d(T)12-18 primer at 42 °C for 1 hour. Total RNA was then converted to cDNA by reverse transcription using T7-(dT)24 primer (Amersham Biosciences, GE Healthcare, NJ) and reverse transcriptase (Invitrogen Co.). Biotinylated cRNA samples were amplified from cDNA by in vitro transcription with an Enzo
High Yield RNA Transcript Labeling System (Enzo Biochem, NY). The fragmented cRNA was then hybridized to a Human Genome Focus Array HG-8500 GeneChip (Affymetrix Inc.). The scanned images were analyzed using GeneSpring 4.0 software (Silicon Genetics, Redwood City, CA). We set a cut off of >=1.5-fold change for “induction” and “repression.” According to Affymetrix GeneChip analysis protocol, “Raw signal value” is useful to screen or select which gene is important to search which refer to the linear data after summarization using a MAS5 summarization algorithm. But it needs to convert to “Normalized signal value” are generated after log transformation and “Baseline transformation”. Baseline transformation is for each probe set the median of the log summarized values from all the samples is calculated and subtracted from each of the samples. This analysis protocol is explained in GeneSpring12.6 manual page 305 to 329 (Affymetrix). Flag analysis, to demonstrate the presence (P) or absence (A) of signals, was evaluated and intensity normalization was performed. Data analysis was then performed using the GeneSpring (Silicone Genetics, Redwood, CA, USA) software package.
4. Immunohistochemistry
Formalin-fixed, paraffin-embedded specimens were subjected to antigen retrieval and endogenous peroxidase blocking (30 min), and rinsed with phosphate-buffered saline (PBS). Immunostaining was performed using Elite ABC kits (Vector, Burlingame, CA, USA) and diaminobenzidine (Kirkegaard & Perry, Gaithersburg, MD, USA) as a chromogen using anti-IL-20 polyclonal antibody (1:400; Santa Cruz Biotech, Santa Cruz, CA, USA). Peroxidase-conjugated rabbit anti-mouse immunoglobulin diluted 1:10 in PBS supplemented with 2-vol% heat-inactivated normal human sera was used. Peroxidase activity was visualised with 0.06% diaminobenzidine (Walter, Kiel, Germany) and 0.01 vol % H2O2.
Results
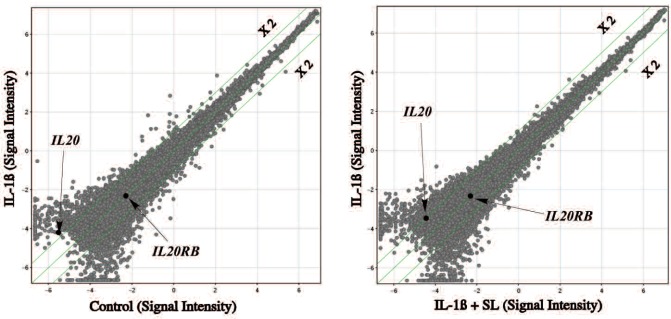
Scatter plot analysis of intensity signals of IL-20 and IL-20R level in MH7A from DNA microarray was shown in Fig. 1. Signal intensity lL-20 gene was shown with 2-fold changes by IL-1ß challenge, and decreased by SL irradiation. However, IL20R was shown to be “Present” in Flag analysis, but did not show a big change.
Fig. 1:
Scatter plot analysis of IL-20 and IL20RB
Table 1 shows the summary of raw mRNA signals of DNA microarray analysis for IL-20 in MH7A cells with or without SL irradiation the median normalization from raw intensity value, and calculated fold changes. As a result, IL-20 gene expression in MH7A was increased as 4-fold by IL-1ß challenge, interestingly, SL irradiation was down regulated of IL-20 gene expression to 2.1 fold change and summarized in Table 1.
Table 1: Raw signal and fold change of IL-20, IL20RA, and IL-20 RB.
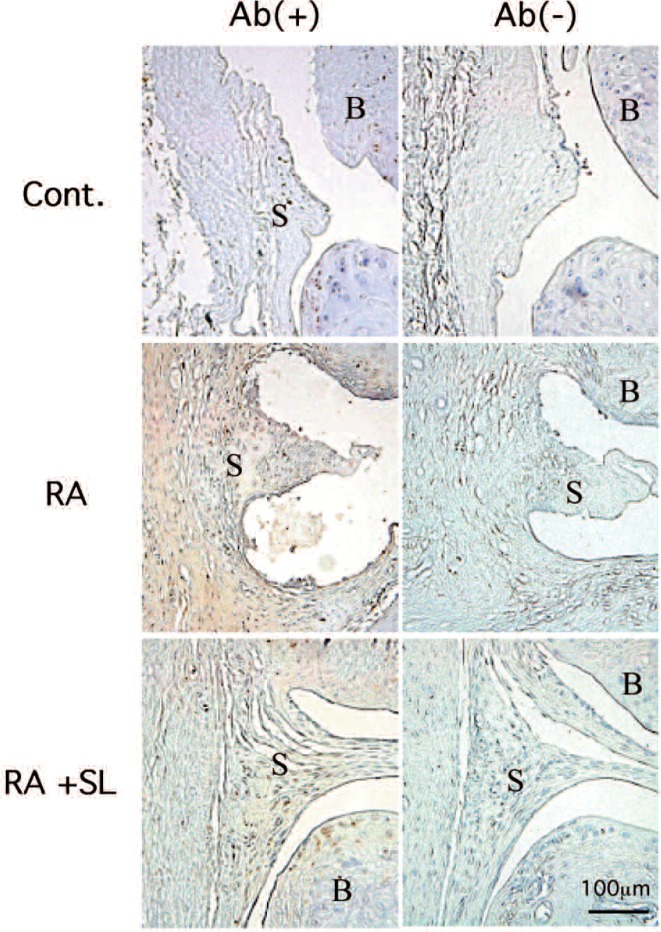
Finally, to confirm the effect of SL irradiation on RA inflammation, phenotypic expression of IL-20 was examined by immunohistochemistry using anti-IL-20 polyclonal antibody. As a result shown in Fig. 2 a significant positive IL-20 staining was found in CIA rat when compared to control, whereas the weak staining was observed in the CIA+SL group when compared to CIA group, suggesting that SL reduced IL-20 production.
Fig. 2:
Immunohistochemical analysis of IL-20
Discussion
IL-20, a member of the IL-10 family, which is preferentially expressed in various tissues with IL-20 receptor 14), Moreover, IL-20 has been implicated to play an important role in several autoimmune diseases including include rheumatoid arthritis (RA) 13). IL-20 signaling through two receptors IL-20RA and IL-20RB 15). It is well known that IL-20 was up-regulated in the synovial fluid and plasma of RA patients, and IL-20 protein was present in mononuclear cells and neutrophil granulocytes in the synovial membrane 16). The rat CIA model demonstrated that IL-20 was involved in the pathogenesis of arthritis, and play important roles at local sites of inflammation 16).
Transcriptional examination demonstrated that an increase in IL-20 gene expression in IL-1ß challenged MH7A, and that was reduced by SL irradiation as in vitro experiment. To confirm this in vivo, the immunohistochemical analysis demonstrated a strong IL-20 staining in whole synovial membrane tissue of CIA rat joint and reduced the staining. These findings suggest that IL-20 production in synovial membrane from RA joint can be reduced by SL irradiation. In this study, we delineate a molecular mechanism of action underlying SL therapy on RA.
The rationale for the use of anti-cytokine therapy in inflammatory joint diseases is based on evidence from studies in vivo, which implicate major cytokines such as IL-1, TNF and IL-6 in RA pathogenesis. Anti-IL-20 monoclonal antibody inhibits the differentiation of osteoclasts and protects against osteoporosis bone loss 17). Thus, the use of SL irradiation for RA may reduce RA inflammation, thereby alleviating joint pain, by reducing the level of IL-20. This mechanism may be more general and underlie the beneficial effects of SL irradiation on inflammatory conditions in RA.
In conclusion therefore, since IL-20 has been identified to be an important proinflamatory cytokine in the pathogenesis of RA, the reduction of IL-20 expression may be one of mechanisms in reduction of inflammation in RA joints by SL irradiation suggesting that SL irradiation may be useful for RA therapy.
References
- 1: Pincus T. (1995): Long-term outcomes in rheumatoid arthritis. Br J Rheumatol, 34:59-73 [PubMed] [Google Scholar]
- 2: Gay S, Gay RE, Koopman WJ. (1993): Molecular and cellular mechanisms of joint destruction in rheumatoid arthritis: two cellular mechanisms explain joint destruction? Ann Rheum Dis, 52: 39-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3: Eastgate JA, Symons JA, Wood NC, Grinlinton FM, di Giovine FS, Duff GW. (1988): Correlation of plasma interleukin 1 levels with disease activity in rheumatoid arthritis. Lancet, 2: 706-709 [DOI] [PubMed] [Google Scholar]
- 4: Abbot SE, Kaul A, Stevens CR, Blake DR. (1992): Isolation and culture of synovial microvascular endothelial cells. Characterization and assessment of adhesion molecule expression. Arthritis Rheum, 35:401-406 [DOI] [PubMed] [Google Scholar]
- 5: Mester E, Mester AF, Mester A. (1985): The biomedical effects of laser application. Lasers Surg Med, 5: 31-39 [DOI] [PubMed] [Google Scholar]
- 6: Brosseau L, Welch V, Wells G, Tugwell P, de Bie R, Gam A, Harman K, Shea B, Morin M. (2000): Low level laser therapy for osteoarthritis and rheumatoid arthritis: a metaanalysis. J Rheumatol. 27:1961-1969 [PubMed] [Google Scholar]
- 7: Shiraishi M, Suzuki K, Nakaji S, Sugawara K, Sugita N, Suzuki KJ, Ohta S. (1999): Effect of linear polarized near-infrared ray irradiation on the chemiluminescence of human neutrophils and serum opsonic activity. Luminescence, 14:239-243 [DOI] [PubMed] [Google Scholar]
- 8: Yokoyama K, Oku T. (1999): Rheumatoid arthritisaffected temporomandibular joint pain analgesia by linear polarized near infrared irradiation. Can J Anaesth, 46: 683-687 [DOI] [PubMed] [Google Scholar]
- 9: Yamazaki M, Miura Y, Tsuboi R, Ogawa H. (2003): Linear polarized infrared irradiation using Super Lizer is an effective treatment for multiple-type alopecia areata. Int J Dermatol, 42:738-740 [DOI] [PubMed] [Google Scholar]
- 10: Ide Y. (2009): Phototherapy for chronic pain treatment. Masui, 58:1401-1406 [PubMed] [Google Scholar]
- 11: Miyazawa K., Mori A., Okudaira H. (1998): Establishment and characterization of a novel human rheumatoid fibroblast-like synoviocyte line, MH7A, immortalized with SV40 T antigen. J Biochem,. 124: 1153-1162 [DOI] [PubMed] [Google Scholar]
- 12: Trentham DE, Townes AS, Kang AH. (1977): Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med, 146: 857-868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13: Hsu YH, Li HH, Hsieh MY, Liu MF, Huang KY, Chin LS, Chen PC, Cheng HH, Chang MS. (2006): Function of interleukin-20 as a proinflammatory molecule in rheumatoid and experimental arthritis. Arthritis Rheum: 54: 2722-2733 [DOI] [PubMed] [Google Scholar]
- 14: Wei CC, Hsu YH, Li HH, Wang YC, Hsieh MY, Chen WY, Hsing CH, Chang MS. (2006): IL-20: biological functions and clinical implications. J Biomed Sci, 13:601-612 [DOI] [PubMed] [Google Scholar]
- 15: Stenderup K, Rosada C, Worsaae A, Clausen JT, Norman DT. (2007): Interleukin-20 as a target in psoriasis treatment. Ann N Y Acad Sci, 1110:368-381 [DOI] [PubMed] [Google Scholar]
- 16: Kragstrup TW1, Otkjaer K, Holm C, Jørgensen A, Hokland M, Iversen L, Deleuran B. (2008): The expression of IL-20 and IL-24 and their shared receptors are increased in rheumatoid arthritis and spondyloarthropathy. Cytokine 41:16-23 [DOI] [PubMed] [Google Scholar]
- 17: Hsu YH. (2011): Anti-IL-20 monoclonal antibody inhibits the differentiation of osteoclasts and protects against osteoporotic bone loss. J Exp Med 208: 1849-1861 [DOI] [PMC free article] [PubMed] [Google Scholar]