Abstract
The right ventricle (RV) is the major determinant of functional state and prognosis in pulmonary arterial hypertension (PAH). RV hypertrophy (RVH) triggered by pressure overload is initially compensatory but often leads to RV failure. Despite similar RV afterload and mass some patients develop adaptive RVH (concentric with retained RV function), whilst others develop maladaptive RVH, characterized by dilatation, fibrosis and RV failure. The differentiation of adaptive versus maladaptive RVH is imprecise but adaptive RVH is associated with better functional capacity and survival. At the molecular level, maladaptive RVH displays greater impairment of angiogenesis, adrenergic signaling and metabolism than adaptive RVH and these derangements often involve the left ventricle. Clinically, maladaptive RVH is characterized by increased NT-proBNP levels, troponin release, elevated catecholamine levels, RV dilatation and late gadolinium-enhancement on magnetic resonance imaging, increased 18fluorodeoxyglucose uptake on positron emission tomography and QTc prolongation on the electrocardiogram. In maladaptive RVH there is reduced inotrope responsiveness due to G-protein receptor kinase (GRK2)-mediated downregulation, desensitization and uncoupling of β-adrenoreceptors. RV ischemia may result from capillary rarefaction and/or decreased right coronary artery perfusion pressure. Maladaptive RVH shares metabolic abnormalities with cancer including aerobic glycolysis (resulting from a FOXO1-mediated transcriptional upregulation of pyruvate dehydrogenase kinase, PDK), and glutaminolysis (reflecting ischemia-induced cMyc activation). Augmentation of glucose oxidation is beneficial in experimental RVH and can be achieved by inhibition of PDK, fatty acid oxidation, or glutaminolysis. Therapeutic targets in RV failure include chamber-specific abnormalities of metabolism, angiogenesis, adrenergic signaling and phosphodiesterase-5 expression. The ability to restore RV function in experimental models challenges the dogma that RV failure is irreversible without regression of pulmonary vascular disease.
Keywords: Right ventricular failure (RVF), aerobic glycolysis, glutaminolysis, G protein receptor kinase 2 (GRK2), Forkhead box protein O1 (FOXO1), adrenoreceptors, pyruvate dehydrogenase (PDH), glutaminolysis, microvascular ischemia, Congenital Heart Disease, fluorodeoxyglucose positron emission tomography (FDG-PET), cardiac magnetic resonance imaging (CMR)
Introduction
The right ventricle (RV) and left ventricle (LV)have discrete embryological origins. The LV develops first, originating from the primary heart field; subsequently the RV develops from precursors cells in the secondary heart field1. The transcriptional regulation of myoblast differentiation also differs between the RV and LV. RV myocytes are directed in their development by chamber-specific transcription factors, such as dHAND and MEF2C2, 3. In contrast, LV myocyte development is guided by Nkx2.5 and eHAND1. The RV’s unique embryology foreshadows differences in its response to pressure and volume overload.
Right ventricular anatomy
The fetal/neonatal RV is a thick-walled chamber that ejects blood at relatively high pressure into a high resistance vascular bed. The wall thickness of both ventricles increases in parallel to ~3.5 mm at term4. Postnatally the pulmonary circulation transitions to a low-pressure circuit and the RV wall thickness remains ~4 mm whilst the LV, faced with 4-times greater afterload, increases in thickness to ~11 mm.
The RV’s crescentic geometry is distinct from the cone-shaped LV. The RV can be divided into 3 segments, including: (1) the inlet, comprised of the tricuspid valve, chordae tendineae, and papillary muscles (2) the trabeculated apical myocardium and (3) the outflow region (called the conus or infundibulum), which has a smooth myocardial surface5. RV contraction starts at the inflow section and progresses towards the outflow tract. A superficial, circumferential layer of RV myocardial fibers is in continuity with the LV fibers, accounting for the systolic motion of the RV free wall toward the interventricular septum. A deeper layer of vertically arranged RV fibers mediates RV systolic shortening. This longitudinal shortening is exploited in echocardiography to measure RV function using the M-mode measurement, tricuspid annular plane systolic excursion (TAPSE). TAPSE predicts RV function and prognosis in adults with Pulmonary Arterial Hypertension (PAH). A TAPSE <18mm indicates a poor prognosis6.
Right ventricular function
After birth, with closure of the ductus arteriosus and foramen ovale, the RV conveys the same cardiac output as the LV, but at ~20% of the LV’s pressure. The RV ejection fraction (EF) in normal children is 53% while the LVEF is 68%7. However, cardiac output is essentially identical, because the RV has slightly larger volumes. In adults the normal RVEF is 62% compared to a normal LVEF of 65%8. RVEF is reduced by half in adults with WHO Group 1 PH (to a mean of 34±10%)9.
The Multi-Ethnic Study of Atherosclerosis (MESA) study used magnetic resonance imaging (MRI) to determine normal values for RV size and function in 4123 normal adults, age 61.5±10.1 years (47.5% males)10. Normal values for RV mass in females and males were 19.2±3.6g and 23.1±4.4g, respectively. Normal values for RV end-diastolic volume in females and males were 108.9±23.2mL and 140.9±29.7mL, respectively. Males had 4% lower RVEF than females. Age was associated with lower RV mass but higher RVEF.
The RV in normal individuals increases cardiac output during exercise. However, at rest, the cardiac output can be maintained at near normal levels without a functional RV, provided that the LV function is normal and there is no pulmonary vascular disease. This is illustrated by two surgical studies in which the RV is removed from the circulation. First, in normal dogs replacement of the RV with a noncontractile Dacron patch reduces cardiac output by only 25%and many animals survive without heart failure. The Dacron patch is pulled toward the septum by the LV and the septum bulges into the RV cavity in systole, creating sufficient force to eject blood into the normal pulmonary circulation11. The second example is the Fontan procedure, performed in patients with tricuspid or pulmonic valve atresia. In the Fontan procedure, all caval flow bypasses the RV and enters directly into the pulmonary circulation. Cardiac output and functional capacity is maintained in these patients, provided there is no pulmonary vascular disease. If performed before age 5-years these patients maintain normal resting cardiac output more than a decade later, although maximal exercise capacity is reduced12.
Rationale for study of the RV
RV function is a major determinant of functional capacity and prognosis when RV afterload is elevated, as in WHO Group 1 PH (PAH)13 or congenital heart diseases, such as pulmonic stenosis14. RV failure (RVF) in PAH differs from LV failure (LVF) in etiology (being more related to increased afterload), prognosis (having higher mortality rates during acute decompensation) and therapy (benefiting from different approved therapies). Compared to LVF the following features are more common in PAH-associated RVF:1) compression of the LV by the enlarged RV, reflecting ventricular interdependence, 2) decreased RV perfusion due to microvascular ischemia and/or reduced epicardial coronary artery perfusion pressure and 3) a high, fixed transpulmonary gradient15. These changes are uncommon in most types of LVF. In adult PAH patients, RV failure requiring inotropic support and admission to an intensive care unit has an inpatient mortality rate over 40%16, 17, far higher than the 13–14% mortality for patients admitted to hospital with LVF requiring inotropes18. A National Heart Lung and Blood Institute-sponsored working group recently highlighted the need to develop a robust basic understanding of the RV’s unique properties, and apply this to advance the understanding of etiology and design of therapies for RV hypertrophy (RVH) and RVF19.
Although the RV is somewhat unique, there are lessons about RVH and RVF to learn from LV hypertrophy (LVH) and LVF. Although the RV has poorer tolerance to sustained afterload than the LV, both ventricles respond to increased afterload with β-receptor downregulation20, 21, increased glycolysis22, 23, and decreased capillary density15, 24. In LVH, proto-oncogenes, such as c-Myc, reactivate fetal gene expression25, including the β-isoform of myosin heavy chain, α-skeletal muscle actin, and atrial natriuretic factor. RVH is also associated with reactivation of the fetal gene package, with activation of c-Myc and a switch to the fetal isoforms of myosin and actin26.
The right ventricle in pulmonary hypertension
Chronic pressure overload, as occurs in World Health Organization (WHO) Categories 1-5 pulmonary hypertension (PH)27, stimulates RVH. RVH can compensate for the increased afterload and maintain cardiac output. However, RVH is rarely fully compensatory and may create RV ischemia and lead to RV failure28.
The ideal means of regressing RVH is reduction in afterload. Unfortunately, approved PH therapies cause only modest reductions in mean pulmonary artery pressure and pulmonary vascular resistance (PVR). There are two circumstances where substantial reductions in PVR demonstrate the expected regression of RVH in response to effective therapy. In a small study of 12 patients with PAH who underwent lung transplantation, there was modest decrease in RV volume after 3 months29. However these changes observed in RVH and RVF after lung transplant for PAH, pale in comparison to that seen in, chronic thromboembolic pulmonary hypertension (CTEPH), where RV function typically returns to normal within weeks after pulmonary endarterectomy (PEA)30. This likely reflects a much more multi-faceted disease process in PAH compared with CTEPH, but also may reflect distinct patterns of the development of RVH. The pattern of recovery seen after PEA for CTEPH and in some PAH patients post-lung transplant indicates that the RV dysfunction is triggered by increased afterload but does not explore the possibility that cardiac-targeted therapies, such as modulators of adrenergic signaling, angiogenesis, fibrosis or metabolism, might serve as a bridge to definitive afterload reduction. This review focuses on identifying pathophysiologic mechanisms that might be exploited to optimize RV function in conditions where RV afterload cannot be corrected,
RV Failure
Clinically, RVF reflects inability of the RV to perfuse the lung circulation adequately to maintain LV filling at low venous/diastolic pressures. Although there is no standard hemodynamic definition, RVF can be characterized by a reduced cardiac index (<2.5 L/min/m2) and increased RV filling pressures (right atrial pressure > 8 mmHg31). The normal values for RV hemodynamics in humans and rodents are contrasted with those observed in PAH in Table 1 & Table 2, respectively. On physical examination, RVF is manifest as an elevation in jugular venous pressure (JVP), reflecting elevated right atrial pressure. Appreciation of an RV lift on palpation of the precordium indicates RV enlargement. A right-sided third heart sound on auscultation indicates a noncompliant and failing RV. In patients with RV dysfunction that are rendered euvolemic on medical therapy the exam may be unremarkable at rest but signs of impaired RV reserve can be elicited by maneuvers that increase venous return to the RV. Kussmaul’s sign (a paradoxical increase in JVP with inspiration) and hepatojugular reflux (a rise in JVP upon pressure over the liver/abdomen) are features of impaired RV reserve. In addition, RVF frequently results in peripheral edema and hepatic congestion on physical examination.
Table 1.
Normal pressure ranges and vascular resistance in humans (adapted from 115 and 116). PAH range is derived from patients with severe Group 1 PH, reference116. In this patient population, 74% of patients were NYHA functional class III and 26% were NYHA functional class IV. This study is chosen because it represents a modern untreated cohort.
| Hemodynamics | Normal Range | PAH Range (mmHg) |
|---|---|---|
| Right atrium - Mean (mmHg) | 1–5 | 11–13 |
| Pulmonary Artery - Mean (mmHg) | 9–20 | 57–61 |
| Pulmonary Capillary Wedge - End-expiratory (mmHg) | 4–12 | 9–11 |
| Systemic artery pressure - Mean (mmHg) | 90–96 | 87–91 |
| Heart beat (bpm) | 60–90 | 84–88 |
| Cardiac index (L/min/m2) | 2.6–4.2 | 1.9–2.3 |
| Pulmonary vascular resistance (dynes sec cm−5) | 20–130 (0.25–1.625 Wood Units) | 1200–1360 (15–17 Wood Units) |
| Systemic vascular resistance (dynes sec cm−5) | 700–1600 (9–20 Wood Units) | 1840–2000 (23–25 Wood Units) |
Table 2.
Normal pressure ranges and vascular resistance in rodents (adapted from 20, 117** represents statistical significance)
| Hemodynamics | Normal rats | Monocrotaline rats |
|---|---|---|
| Pulmonary artery systolic pressure (mmHg) | 25±1 | 60±5** |
| Pulmonary artery diastolic pressure (mmHg) | 3±1 | 13±2** |
| Systemic artery mean pressure (mmHg) | 110±8 | 95±6 |
| Heart rate (bpm) | 320±7 | 294±5** |
| CO (ml/min) | 110±5 | 76±5** |
| Pulmonary vascular resistance (dynesseccm−5) | 0.07±0.01 | 0.44±0.08** |
| Systemic vascular resistance (dynesseccm−5) | 0.85±0.12 | 1.01±0.6 |
Although elevated afterload, due to pulmonary vascular disease, initiates RVH in PAH, it is the decline in RV function (manifest as reduced RVEF and RV dilatation)that best predicts adverse prognosis. Consistent with this, impaired RVEF predicts clinical worsening in PAH more accurately than elevated PVR32. In fact, the RV response to therapy is the key determinant of clinical outcomes in PAH. Even though PAH therapies target pulmonary vasoconstriction, survival has been shown to be significantly associated with changes in RVEF, whereas therapeutic changes in PVR or CO demonstrated little relationship to survival. 5-year survival exceeds 90% in PAH patients with a stable or increased RVEF and an accompanying fall in PVR. However, in some patients, despite a demonstrable therapeutic decrease in afterload, there is continued deterioration in RV function and increased RV dimensions. Increases in RV end-systolic and end-diastolic volumes correlate with mortality, an association which may be related to the Law of Laplace (higher wall tension related to chamber dilatation) combined with increased intraluminal pressure33. Thus, the RV may ultimately decompensate because of persistently elevated wall stress. The variability in RV response between patients may suggest intrinsic genetic or epigenetic differences in susceptibility to decompensation. Ventricular interdependence is also important when considering the status of the RV in PAH. Inadequate RV perfusion and impingement of the pressure and volume overloaded RV on the LV, leads to LV under-filling, which reduces cardiac output. The resulting phenotype, a small LV and a dilated RV, portends poor prognosis34. In severe RVF, low cardiac output can reduce pulmonary artery pressure, which portends a worsening prognosis35. Reduction in RVEF and/or late gadolinium enhancement (LGE) at the RV-LV septal hinge points on MRI predicts clinical worsening in PAH36.
Adaptive versus Maladaptive RVH
There is heterogeneity in RVH in terms of its effects on cardiac output and the likelihood of progression to RVF that is not explained by differences in RV mass or RV pressure overload. Some patients have maladaptive forms of RVH and rapidly decompensate whilst others remain stable for decades, despite similar elevations in RV pressure and RVH22 (Figure 1A). Within the WHO classification there is subclass heterogeneity in the predilection to RVF, even within the relatively homogenous Group 1 patients. RVF is much less prevalent and occurs later in those with congenital heart disease (i.e. Eisenmenger’s syndrome37) versus in those with scleroderma-associated PAH38, 39. Patients with RV pressure overload without pulmonary vascular disease, such as those with pulmonic stenosis, retain concentrically hypertrophied, contractile RVs for decades40–42.
Figure 1.
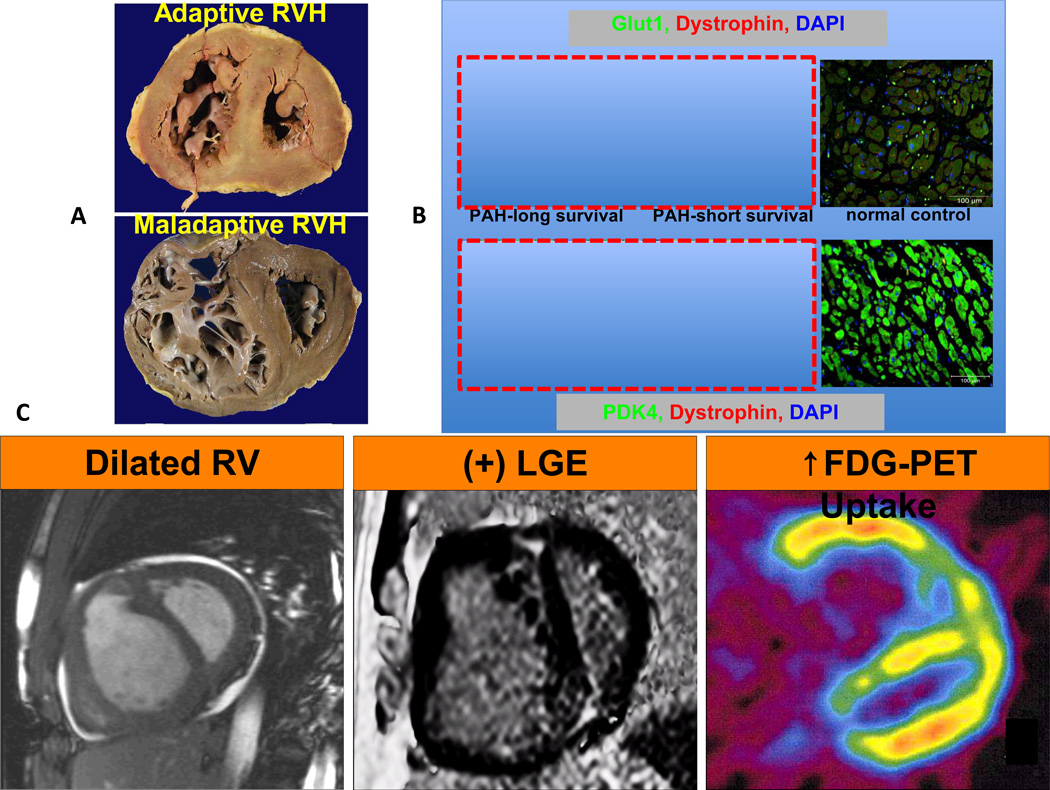
Increased glycolysis in the right ventricle (RV) in right ventricular hypertrophy (RVH) in PAH patients. (A) The cross sections of RVs from patients with adaptive versus maladaptive RVH. RV chambers are enlarged in both patients however adaptive RVH is concentric with less dilatation and fibrosis. (B) Immunostaining shows up-regulation of Glut1 and PDK4 expression in RV myocytes and is less profound in the PAH patient with adaptive RVH. (C) Imaging modalities showing RV dilatation in MRI, RV fibrosis in MRI and increased FDG uptake in PET scan. The figure is partially adapted from references22, 36, 45with permission.
Animal models of RVH recapitulate this separation into adaptive and maladaptive categories, as judged by their exercise performance and survival20. RVH in rats post pulmonary artery banding (PAB), which models pulmonic stenosis, is better tolerated, than RVH induced by endothelial toxins, such as monocrotaline or the vascular endothelial growth factor receptor (VEGF-R) antagonist, Sugen 5416, plus chronic hypoxia (CH+SU)43. That RV failure is more prevalent in PAH models with endothelial injury, suggests a potential role for endothelial dysfunction in the coronary vasculature of the RV itself.
Using monocrotaline rats, Sutendra et al have studied the transition period between adaptive and maladaptive RVH44. In this work, the transition to maladaptive RVH is associated with a decrease in RV HIF1α, a decline in angiogenesis, a fall in glucose uptake and a reversion towards normal metabolism. The authors argue that metabolic shift observed in the RV is not sustained throughout the progression of RV failure. A rise in mitochondrial-derived ROS potentially results in HIF1α inhibition, thereby suppressing angiogenesis. The resultant ischemia from the decrease in angiogenesis may contribute to the rapid deterioration of RV function in maladaptive RVH.
Although this is an elegant theory, the time course over which this maladaptive metabolism regresses in undefined and the clinical relevance is unclear. In the limited data available patients with advanced PAH manifest persistent glycolytic shift on FDG-PET22, 45. Moreover, there is disagreement regarding the predominant transcription factor involved in aerobic glycolysis in the heart. HIF1α is not consistently upregulated in our rodent RVH experiments and HIF1α is not known to upregulate transcription of PDK4, the predominant cardiac form of PDK that leads to aerobic glycolysis. In contrast to the heart, HIF1α is upregulated in the lung in PAH models46 (relating to epigenetic silencing of SOD247). In the lung vasculature, HIF1α appears to account for aerobic glycolysis; in contrast, the metabolic remodeling in the RV appears to be more related to pathologic activation of FOXO1 and c-Myc26, 48. Emerging concepts regarding the molecular basis for these adaptive and maladaptive RVH phenotypes and their therapeutic implications are discussed subsequently.
There are many factors that determine whether RVH will be well tolerated, such as the presence and severity of RV fibrosis, ischemia, autonomic dysregulation, and metabolic changes (Figure 1B). Adaptive RVH is provisionally defined by preservation of normal cardiac output, RVEF, RV filling pressures and exercise capacity. It is usually characterized by concentric hypertrophy with minimal RV dilatation or fibrosis. Conversely, the maladaptive phenotype can be defined by significant reductions in cardiac output, RVEF with elevation of RV filling pressures and reduced exercise capacity. Maladaptive RVH commonly displays RV fibrosis and dilatation (Figure 1C). While these definitions are imprecise, there is progress towards more rigorous, molecular fingerprints of these RVH phenotypes. Several abnormalities appear common to maladaptive RVH, including RV ischemia49 and two forms of cancer metabolism, aerobic glycolysis22, 45, 50 and glutaminolysis26. In addition maladaptive RVH shows greater impairment of angiogenesis, manifest as capillary rarefaction and decreased expression of angiogenic genes (VEGF, IGF-1, apelin and angiopoeitin-1). There is also greater dysregulation of the autonomic nervous system in maladaptive RVH with a broad downregulation and sensitization of α, β and dopaminergic receptors in the RV myocytes45, 50–52. Finally, in maladaptive RVH changes in RV perfusion, angiogenesis, adrenergic signaling and metabolism tend to involve the LV whereas they tend to be confined to the RV in adaptive RVH. Although these changes have yet to shape clinical practice, they do offer opportunities for research and suggests new therapeutic strategies (Figure 2).
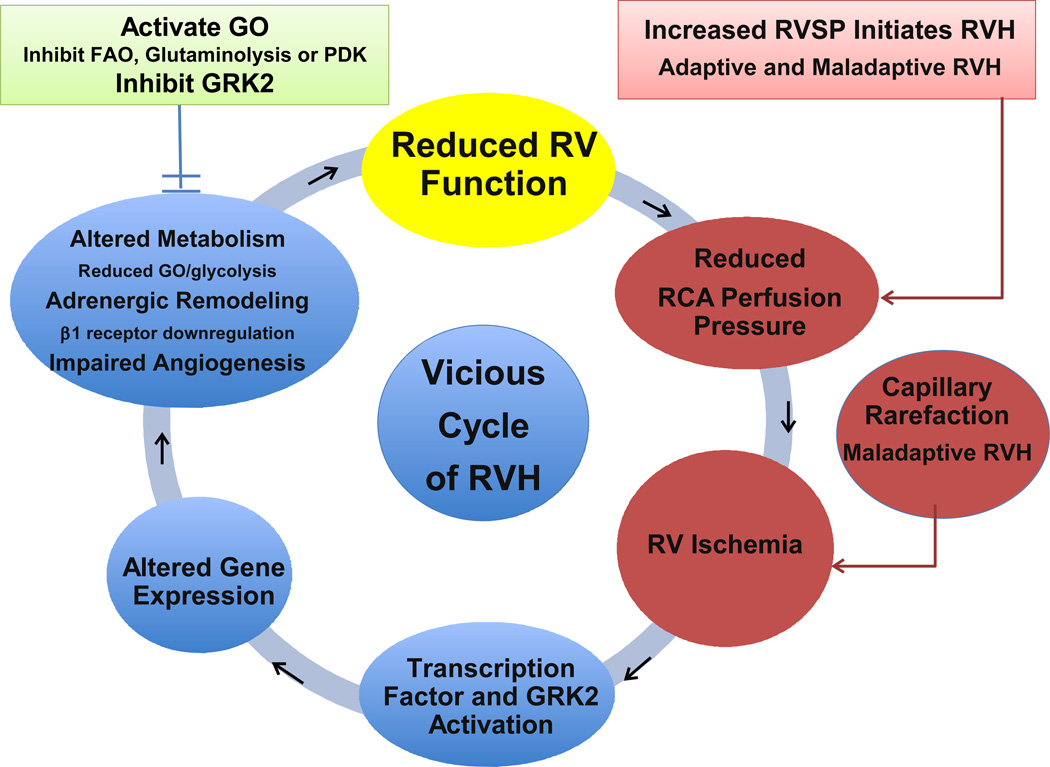
Figure 2.
Vicious cycle of right ventricular failure including metabolic changes and RV ischemia.
Right Ventricular ischemia
RV dysfunction in PAH may reflect chronic reduction in RV perfusion in the presence of viable myocardium, representing a form of myocardial hibernation. Evidence of RV ischemia in PAH includes angina-like chest pain, ischemia on nuclear perfusion stress imaging53, and increased RV uptake of 18fluorodeoxyglucose on positron emission tomography (FDG-PET)45, 54. Evidence of ischemia, such as elevation of troponin levels, indicates poor prognosis49, 55. Ischemia is also relevant to RVF in congenital heart disease. Late failure of the systemic RV after the Mustard repair of transposition of the great arteries is associated with impaired myocardial flow reserve56–58. Nuclear perfusion scans commonly demonstrate perfusion defects with concordant regional wall motion abnormalities after repair of transposition of the great arteries56.
RV ischemia may reflect reduced right coronary artery (RCA) perfusion pressure, and/or decreased coronary flow reserve59. The low RV systolic pressure (RVSP) in normal individuals permits filling of the RCA during both systole and diastole. In RV pressure overload, the systolic perfusion gradient (Aortic pressuresystole-RVSP)may be eliminated and the diastolic RCA perfusion pressure (Aortic pressurediastole-RV end diastolic pressure) be reduced, thereby impairing RCA flow60, 61. RV contractile function remains constant until RCA perfusion pressures fall below 50 mmHg62.
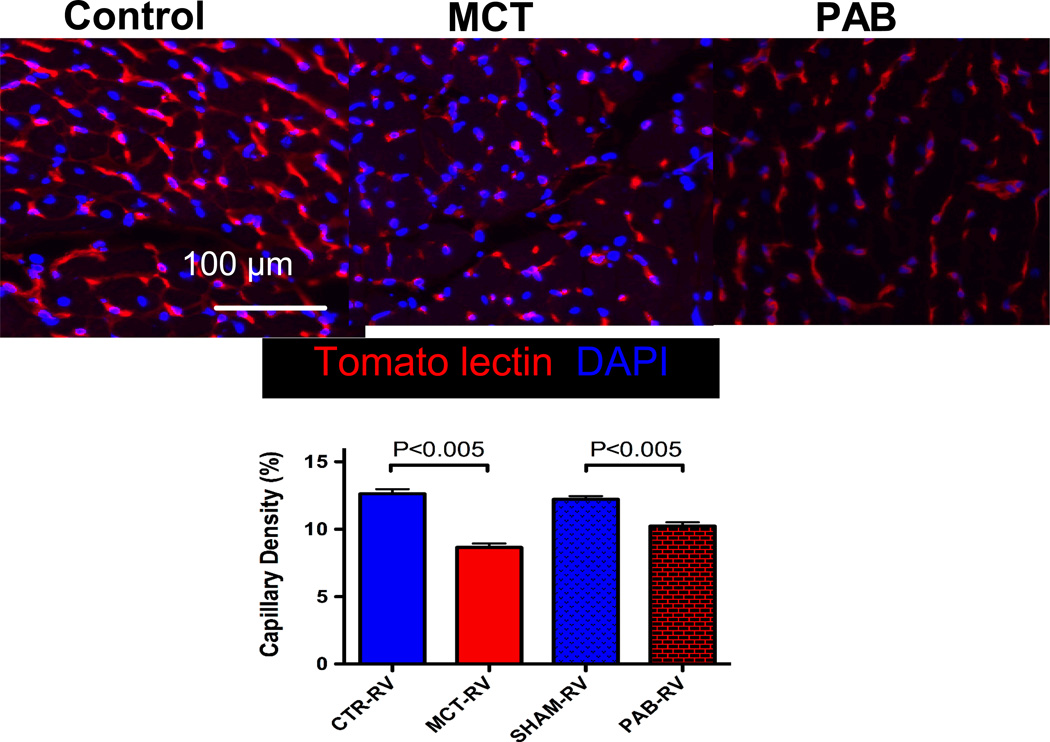
It has been suggested that an additional cause of ischemia contributes to RVF in PAH, namely capillary rarefaction, defined as a reduction in the density of capillaries and small intramyocardial arterioles in the RV15. RV capillary density is reduced in CH+SU and monocrotaline RV with little change in PAB RVs15, 26 (Figure 3). RV capillary rarefaction may occur in PAH patients, particularly those with scleroderma PAH26. Since CH+SU and monocrotaline rats have similar elevation in RVSP and similar RVH as PAB rats, their reduced functional capacity and greater mortality suggests that ischemia cannot be explained solely by diminished RCA perfusion pressure50.
Figure 3.
Decreased capillary density in animal models of maladaptive PAH but not in adaptive PAH.
Correctly identifying the cause of RV ischemia in RVH has therapeutic ramifications. If RVF were initiated primarily by a drop in coronary perfusion pressure then strategies such as infusion of phenylephrine to increase the aortic-RV pressure gradient to drive coronary perfusion might be rational. However, if capillary rarefaction contributes to RV ischemia it would be more logical to treat RV failure by pharmacologically changing metabolism to “do more with less” or by enhancing RV angiogenesis. In rodent models capillary rarefaction observed in PAH rats appears to be reversible with β-blockers63. It is likely that ischemia precipitates many of the metabolic changes that occur in RVH.
Metabolism of the right ventricle in PAH
Aerobic Glycolysis
In the fetal heart, where circulating fatty acid levels are low, glycolysis and glucose oxidation are the major sources of ATP production64. In the adult heart fatty acid oxidation (FAO) becomes the predominant energy source (60–90%) but glucose metabolism continues to contribute 10%-40% of ATP production65.
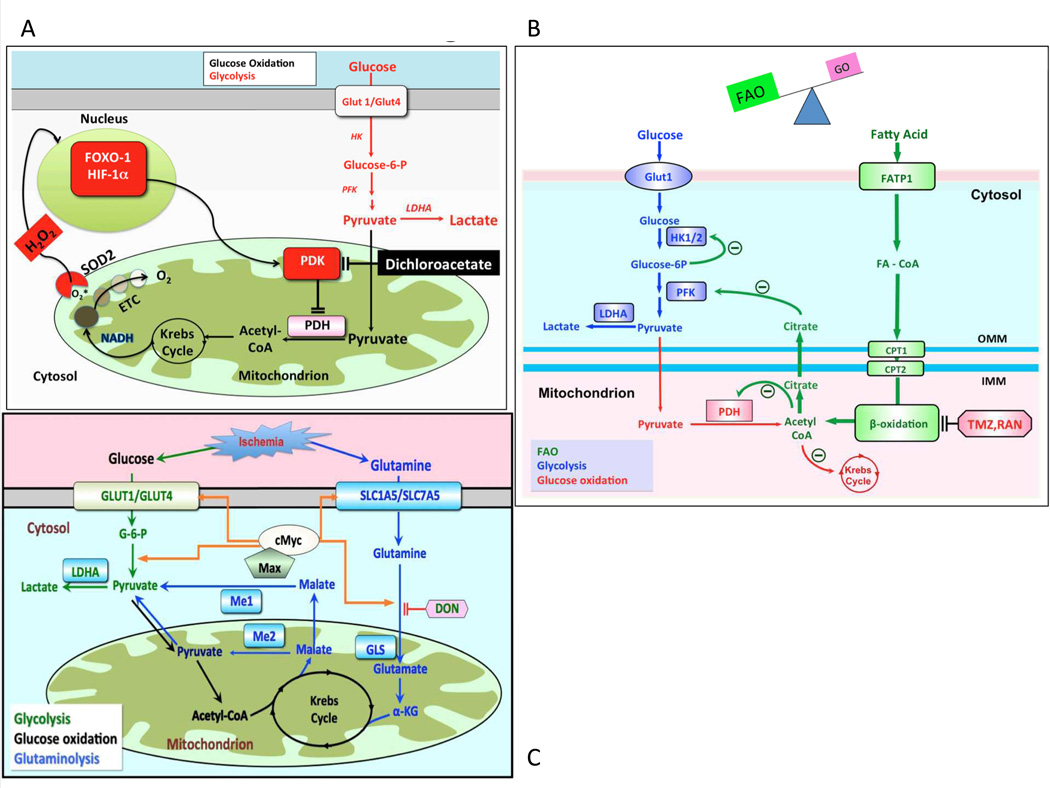
In RVH the metabolic fate of glucose is altered because mitochondrial metabolism is actively (although reversibly) suppressed66. Glucose metabolism starts with cytosolic glycolysis, which ultimately converts glucose to pyruvate. In the normal adult RV myocyte, pyruvate is transferred to the mitochondria where it serves as substrate for pyruvate dehydrogenase (PDH).If the PDH complex in mitochondria is active, pyruvate is converted to acetyl CoA, fueling Krebs cycle and providing electron donors (NADH and FADH) for the electron transport chain and ATP generation67.
Pyruvate dehydrogenase kinase (PDK), a key inhibitory regulator of PDH (and thus of glucose oxidation), is transcriptionally upregulated in RVH (Figure 1B). All 4 PDK isoforms inhibit PDH by phosphorylating its E1-∝ subunit. The predominant cardiac PDK isoforms in the RV are PDK2 and PDK448. When PDH is inhibited, supply of electron donors to Krebs’ cycle is limited, which reduces energy production68. This PDK-mediated metabolic switch is associated with decreased RV contractility and reduced cardiac output50.
The shift to aerobic glycolysis in RVH has several consequences (Figure 4A). First, lactate is produced, resulting in acidosis, which impairs RV function. Second, only 2 ATP molecules/glucose are obtained per mole of glucose, compared to the 32 ATP generated during glucose oxidation50. To support the increased glycolysis required to maintain energy homeostasis there is marked upregulation of glucose uptake, which can be detected by FDG-PET scans in RVH45, 54, 66 (Figure 1C).
Figure 4.
A: Mechanism of impaired glucose oxidation and enhanced glycolysis in RVH. In RVH, activation of various transcription factors, including FOXO1, cMyc and HIF-1α upregulates expression of many glycolytic gene. A common finding in RVH is increased PDK expression, which inhibits PDH and reduces mitochondrial respiration. PDK activation also occurs in the lung in PAH, although the transcriptional regulation and isoform specificity may differ than that seen in the RV. Dichloroacetate inhibits PDK and thereby promotes glucose oxidation and inhibits glycolysis. ETC = electron transport chain, HK = hexokinase, H2O2 = hydrogen peroxide, LDHA = lactate dehydrogrenase A, PFK = phosphofructokinase. Adapted from 50
B: The Randle cycle in RVH The inhibition of β-FAO by trimetazidine and ranolazine increases PDH activity and improves GO. This reciprocal relationship between GO and FAO is referred to as the Randle cycle. Adapted with permission from 75.
C: Proposed mechanism of glutaminolysis in RVH. RV ischemia and capillary rarefaction activate cMyc and Max, which increases glutamine uptake and production of α-ketoglutarate (α-KG). α-KG enters Krebs’ cycle leading to production of malate. Krebs’ cycle-derived malate generates cytosolic pyruvate, which is converted by lactate dehydrogenase A (LDHA) to lactate. In conditions of high glutaminolysis GO is inhibited. Reproduced from 26 (Illustration Credit: Ben Smith).
Increased RV glucose uptake, reflective of increased glycolysis, has been shown in a small series of PAH patients undergoing FDG-PET45, 54. Moreover, there is some evidence that effective reduction of afterload reduces RV uptake of FDG45. Likewise, in experimental RVH, there is increased RV glycolysis, evidenced by direct measurement of metabolism in isolated RV working heart and increased uptake of FDG-PET in vivo45.
In a case report comparing a long-term PAH survivor with an individual who rapidly decompensated from RV failure, the markers of aerobic glycolysis (the glucose transporter, glut 1, and PDK4) were less elevated in the adaptive versus the maladaptive RVH patient22. Perhaps the hypokinetic RV in maladaptive RVH reflects a form of myocardial hibernation, precipitated by impaired RV perfusion and metabolic shifts in the RV myocytes22 (Figure 2).
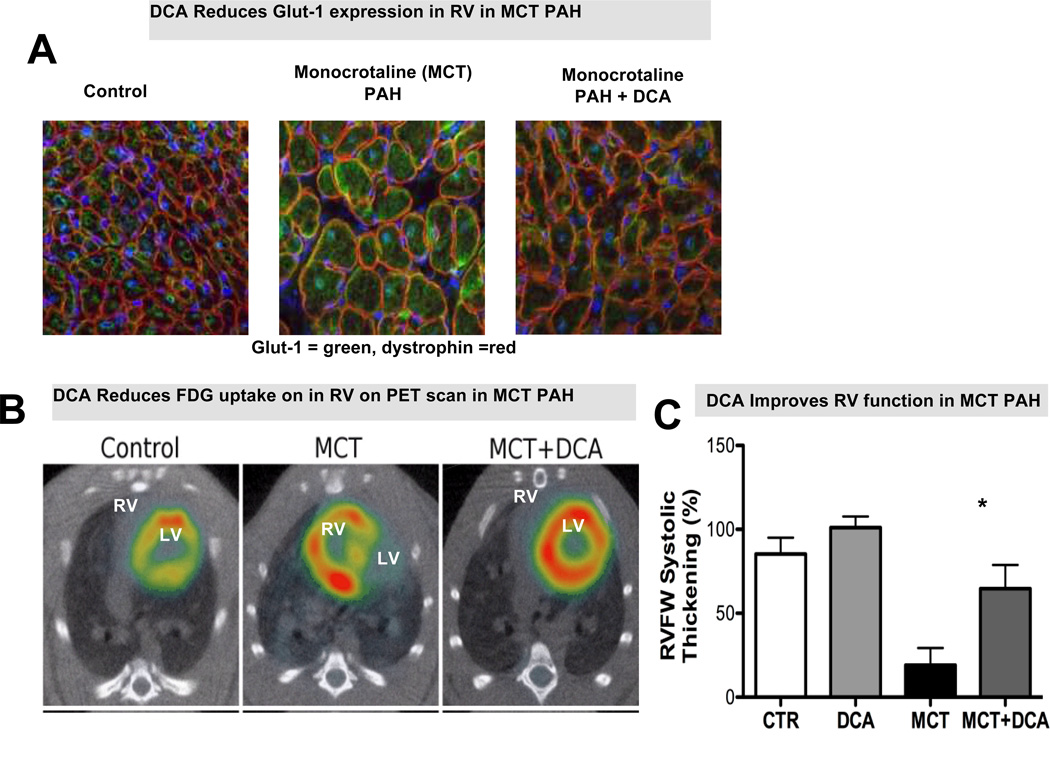
In rodents, PDH is inhibited more in maladaptive monocrotaline RVH than in adaptive PAB RVH22. Dichloroacetate (DCA), a PDK inhibitor, reduces PDH phosphorylation and partially restores RV contractility in rodent models of PAH50, 69,70 (Figure 5). The possibility that RV function can be improved by metabolically targeted therapies, even without reducing the afterload, is intriguing. There is a similar metabolic shift towards aerobic glycolysis in the lung vasculature in PAH66, 71. Thus a PDK inhibitor might be expected to have beneficial effects on both the RV and lung circulation54, 66, 69. A therapeutic strategy of enhancing glucose oxidation has the potential to dramatically change the treatment paradigms of PAH patients with RV failure. DCA has been used safely in children with lactic acidosis72 and in adults with glioblastoma multiforme73, with the main toxicity being reversible peripheral neuropathy. A phase 1 clinical trial is currently assessing DCA in PAH (Dichloroacetate (DCA) for the Treatment of Pulmonary Arterial Hypertension; NCT01083524)74.
Figure 5.
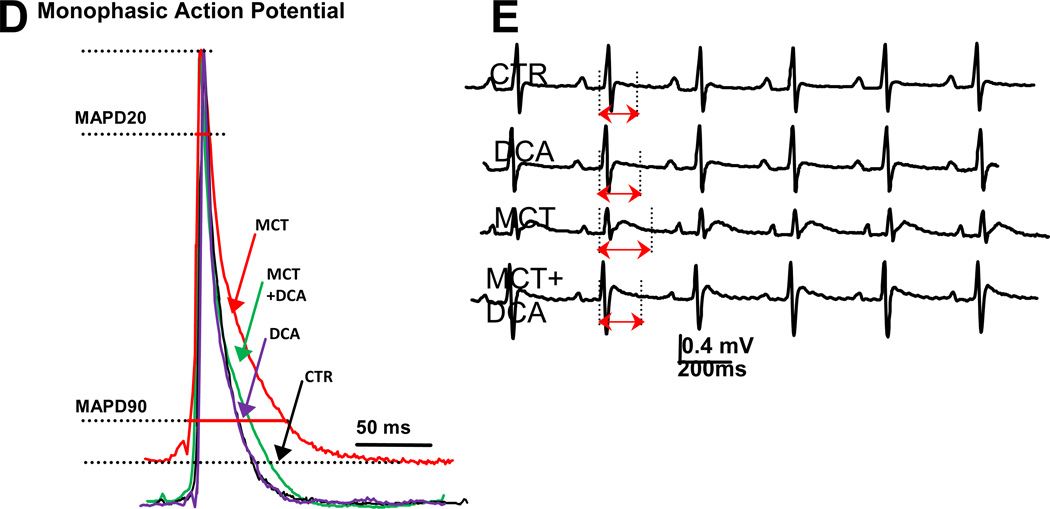
(A)DCA Reduces Glut-1 expression in RV in monocrotaline-PAH. (B) DCA Reduces FDG uptake on in RV on PET scan in monocrotaline-PAH. (C) DCA Improves RV function in monocrotaline-PAH.(D)Representative traces and mean data showing MAPD20 and MAPD90 are significantly prolonged in the monocrotaline (MCT) group vscontrol (CTR) and that repolarization is improved by DCA. (E) Representative traces and mean data of lead II surface ECG. DCA reduces the QTc prolongation in RVH. Red highlighted arrows indicate QTc intervals. Adapted with permission from 50, 75
Pathologic activation of transcription factors in the RV in PAH (e.g. HIF1α, and FOXO1) leads to changes in metabolism, notably activation of PDK2 and PDK4. This decreases expression of repolarizing voltage-gated potassium channels (Kv), such as Kv1.5, in cardiac myocytes. In rodent models of RVH this ionic remodeling results in prolongation of the RV’s monophasic action potential duration and mild QTc interval prolongation on the surface electrocardiogram. Therapy with the PDK inhibitor DCA restores oxidative glucose metabolism and restores Kv channel expression leading to normalization of QTc intervals (Figure 5D, 5E)75. In PAH patients QTc intervals are also prolonged compared with normal subjects (454.8±29 ms vs. 429.8±18 ms) and the QTc interval correlates directly with increasing RV end-diastolic volume and mass and inversely with RVEF. QTc interval is thus a potential simple biomarker of RVH. Prognostically, a QTc interval >480 ms portends decreased survival in PAH76.
Fatty Acid Oxidation and the Randle Cycle
There is a reciprocal relationship between the two major oxidative metabolic pathways, such that inhibiting FAO increases the glucose oxidation. This is called the Randle cycle77 (Figure 4B). A therapeutic strategy of enhancing glucose oxidation by inhibiting FAO might be beneficial in RVH since FAO uses 12% more oxygen than glucose oxidation to generate the same amount of ATP78. FAO’s demand for oxygen may be difficult to sustain in the presence of RV ischemia. Partial inhibitors of fatty acid oxidation (pFOXi) are approved for human use for several cardiovascular indications. Trimetazidine, a long-chain 3-ketoacyl coenzyme A thiolase, is used in Europe to treat refractory ischemia in patients with coronary artery disease79–82. Another pFOXi, ranolazine, is approved for refractory ischemia in America83–85 (although there is some controversy whether it works through inhibition of FAO and activation of PDH86–88).
The reciprocal relationship between FAO and glucose oxidation reflects (in part) citrate production during FAO. Citrate inhibits phosphofructokinase (PFK), causing accumulation of glucose-6-phosphate, which inhibits hexokinase and glucose oxidation. In addition, acetyl CoA, generated from FAO, inhibits PDH. FAO inhibition can reduce these inhibitory mechanisms and enhance glucose oxidation. A study of trimetazidine and ranolazine in rats with PAB-induced RVH showed that pFOXi increased cardiac output and treadmill exercise capacity, suggesting that the increased FAO in PAB-RVH is maladaptive75. FAO inhibitors elevated RV ATP and increased glucose oxidation, reflecting activation of the Randle cycle75. Like PDK inhibitors, pFOXi also partially regressed RVH and increased cardiac output75. The beneficial effect of trimetazidine has also been shown in monocrotaline-induced RVH89. In this model, trimetazidine enhanced cardiac mitochondrial function and increased oxygen consumption, while reducing the formation of oxygen free radicals90.
PET studies have shown that in patients with idiopathic dilated cardiomyopathy, trimetazidine decreases FAO and causes a compensatory increase in glucose oxidation91. Whether combining PDK inhibitors and pFOXi would have additive or synergistic benefit in RVH has not been studied. It should also be noted that FAO is not increased in all models of RVH48.
Glutaminolysis
The Warburg phenomenon (aerobic glycolysis) and glutaminolysis are metabolic pathways that are common in cancer and permit rapid cell growth without apoptosis92. Perhaps in maladaptive RVH these cancer metabolic pathways permit inappropriate myocyte enlargement. We recently examined the possibility that glutaminolysis might also occur in RVH. In comparisons of monocrotaline-RVH and PAB-RVH we noted the monocrotaline model had greater ischemia as determined by larger reductions in RV VEGFα expression, greater reduction in coronary blood flow and reduced RV microvascular density. Consistent with increased glutaminolysis in monocrotaline-RVH, RV expression of glutamine transporters (SLC1A5 and SLC7A5) and mitochondrial malic enzyme were elevated. This is analogous to the upregulation of glut-1 transporter that is seen in aerobic glycolysis50. In both scenarios, transporter upregulation ensures that substrate provision is not a limiting factor in metabolic capacity. Direct measurement of metabolism in the RV working heart model, using a dual isotope technique, demonstrated a 6-fold increase in 14C-glutamine metabolism in monocrotaline-RVH, which was not seen in PAB. As with aerobic glycolysis, glutaminolysis appears to be maladaptive.In vivo, the glutamine antagonist,6-Diazo-5-oxo-L-norleucine (DON), at doses that inhibited glutaminolysis, increased glucose oxidation and elevated cardiac output. Longer-term therapy with DON restored PDH activity, reduced RVH and increased cardiac output. The transcriptional basis for this RV metabolic pathway appears to be activation of the cMyc-Max pathway, perhaps as a consequence of RV ischemia. Glutaminolysis may be a therapeutic target in maladaptive RVH, although DON has nonspecific systemic toxicity (Figure 4C).
Right Ventricular Sympathetic activation in PAH
Dopamine and dobutamine are commonly used as inotropic agents to treat acute RVH in PAH patients. Some centers also use the pure α-adrenergic agonist, phenylephrine, as a vasopressor, to increase coronary perfusion pressure. Dobutamine and dopamine primarily exert their inotropic effects by stimulating β1-adrenergic receptors (β1-AR) but dopamine has some reliance on α-adrenergic receptors (α-AR) at higher doses (10–20 µg/Kg/min). However, the choice of inotropes for RVF is highly variable amongst practitioners, even at a single institution, and inotrope use is also associated with extremely high mortality17. This may reflect the dire condition of PAH patients that suggests the need for inotropic support but should provoke the question whether catecholamines might actually worsen prognosis in RV failure. The latter interpretation is possible, since the adrenergic system is arguably maximally activated in RV failure in PAH20. PAH patients with RVF have high circulating catecholamine levels and lose the normal ability to augment catecholamine levels with exercise93. Autonomic activation, loss of inotropy to β-AR agonists and downregulation of β1-AR expression also occurs in maladaptive rat PAH models94, 95.
In a canine RVF model, which combined PAB and tricuspid valve avulsion, downregulation of the β-AR was confined to the RV and resulted in chamber specific reduction of isoproterenol-induced cAMP production96. In humans with PAH and RVF, RV β-AR density is decreased and the response to inotropes is similarly impaired97. However, whereas Bristow et al found no impairment of LV β-AR signaling in human RVF associated with PAH, β-AR density decreases in the non-hypertrophied LV in monocrotaline RVH98, a finding that was recently reproduced20.
We recently discovered a broad downregulation of adrenoreceptors in rodent RVH, including α1, β1 and dopamine (1–5) receptors20. While changes occurred in all forms of RVH, the adrenoreceptor downregulation was more severe in maladaptive RVH, and extended to the LV. The cause of this broad downregulation of adrenergic receptor expression and function was activation of G protein receptor kinase (GRK2) (also called β-adrenergic receptor kinase 1 (BARK1)). Interestingly, GRK2 activity was as high in RVH at baseline as could be stimulated by catecholamines in normal RVs. This suggests a near maximal receptor downregulation and desensitization occurs in RVH.
β1-receptor uncoupling and downregulation reduced the RV response to all inotropes in RVH, perhaps indicating why patients with PAH and RVF respond poorly to inotrope infusion. In rodent models, dobutamine was superior to dopamine in terms of its ability to increase RV contractility in RV Langendorff models and in vivo. Dobutamine’s superiority was associated with its superior coupling to adenylyl cyclase (evident as a greater increase in cAMP levels). Interrupting Gβγ-signaling, using gallein, inhibits GRK2 activity and improved cardiac function when administered chronically in vivo20.
In left heart failure, β-blockers improve survival and LV function99. However, β-blockers are not used clinically in PAH and concerns about their safety exist. However, the α/β blocker carvedilol and the β-blocker propranolol have been shown to regress RVH and lower RVSP in experimental models of chronic hypoxic pulmonary hypertension and in the CH+SU model100. Small clinical trials have demonstrated that β-blockade with carvedilol can improve RV systolic function101and a clinical trial of β-blockers for RVH in WHO Group 1 PH is underway102.
Phosphodiesterase-5 and Endothelinin RVH
In the normal heart quantitative differences in expression of many pumps and transporters exists between the RV versus LV103. This is a reminder that there may be other chamber-selective therapeutic targets in RVH. Sildenafil, a phosphodiesterase (PDE) 5 inhibitor has been found to have a direct RV inotropic effect104, 105. Interestingly PDE5 is not present in the normal RV myocytes but is induced during RVH, both in rodents and humans104. By inhibiting PDE5, sildenafil increases cGMP levels, which then inhibits PDE3. Sildenafil’s modest inotropic effects are due (in part) to this indirect inhibition of PDE3. Thus there are mechanistic similarities between sildenafil’s actions in RVH and the more potent direct PDE3 inhibitor of milrinone105. This de novo appearance of a selective RV target accounts in part for sildenafil’s ability to increase cardiac output in PAH105.
Patients with PAH also have up-regulation of the RV myocardial endothelin axis, which may be a compensatory mechanism to increase contractility and cardiac output in the setting of the increased afterload observed. In the working heart model, endothelin receptor antagonists (ERAs) decrease contractility106. This is of interest because of the published trials failing to show a benefit of ERAs in left heart failure107 although ERAs have demonstrated an established clinical improvement in PAH108, 109.
Both the effects of PDE5 inhibitors and ERAs on the RV were unanticipated by PAH trials which focused on the effects of these drugs on the pulmonary vasculature. Future trials should directly examine the effects of putative PAH therapies on the RV, to detect both benefit and harm110.
Right ventricular fibrosis
In adult patients with PAH, late gadolinium-enhancement on MRI at the RV insertion points is likely evidence of localized fibrosis and is associated with worsened prognosis111. In children with congenital heart disease, fibrosis may also be an important determinant of RVF.
Whether trials should be performed to reduce RV fibrosis is unclear. There are several potential means by which fibrosis could be inhibited, such as using inhibitors of the renin-angiotensin-aldosterone system, including angiotensin receptor blockers or mineralocorticoid antagonists112. A study in patients with congenital heart disease and a systemic RV tested the ability of the angiotensin receptor blocker, losartan, to improve cardiac function. In this study, losartan failed to improve hemodynamics or exercise capacity113. In PAH, the aldosterone pathway has been identified as a potential therapeutic target114.
Conclusions
Although a cure for PAH will require regression of pulmonary vascular lesions or transplantation, substantial improvement in longevity and functional state might be achieved by an effective treatment for RV failure. Hopefully an increased understanding of adrenergic, angiogenic, fibrotic and metabolic derangements in the RV in PAH will offer new therapeutic targets to enhance RV function (Figure 2).
Supplementary Material
A Patient Asks Questions….
I met this patient in the PAH group meeting… She has lower pulmonary artery pressures than I do, but she is much sicker….What does that mean; how is it possible?
At first, this appears to be a paradox; one would assume that higher lung blood pressure would mean more advanced disease and more symptoms. However, as it was discussed in the first paper in this collection, the symptoms in PAH (i.e. shortness of breath) are not caused by the pressure in the arteries of the lungs, but by the function of the right chambers of the heart (right ventricle). At some point the muscle of the right ventricle starts getting exhausted due to pumping against higher than normal pressures. Its contractile strength is suppressed, causing a decrease in the amount of blood ejected with each contraction; and thus decrease in the amount of blood (and thus oxygen) that reaches the organs of the body, generating the sensation of shortness of breath. However, as the contractile power of the heart muscle decreases, so does the pressure of the blood that it ejects; in other words, the pressure in the lung blood vessels decreases. This is similar to the decrease in the pressure of the water at a water hose, not because there is narrowing of the hose lumen but because the pressure in the water pump feeding the hose is decreasing.
This is a very important realization that sometimes may even confuse physicians. For example, let’s say that a therapy is initiated to treat PAH aiming to decrease the narrowing of lung blood vessels. Let’s assume that this therapy may also unexpectedly suppress the function of the heart muscle in the right ventricle. Such unexpected effects (sometimes called “off target effects”) are much more common than we assume in Medicine. In this case, the pressures in the lung arteries will decrease not because the therapy improved the function of the blood vessels, but because it adversely decreased the contractile power of the heart. While the pressures in tests (for example, an echocardiogram) may appear to be decreasing, the patient will actually feel worse. If the patient does not communicate well with the treating physician and if the treating physician does not look at the “big picture”, he/she may actually prescribe an increase in the dose of the therapy, rather than stopping it. This would obviously make things even worse.
This is why it is important to approach the right ventricle in parallel to the lung vessels in PAH, an idea that is changing the way that we approach PAH. This article discusses many mechanisms that may explain why the right ventricle may worsen and perhaps why it may worsen in one patient but not another. In our patient’s question, the one with lower pressures was feeling worse because she has worse right ventricular function. What makes the right ventricle start deteriorating at some point and why this happens earlier in some patients, is one of the most critical questions that we need to answer in PAH. Understanding this concept may also help the patients to better understand their symptoms and their response to standard or investigative therapies.
Acknowledgements
We apologize to colleagues whose work could not be cited due to space limitations.
Funding Sources:
This work is supported by NIH-RO1-HL071115 and 1RC1HL099462 (SLA).
Non-standard Abbreviations and Acronyms
- CH+SU
Chronic hypoxia plus Sugen 5416
- CTEPH
Chronic Thromboembolic Pulmonary Hypertension
- DCA
Dichloroacetate
- EF
Ejection fraction
- ERA
Endothelin receptor antagonist
- FAO
Fatty acid oxidation
- FDG-PET
18fluorodeoxyglucose positron emission tomography
- GRK2
G protein receptor kinase
- HIF1α
Hypoxia-inducible factor 1 alpha
- LGE
Late gadolinium enhancement
- LV
Left ventricle
- LVF
Left ventricular failure
- LVH
Left ventricular failure
- MRI
Magnetic Resonance Imaging
- PAH
Pulmonary Arterial Hypertension
- PDE
Phosphodiesterase
- PDH
Pyruvate dehydrogenase
- PDK
Pyruvate dehydrogenase kinase
- PEA
Pulmonary Endarterectomy
- PH
Pulmonary Hypertension
- PVR
Pulmonary vascular resistance
- RCA
Right coronary artery
- RV
Right ventricle
- RVF
Right ventricular failure
- RVH
Right ventricular hypertrophy
- TAPSE
Tricuspid annular plane systolic excursion
- VEGF
Vascular endothelial growth factor receptor;
Footnotes
Disclosures:
None.
References
- 1.Srivastava D, Olson EN. A genetic blueprint for cardiac development. Nature. 2000;407:221–226. doi: 10.1038/35025190. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Ryan JJ, Rich S, Nathan S, Tsai WK, Patel AR, Fang YH, Piao L. Muscle: Fundamental Biology and Mechanisms of Disease. Academic Press; 2012. The right ventricle: Reemergence of the forgotten ventricle; pp. 537–553. [Google Scholar]
- 3.Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of isl1 and gata factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- 4.Firpo C, Hoffman JI, Silverman NH. Evaluation of fetal heart dimensions from 12 weeks to term. Am J Cardiol. 2001;87:594–600. doi: 10.1016/s0002-9149(00)01437-5. [DOI] [PubMed] [Google Scholar]
- 5.Bronicki RA, Baden HP. Pathophysiology of right ventricular failure in pulmonary hypertension. Pediatr Crit Care Med. 2010;11:S15–S22. doi: 10.1097/PCC.0b013e3181c7671c. [DOI] [PubMed] [Google Scholar]
- 6.Forfia PR, Fisher MR, Mathai SC, Housten-Harris T, Hemnes AR, Borlaug BA, Chamera E, Corretti MC, Champion HC, Abraham TP, Girgis RE, Hassoun PM. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med. 2006;174:1034–1041. doi: 10.1164/rccm.200604-547OC. [DOI] [PubMed] [Google Scholar]
- 7.Hurwitz RA, Treves S, Kuruc A. Right ventricular and left ventricular ejection fraction in pediatric patients with normal hearts: First-pass radionuclide angiocardiography. Am Heart J. 1984;107:726–732. doi: 10.1016/0002-8703(84)90321-1. [DOI] [PubMed] [Google Scholar]
- 8.Rominger MB, Bachmann GF, Pabst W, Rau WS. Right ventricular volumes and ejection fraction with fast cine mr imaging in breath-hold technique: Applicability, normal values from 52 volunteers, and evaluation of 325 adult cardiac patients. J Magn Reson Imaging. 1999;10:908–918. doi: 10.1002/(sici)1522-2586(199912)10:6<908::aid-jmri2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 9.Hoeper MM, Tongers J, Leppert A, Baus S, Maier R, Lotz J. Evaluation of right ventricular performance with a right ventricular ejection fraction thermodilution catheter and mri in patients with pulmonary hypertension. Chest. 2001;120:502–507. doi: 10.1378/chest.120.2.502. [DOI] [PubMed] [Google Scholar]
- 10.Kawut SM, Lima JA, Barr RG, Chahal H, Jain A, Tandri H, Praestgaard A, Bagiella E, Kizer JR, Johnson WC, Kronmal RA, Bluemke DA. Sex and race differences in right ventricular structure and function: The multi-ethnic study of atherosclerosis-right ventricle study. Circulation. 2011;123:2542–2551. doi: 10.1161/CIRCULATIONAHA.110.985515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawatani S, Mandell G, Kusaba E, Schraut W, Cascade P, Wajszczuk WJ, Kantrowitz A. Ventricular performance following ablation and prosthetic replacement of right ventricular myocardium. Trans Am Soc Artif Intern Organs. 1974;20B:629–636. [PubMed] [Google Scholar]
- 12.Driscoll DJ, Offord KP, Feldt RH, Schaff HV, Puga FJ, Danielson GK. Five- to fifteen-year follow-up after fontan operation. Circulation. 1992;85:469–496. doi: 10.1161/01.cir.85.2.469. [DOI] [PubMed] [Google Scholar]
- 13.Sztrymf B, Souza R, Bertoletti L, Jais X, Sitbon O, Price LC, Simonneau G, Humbert M. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J. 2009 doi: 10.1183/09031936.00070209. [DOI] [PubMed] [Google Scholar]
- 14.Brickner ME, Hillis LD, Lange RA. Congenital heart disease in adults. First of two parts. N Engl J Med. 2000;342:256–263. doi: 10.1056/NEJM200001273420407. [DOI] [PubMed] [Google Scholar]
- 15.Bogaard HJ, Natarajan R, Henderson SC, Long CS, Kraskauskas D, Smithson L, Ockaili R, McCord JM, Voelkel NF. Chronic pulmonary artery pressure elevation is insufficient to explain right heart failure. Circulation. 2009;120:1951–1960. doi: 10.1161/CIRCULATIONAHA.109.883843. [DOI] [PubMed] [Google Scholar]
- 16.Sztrymf B, Souza R, Bertoletti L, Jais X, Sitbon O, Price LC, Simonneau G, Humbert M. Prognostic factors of acute heart failure in patients with pulmonary arterial hypertension. Eur Respir J. 2010;35:1286–1293. doi: 10.1183/09031936.00070209. [DOI] [PubMed] [Google Scholar]
- 17.Campo A, Mathai SC, Le Pavec J, Zaiman AL, Hummers LK, Boyce D, Housten T, Lechtzin N, Chami H, Girgis RE, Hassoun PM. Outcomes of hospitalisation for right heart failure in pulmonary arterial hypertension. Eur Respir J. 2011;38:359–367. doi: 10.1183/09031936.00148310. [DOI] [PubMed] [Google Scholar]
- 18.Abraham WT, Adams KF, Fonarow GC, Costanzo MR, Berkowitz RL, LeJemtel TH, Cheng ML, Wynne J. In-hospital mortality in patients with acute decompensated heart failure requiring intravenous vasoactive medications: An analysis from the acute decompensated heart failure national registry (adhere) J Am Coll Cardiol. 2005;46:57–64. doi: 10.1016/j.jacc.2005.03.051. [DOI] [PubMed] [Google Scholar]
- 19.Voelkel NF, Quaife RA, Leinwand LA, Barst RJ, McGoon MD, Meldrum DR, Dupuis J, Long CS, Rubin LJ, Smart FW, Suzuki YJ, Gladwin M, Denholm EM, Gail DB. Right ventricular function and failure: Report of a national heart, lung, and blood institute working group on cellular and molecular mechanisms of right heart failure. Circulation. 2006;114:1883–1891. doi: 10.1161/CIRCULATIONAHA.106.632208. [DOI] [PubMed] [Google Scholar]
- 20.Piao L, Fang YH, Parikh KS, Ryan JJ, D’Souza KM, Theccanat T, Toth PT, Pogoriler J, Paul J, Blaxall BC, Akhter SA, Archer SL. Grk2-mediated inhibition of adrenergic and dopaminergic signaling in right ventricular hypertrophy: Therapeutic implications in pulmonary hypertension. Circulation. 2012;126:2859–2869. doi: 10.1161/CIRCULATIONAHA.112.109868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bristow MR, Minobe W, Rasmussen R, Larrabee P, Skerl L, Klein JW, Anderson FL, Murray J, Mestroni L, Karwande SV, et al. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. J Clin Invest. 1992;89:803–815. doi: 10.1172/JCI115659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rich S, Pogoriler J, Husain AN, Toth PT, Gomberg-Maitland M, Archer SL. Long-term effects of epoprostenol on the pulmonary vasculature in idiopathic pulmonary arterial hypertension. Chest. 2010;138:1234–1239. doi: 10.1378/chest.09-2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz A, Lee KS. Study of heart mitochondria and glycolytic metabolism in experimentally induced cardiac failure. Circ Res. 1962;10:321–332. doi: 10.1161/01.res.10.3.321. [DOI] [PubMed] [Google Scholar]
- 24.Sabri A, Samuel JL, Marotte F, Poitevin P, Rappaport L, Levy BI. Microvasculature in angiotensin ii-dependent cardiac hypertrophy in the rat. Hypertension. 1998;32:371–375. doi: 10.1161/01.hyp.32.2.371. [DOI] [PubMed] [Google Scholar]
- 25.Izumo S, Nadal-Ginard B, Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988;85:339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Piao L, Fang YH, Parikh KS, Ryan JJ, Toth PT, Archer SL. Cardiac glutaminolysis: A maladaptive cancer metabolism pathway in the right ventricle in pulmonary hypertension. J Mol Med (Berl) 2013 doi: 10.1007/s00109-013-1064-7. accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Simonneau G, Robbins IM, Beghetti M, Channick RN, Delcroix M, Denton CP, Elliott CG, Gaine SP, Gladwin MT, Jing ZC, Krowka MJ, Langleben D, Nakanishi N, Souza R. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol. 2009;54:S43–S54. doi: 10.1016/j.jacc.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 28.Guarracino F, Cariello C, Danella A, Doroni L, Lapolla F, Vullo C, Pasquini C, Stefani M. Right ventricular failure: Physiology and assessment. Minerva Anestesiol. 2005;71:307–312. [PubMed] [Google Scholar]
- 29.Ritchie M, Waggoner AD, Davila-Roman VG, Barzilai B, Trulock EP, Eisenberg PR. Echocardiographic characterization of the improvement in right ventricular function in patients with severe pulmonary hypertension after single-lung transplantation. J Am Coll Cardiol. 1993;22:1170–1174. doi: 10.1016/0735-1097(93)90433-2. [DOI] [PubMed] [Google Scholar]
- 30.Jamieson SW, Kapelanski DP, Sakakibara N, Manecke GR, Thistlethwaite PA, Kerr KM, Channick RN, Fedullo PF, Auger WR. Pulmonary endarterectomy: Experience and lessons learned in 1,500 cases. Ann Thorac Surg. 2003;76:1457–1462. doi: 10.1016/s0003-4975(03)00828-2. discussion 1462-1454. [DOI] [PubMed] [Google Scholar]
- 31.Hoeper MM, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. Am J Respir Crit Care Med. 2011;184:1114–1124. doi: 10.1164/rccm.201104-0662CI. [DOI] [PubMed] [Google Scholar]
- 32.van de Veerdonk MC, Kind T, Marcus JT, Mauritz GJ, Heymans MW, Bogaard HJ, Boonstra A, Marques KM, Westerhof N, Vonk-Noordegraaf A. Progressive right ventricular dysfunction in patients with pulmonary arterial hypertension responding to therapy. J Am Coll Cardiol. 2011;58:2511–2519. doi: 10.1016/j.jacc.2011.06.068. [DOI] [PubMed] [Google Scholar]
- 33.Girgis RE. Predicting long-term survival in pulmonary arterial hypertension: More than just pulmonary vascular resistance. J Am Coll Cardiol. 2011;58:2520–2521. doi: 10.1016/j.jacc.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 34.van Wolferen SA, Marcus JT, Boonstra A, Marques KM, Bronzwaer JG, Spreeuwenberg MD, Postmus PE, Vonk-Noordegraaf A. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J. 2007;28:1250–1257. doi: 10.1093/eurheartj/ehl477. [DOI] [PubMed] [Google Scholar]
- 35.Gaine S. Pulmonary hypertension. Jama. 2000;284:3160–3168. doi: 10.1001/jama.284.24.3160. [DOI] [PubMed] [Google Scholar]
- 36.Freed BH, Gomberg-Maitland M, Chandra S, Mor-Avi V, Rich S, Archer SL, Jamison EB, Jr, Lang RM, Patel AR. Late gadolinium enhancement cardiovascular magnetic resonance predicts clinical worsening in patients with pulmonary hypertension. J Cardiovasc Magn Reson. 2012;14:11. doi: 10.1186/1532-429X-14-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hopkins WE, Ochoa LL, Richardson GW, Trulock EP. Comparison of the hemodynamics and survival of adults with severe primary pulmonary hypertension or eisenmenger syndrome. J Heart Lung Transplant. 1996;15:100–105. [PubMed] [Google Scholar]
- 38.Kawut SM, Taichman DB, Archer-Chicko CL, Palevsky HI, Kimmel SE. Hemodynamics and survival in patients with pulmonary arterial hypertension related to systemic sclerosis. Chest. 2003;123:344–350. doi: 10.1378/chest.123.2.344. [DOI] [PubMed] [Google Scholar]
- 39.Kuhn KP, Byrne DW, Arbogast PG, Doyle TP, Loyd JE, Robbins IM. Outcome in 91 consecutive patients with pulmonary arterial hypertension receiving epoprostenol. Am J Respir Crit Care Med. 2003;167:580–586. doi: 10.1164/rccm.200204-333OC. [DOI] [PubMed] [Google Scholar]
- 40.Oosterhof T, Tulevski II, Vliegen HW, Spijkerboer AM, Mulder BJ. Effects of volume and/or pressure overload secondary to congenital heart disease (tetralogy of fallot or pulmonary stenosis) on right ventricular function using cardiovascular magnetic resonance and b-type natriuretic peptide levels. Am J Cardiol. 2006;97:1051–1055. doi: 10.1016/j.amjcard.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 41.Haddad F, Doyle R, Murphy DJ, Hunt SA. Right ventricular function in cardiovascular disease, part ii: Pathophysiology, clinical importance, and management of right ventricular failure. Circulation. 2008;117:1717–1731. doi: 10.1161/CIRCULATIONAHA.107.653584. [DOI] [PubMed] [Google Scholar]
- 42.Hopkins WE, Waggoner AD. Severe pulmonary hypertension without right ventricular failure: The unique hearts of patients with eisenmenger syndrome. Am J Cardiol. 2002;89:34–38. doi: 10.1016/s0002-9149(01)02159-2. [DOI] [PubMed] [Google Scholar]
- 43.Oka M, Homma N, Taraseviciene-Stewart L, Morris KG, Kraskauskas D, Burns N, Voelkel NF, McMurtry IF. Rho kinase-mediated vasoconstriction is important in severe occlusive pulmonary arterial hypertension in rats. Circulation research. 2007;100:923–929. doi: 10.1161/01.RES.0000261658.12024.18. [DOI] [PubMed] [Google Scholar]
- 44.Sutendra G, Dromparis P, Paulin R, Zervopoulos S, Haromy A, Nagendran J, Michelakis ED. A metabolic remodeling in right ventricular hypertrophy is associated with decreased angiogenesis and a transition from a compensated to a decompensated state in pulmonary hypertension. J Mol Med (Berl) 2013 doi: 10.1007/s00109-013-1059-4. [DOI] [PubMed] [Google Scholar]
- 45.Oikawa M, Kagaya Y, Otani H, Sakuma M, Demachi J, Suzuki J, Takahashi T, Nawata J, Ido T, Watanabe J, Shirato K. Increased [18f]fluorodeoxyglucose accumulation in right ventricular free wall in patients with pulmonary hypertension and the effect of epoprostenol. J Am Coll Cardiol. 2005;45:1849–1855. doi: 10.1016/j.jacc.2005.02.065. [DOI] [PubMed] [Google Scholar]
- 46.Bonnet S, Michelakis ED, Porter CJ, Andrade-Navarro MA, Thebaud B, Haromy A, Harry G, Moudgil R, McMurtry MS, Weir EK, Archer SL. An abnormal mitochondrial-hypoxia inducible factor-1alpha-kv channel pathway disrupts oxygen sensing and triggers pulmonary arterial hypertension in fawn hooded rats: Similarities to human pulmonary arterial hypertension. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- 47.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: A basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–2671. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Piao L, Sidhu VK, Fang YH, Ryan JJ, Parikh KS, Hong Z, Toth PT, Morrow E, Kutty S, Lopaschuk GD, Archer SL. Foxo1-mediated upregulation of pyruvate dehydrogenase kinase-4 (pdk4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: Therapeutic benefits of dichloroacetate. J Mol Med (Berl) 2013;91:333–346. doi: 10.1007/s00109-012-0982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kurzyna M, Zylkowska J, Fijalkowska A, Florczyk M, Wieteska M, Kacprzak A, Burakowski J, Szturmowicz M, Wawrzynska L, Torbicki A. Characteristics and prognosis of patients with decompensated right ventricular failure during the course of pulmonary hypertension. Kardiol Pol. 2008;66:1033–1039. discussion 1040-1031. [PubMed] [Google Scholar]
- 50.Piao L, Fang YH, Cadete VJ, Wietholt C, Urboniene D, Toth PT, Marsboom G, Zhang HJ, Haber I, Rehman J, Lopaschuk GD, Archer SL. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: Resuscitating the hibernating right ventricle. J Mol Med (Berl) 2010;88:47–60. doi: 10.1007/s00109-009-0524-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morimitsu T, Miyahara Y, Sinboku H, Ikeda S, Naito T, Nishijima K, Takao M. Iodine-123-metaiodobenzylguanidine myocardial imaging in patients with right ventricular pressure overload. J Nucl Med. 1996;37:1343–1346. [PubMed] [Google Scholar]
- 52.Drake JI, Bogaard HJ, Mizuno S, Clifton B, Xie B, Gao Y, Dumur CI, Fawcett P, Voelkel NF, Natarajan R. Molecular signature of a right heart failure program in chronic severe pulmonary hypertension. Am J Respir Cell Mol Biol. 2011;45:1239–1247. doi: 10.1165/rcmb.2010-0412OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomez A, Bialostozky D, Zajarias A, Santos E, Palomar A, Martinez ML, Sandoval J. Right ventricular ischemia in patients with primary pulmonary hypertension. J Am Coll Cardiol. 2001;38:1137–1142. doi: 10.1016/s0735-1097(01)01496-6. [DOI] [PubMed] [Google Scholar]
- 54.Lundgrin EL, Park MM, Sharp J, Tang WH, Thomas JD, Asosingh K, Comhair SA, DiFilippo FP, Neumann DR, Davis L, Graham BB, Tuder RM, Dostanic I, Erzurum SC. Fasting 2-deoxy-2-[18f]fluoro-d-glucose positron emission tomography to detect metabolic changes in pulmonary arterial hypertension hearts over 1 year. Ann Am Thorac Soc. 2013;10:1–9. doi: 10.1513/AnnalsATS.201206-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heresi GA, Tang WH, Aytekin M, Hammel J, Hazen SL, Dweik RA. Sensitive cardiac troponin i predicts poor outcomes in pulmonary arterial hypertension. Eur Respir J. 2012;39:939–944. doi: 10.1183/09031936.00067011. [DOI] [PubMed] [Google Scholar]
- 56.Millane T, Bernard EJ, Jaeggi E, Howman-Giles RB, Uren RF, Cartmill TB, Hawker RE, Celermajer DS. Role of ischemia and infarction in late right ventricular dysfunction after atrial repair of transposition of the great arteries. J Am Coll Cardiol. 2000;35:1661–1668. doi: 10.1016/s0735-1097(00)00585-4. [DOI] [PubMed] [Google Scholar]
- 57.Singh TP, Humes RA, Muzik O, Kottamasu S, Karpawich PP, Di Carli MF. Myocardial flow reserve in patients with a systemic right ventricle after atrial switch repair. J Am Coll Cardiol. 2001;37:2120–2125. doi: 10.1016/s0735-1097(01)01283-9. [DOI] [PubMed] [Google Scholar]
- 58.Hornung TS, Kilner PJ, Davlouros PA, Grothues F, Li W, Gatzoulis MA. Excessive right ventricular hypertrophic response in adults with the mustard procedure for transposition of the great arteries. Am J Cardiol. 2002;90:800–803. doi: 10.1016/s0002-9149(02)02619-x. [DOI] [PubMed] [Google Scholar]
- 59.Vogel-Claussen J, Skrok J, Shehata ML, Singh S, Sibley CT, Boyce DM, Lechtzin N, Girgis RE, Mathai SC, Goldstein TA, Zheng J, Lima JA, Bluemke DA, Hassoun PM. Right and left ventricular myocardial perfusion reserves correlate with right ventricular function and pulmonary hemodynamics in patients with pulmonary arterial hypertension. Radiology. 2011;258:119–127. doi: 10.1148/radiol.10100725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Wolferen SA, Marcus JT, Westerhof N, Spreeuwenberg MD, Marques KM, Bronzwaer JG, Henkens IR, Gan CT, Boonstra A, Postmus PE, Vonk-Noordegraaf A. Right coronary artery flow impairment in patients with pulmonary hypertension. Eur Heart J. 2008;29:120–127. doi: 10.1093/eurheartj/ehm567. [DOI] [PubMed] [Google Scholar]
- 61.Vlahakes GJ, Baer RW, Uhlig PN, Verrier ED, Bristow JD, Hoffmann JI. Adrenergic influence in the coronary circulation of conscious dogs during maximal vasodilation with adenosine. Circ Res. 1982;51:371–384. doi: 10.1161/01.res.51.3.371. [DOI] [PubMed] [Google Scholar]
- 62.Bian X, Williams AG, Jr, Gwirtz PA, Downey HF. Right coronary autoregulation in conscious, chronically instrumented dogs. Am J Physiol. 1998;275:H169–H175. doi: 10.1152/ajpheart.1998.275.1.H169. [DOI] [PubMed] [Google Scholar]
- 63.Bogaard HJ, Natarajan R, Mizuno S, Abbate A, Chang PJ, Chau VQ, Hoke NN, Kraskauskas D, Kasper M, Salloum FN, Voelkel NF. Adrenergic receptor blockade reverses right heart remodeling and dysfunction in pulmonary hypertensive rats. Am J Respir Crit Care Med. 2010;182:652–660. doi: 10.1164/rccm.201003-0335OC. [DOI] [PubMed] [Google Scholar]
- 64.Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: A strong hypothesis. Heart Fail Rev. 2007;12:331–343. doi: 10.1007/s10741-007-9034-1. [DOI] [PubMed] [Google Scholar]
- 65.Neely JR, Morgan HE. Relationship between carbohydrate and lipid metabolism and the energy balance of heart muscle. Annu Rev Physiol. 1974;36:413–459. doi: 10.1146/annurev.ph.36.030174.002213. [DOI] [PubMed] [Google Scholar]
- 66.Marsboom G, Wietholt C, Haney CR, Toth PT, Ryan JJ, Morrow E, Thenappan T, Bache-Wiig P, Piao L, Paul J, Chen CT, Archer SL. Lung (1)(8)f-fluorodeoxyglucose positron emission tomography for diagnosis and monitoring of pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:670–679. doi: 10.1164/rccm.201108-1562OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res. 1997;33:243–257. doi: 10.1016/s0008-6363(96)00245-3. [DOI] [PubMed] [Google Scholar]
- 68.Sugden MC, Langdown ML, Harris RA, Holness MJ. Expression and regulation of pyruvate dehydrogenase kinase isoforms in the developing rat heart and in adulthood: Role of thyroid hormone status and lipid supply. Biochem J. 2000;352(Pt 3):731–738. [PMC free article] [PubMed] [Google Scholar]
- 69.Michelakis ED, McMurtry MS, Wu XC, Dyck JR, Moudgil R, Hopkins TA, Lopaschuk GD, Puttagunta L, Waite R, Archer SL. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats: Role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- 70.Nagendran J, Gurtu V, Fu DZ, Dyck JR, Haromy A, Ross DB, Rebeyka IM, Michelakis ED. A dynamic and chamber-specific mitochondrial remodeling in right ventricular hypertrophy can be therapeutically targeted. J Thorac Cardiovasc Surg. 2008;136:168–178. doi: 10.1016/j.jtcvs.2008.01.040. 178 e161-163. [DOI] [PubMed] [Google Scholar]
- 71.Hagan G, Southwood M, Treacy C, Ross RM, Soon E, Coulson J, Sheares K, Screaton N, Pepke-Zaba J, Morrell NW, Rudd JH. (18)fdg pet imaging can quantify increased cellular metabolism in pulmonary arterial hypertension: A proof-of-principle study. Pulm Circ. 2011;1:448–455. doi: 10.4103/2045-8932.93543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stacpoole PW, Kerr DS, Barnes C, Bunch ST, Carney PR, Fennell EM, Felitsyn NM, Gilmore RL, Greer M, Henderson GN, Hutson AD, Neiberger RE, O’Brien RG, Perkins LA, Quisling RG, Shroads AL, Shuster JJ, Silverstein JH, Theriaque DW, Valenstein E. Controlled clinical trial of dichloroacetate for treatment of congenital lactic acidosis in children. Pediatrics. 2006;117:1519–1531. doi: 10.1542/peds.2005-1226. [DOI] [PubMed] [Google Scholar]
- 73.Michelakis ED, Sutendra G, Dromparis P, Webster L, Haromy A, Niven E, Maguire C, Gammer TL, Mackey JR, Fulton D, Abdulkarim B, McMurtry MS, Petruk KC. Metabolic modulation of glioblastoma with dichloroacetate. Science translational medicine. 2010;2 doi: 10.1126/scitranslmed.3000677. 31ra34. [DOI] [PubMed] [Google Scholar]
- 74.ClinicalTrials.gov. Dichloroacetate (dca) for the treatment of pulmonary arterial hypertension. NCT01083524. http://clinicaltrials.gov/ct2/show/NCT01083524.
- 75.Fang YH, Piao L, Hong Z, Toth PT, Marsboom G, Bache-Wiig P, Rehman J, Archer SL. Therapeutic inhibition of fatty acid oxidation in right ventricular hypertrophy: Exploiting randle’s cycle. J Mol Med (Berl) 2012;90:31–43. doi: 10.1007/s00109-011-0804-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rich JD, Thenappan T, Freed B, Patel AR, Thisted RA, Childers R, Archer SL. Qtc prolongation is associated with impaired right ventricular function and predicts mortality in pulmonary hypertension. Int J Cardiol. 2012 doi: 10.1016/j.ijcard.2012.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- 78.Abozguia K, Clarke K, Lee L, Frenneaux M. Modification of myocardial substrate use as a therapy for heart failure. Nat Clin Pract Cardiovasc Med. 2006;3:490–498. doi: 10.1038/ncpcardio0583. [DOI] [PubMed] [Google Scholar]
- 79.Grabczewska Z, Bialoszynski T, Szymanski P, Sukiennik A, Swiatkiewicz I, Kozinski M, Kochman W, Grzesk G, Kubica J. The effect of trimetazidine added to maximal anti-ischemic therapy in patients with advanced coronary artery disease. Cardiol J. 2008;15:344–350. [PubMed] [Google Scholar]
- 80.Gunes Y, Guntekin U, Tuncer M, Sahin M. Improved left and right ventricular functions with trimetazidine in patients with heart failure: A tissue doppler study. Heart Vessels. 2009;24:277–282. doi: 10.1007/s00380-008-1118-x. [DOI] [PubMed] [Google Scholar]
- 81.Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme a thiolase. Circ Res. 2000;86:580–588. doi: 10.1161/01.res.86.5.580. [DOI] [PubMed] [Google Scholar]
- 82.Rosano GM, Vitale C, Sposato B, Mercuro G, Fini M. Trimetazidine improves left ventricular function in diabetic patients with coronary artery disease: A double-blind placebo-controlled study. Cardiovasc Diabetol. 2003;2:16. doi: 10.1186/1475-2840-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fragasso G, Spoladore R, Cuko A, Palloshi A. Modulation of fatty acids oxidation in heart failure by selective pharmacological inhibition of 3-ketoacyl coenzyme-a thiolase. Curr Clin Pharmacol. 2007;2:190–196. doi: 10.2174/157488407781668776. [DOI] [PubMed] [Google Scholar]
- 84.Stanley WC. Partial fatty acid oxidation inhibitors for stable angina. Expert Opin Investig Drugs. 2002;11:615–629. doi: 10.1517/13543784.11.5.615. [DOI] [PubMed] [Google Scholar]
- 85.Wang P, Fraser H, Lloyd SG, McVeigh JJ, Belardinelli L, Chatham JC. A comparison between ranolazine and cvt-4325, a novel inhibitor of fatty acid oxidation, on cardiac metabolism and left ventricular function in rat isolated perfused heart during ischemia and reperfusion. J Pharmacol Exp Ther. 2007;321:213–220. doi: 10.1124/jpet.106.115519. [DOI] [PubMed] [Google Scholar]
- 86.Clarke B, Wyatt KM, McCormack JG. Ranolazine increases active pyruvate dehydrogenase in perfused normoxic rat hearts: Evidence for an indirect mechanism. J Mol Cell Cardiol. 1996;28:341–350. doi: 10.1006/jmcc.1996.0032. [DOI] [PubMed] [Google Scholar]
- 87.McCormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation. 1996;93:135–142. doi: 10.1161/01.cir.93.1.135. [DOI] [PubMed] [Google Scholar]
- 88.Samudio I, Harmancey R, Fiegl M, Kantarjian H, Konopleva M, Korchin B, Kaluarachchi K, Bornmann W, Duvvuri S, Taegtmeyer H, Andreeff M. Pharmacologic inhibition of fatty acid oxidation sensitizes human leukemia cells to apoptosis induction. J Clin Invest. 2010;120:142–156. doi: 10.1172/JCI38942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Guarnieri C, Muscari C. Beneficial effects of trimetazidine on mitochondrial function and superoxide production in the cardiac muscle. Cardiovasc Drugs Ther. 1990;4(Suppl 4):814–815. doi: 10.1007/BF00051282. [DOI] [PubMed] [Google Scholar]
- 90.Guarnieri C, Muscari C. Beneficial effects of trimetazidine on mitochondrial function and superoxide production in the cardiac muscle of monocrotaline-treated rats. Biochem Pharmacol. 1988;37:4685–4688. doi: 10.1016/0006-2952(88)90338-3. [DOI] [PubMed] [Google Scholar]
- 91.Tuunanen H, Engblom E, Naum A, Nagren K, Scheinin M, Hesse B, Juhani Airaksinen KE, Nuutila P, Iozzo P, Ukkonen H, Opie LH, Knuuti J. Trimetazidine, a metabolic modulator, has cardiac and extracardiac benefits in idiopathic dilated cardiomyopathy. Circulation. 2008;118:1250–1258. doi: 10.1161/CIRCULATIONAHA.108.778019. [DOI] [PubMed] [Google Scholar]
- 92.Dang CV. Glutaminolysis: Supplying carbon or nitrogen or both for cancer cells? Cell Cycle. 2010;9:3884–3886. doi: 10.4161/cc.9.19.13302. [DOI] [PubMed] [Google Scholar]
- 93.Nootens M, Kaufmann E, Rector T, Toher C, Judd D, Francis GS, Rich S. Neurohormonal activation in patients with right ventricular failure from pulmonary hypertension: Relation to hemodynamic variables and endothelin levels. J Am Coll Cardiol. 1995;26:1581–1585. doi: 10.1016/0735-1097(95)00399-1. [DOI] [PubMed] [Google Scholar]
- 94.Brown L, Miller J, Dagger A, Sernia C. Cardiac and vascular responses after monocrotaline-induced hypertrophy in rats. Journal of cardiovascular pharmacology. 1998;31:108–115. doi: 10.1097/00005344-199801000-00016. [DOI] [PubMed] [Google Scholar]
- 95.Usui S, Yao A, Hatano M, Kohmoto O, Takahashi T, Nagai R, Kinugawa K. Upregulated neurohumoral factors are associated with left ventricular remodeling and poor prognosis in rats with monocrotaline-induced pulmonary arterial hypertension. Circulation journal : official journal of the Japanese Circulation Society. 2006;70:1208–1215. doi: 10.1253/circj.70.1208. [DOI] [PubMed] [Google Scholar]
- 96.Fan TH, Liang CS, Kawashima S, Banerjee SP. Alterations in cardiac beta-adrenoceptor responsiveness and adenylate cyclase system by congestive heart failure in dogs. European journal of pharmacology. 1987;140:123–132. doi: 10.1016/0014-2999(87)90798-9. [DOI] [PubMed] [Google Scholar]
- 97.Bristow MR, Minobe W, Rasmussen R, Larrabee P, Skerl L, Klein JW, Anderson FL, Murray J, Mestroni L, Karwande SV, Fowler M, Ginsburg R. Beta-adrenergic neuroeffector abnormalities in the failing human heart are produced by local rather than systemic mechanisms. Journal of Clinical Investigation. 1992;89:803–815. doi: 10.1172/JCI115659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ishikawa S, Honda M, Yamada S, Morioka S, Moriyama K. Biventricular down-regulation of beta-adrenergic receptors in right ventricular hypertrophy induced by monocrotaline. Japanese Circulation Journal-English Edition. 1991;55:1077–1085. doi: 10.1253/jcj.55.1077. [DOI] [PubMed] [Google Scholar]
- 99.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol european trial (comet): Randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 100.Tual L, Morel OE, Favret F, Fouillit M, Guernier C, Buvry A, Germain L, Dhonneur G, Bernaudin JF, Richalet JP. Carvedilol inhibits right ventricular hypertrophy induced by chronic hypobaric hypoxia. Pflugers Arch. 2006;452:371–379. doi: 10.1007/s00424-006-0058-5. [DOI] [PubMed] [Google Scholar]
- 101.Quaife RA, Christian PE, Gilbert EM, Datz FL, Volkman K, Bristow MR. Effects of carvedilol on right ventricular function in chronic heart failure. Am J Cardiol. 1998;81:247–250. doi: 10.1016/s0002-9149(97)00874-6. [DOI] [PubMed] [Google Scholar]
- 102. Http://clinicaltrials.Gov/ct2/show/nct01246037.
- 103.Chugh SS, Whitesel S, Turner M, Roberts CT, Jr, Nagalla SR. Genetic basis for chamber-specific ventricular phenotypes in the rat infarct model. Cardiovasc Res. 2003;57:477–485. doi: 10.1016/s0008-6363(02)00703-4. [DOI] [PubMed] [Google Scholar]
- 104.Nagendran J, Archer SL, Soliman D, Gurtu V, Moudgil R, Haromy A, St Aubin C, Webster L, Rebeyka IM, Ross DB, Light PE, Dyck JR, Michelakis ED. Phosphodiesterase type 5 is highly expressed in the hypertrophied human right ventricle, and acute inhibition of phosphodiesterase type 5 improves contractility. Circulation. 2007;116:238–248. doi: 10.1161/CIRCULATIONAHA.106.655266. [DOI] [PubMed] [Google Scholar]
- 105.Archer SL, Michelakis ED. Phosphodiesterase type 5 inhibitors for pulmonary arterial hypertension. The New England journal of medicine. 2009;361:1864–1871. doi: 10.1056/NEJMct0904473. [DOI] [PubMed] [Google Scholar]
- 106.Nagendran J, Sutendra G, Paterson I, Champion HC, Webster L, Chiu B, Haromy A, Rebeyka IM, Ross DB, Michelakis ED. Endothelin axis is upregulated in human and rat right ventricular hypertrophy. Circ Res. 2013;112:347–354. doi: 10.1161/CIRCRESAHA.111.300448. [DOI] [PubMed] [Google Scholar]
- 107.Packer M, McMurray J, Massie BM, Caspi A, Charlon V, Cohen-Solal A, Kiowski W, Kostuk W, Krum H, Levine B, Rizzon P, Soler J, Swedberg K, Anderson S, Demets DL. Clinical effects of endothelin receptor antagonism with bosentan in patients with severe chronic heart failure: Results of a pilot study. J Card Fail. 2005;11:12–20. doi: 10.1016/j.cardfail.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 108.Rubin LJ, Badesch DB, Barst RJ, Galie N, Black CM, Keogh A, Pulido T, Frost A, Roux S, Leconte I, Landzberg M, Simonneau G. Bosentan therapy for pulmonary arterial hypertension. N Engl J Med. 2002;346:896–903. doi: 10.1056/NEJMoa012212. [DOI] [PubMed] [Google Scholar]
- 109.Sitbon O, Badesch DB, Channick RN, Frost A, Robbins IM, Simonneau G, Tapson VF, Rubin LJ. Effects of the dual endothelin receptor antagonist bosentan in patients with pulmonary arterial hypertension: A 1-year follow-up study. Chest. 2003;124:247–254. doi: 10.1378/chest.124.1.247. [DOI] [PubMed] [Google Scholar]
- 110.Archer SL. Riociguat for pulmonary hypertension--a glass half full. N Engl J Med. 2013;369:386–388. doi: 10.1056/NEJMe1306684. [DOI] [PubMed] [Google Scholar]
- 111.Shehata ML, Lossnitzer D, Skrok J, Boyce D, Lechtzin N, Mathai SC, Girgis RE, Osman N, Lima JA, Bluemke DA, Hassoun PM, Vogel-Claussen J. Myocardial delayed enhancement in pulmonary hypertension: Pulmonary hemodynamics, right ventricular function, and remodeling. AJR Am J Roentgenol. 2011;196:87–94. doi: 10.2214/AJR.09.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Beggah AT, Escoubet B, Puttini S, Cailmail S, Delage V, Ouvrard-Pascaud A, Bocchi B, Peuchmaur M, Delcayre C, Farman N, Jaisser F. Reversible cardiac fibrosis and heart failure induced by conditional expression of an antisense mrna of the mineralocorticoid receptor in cardiomyocytes. Proc Natl Acad Sci U S A. 2002;99:7160–7165. doi: 10.1073/pnas.102673599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, Marcotte F, Mercier LA. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: A multicenter, randomized, placebo-controlled clinical trial. Circulation. 2005;112:2411–2416. doi: 10.1161/CIRCULATIONAHA.105.543470. [DOI] [PubMed] [Google Scholar]
- 114.Maron BA, Zhang YY, White K, Chan SY, Handy DE, Mahoney CE, Loscalzo J, Leopold JA. Aldosterone inactivates the endothelin-b receptor via a cysteinyl thiol redox switch to decrease pulmonary endothelial nitric oxide levels and modulate pulmonary arterial hypertension. Circulation. 2012;126:963–974. doi: 10.1161/CIRCULATIONAHA.112.094722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Grossman W. Pressure measurement. In: Grossman W, Baim DS, editors. Cardiac Catheterization, Angiography, and Intervention. 7th ed. Philadelphia: Lea & Febiger; 2006. pp. 13–35. [Google Scholar]
- 116.Barst RJ, Rubin LJ, Long WA, McGoon MD, Rich S, Badesch DB, Groves BM, Tapson VF, Bourge RC, Brundage BH, Koerner SK, Langleben D, Keller CA, Murali S, Uretsky BF, Clayton LM, Jobsis MM, Blackburn SD, Shortino D, Crow JW. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. N Engl J Med. 1996;334:296–301. doi: 10.1056/NEJM199602013340504. [DOI] [PubMed] [Google Scholar]
- 117.Ryan JJ, Marsboom G, Fang YH, Toth PT, Morrow E, Luo N, Piao L, Hong Z, Ericson K, Zhang HJ, Han M, Haney CR, Chen CT, Sharp WW, Archer SL. Pgc1alpha-mediated mitofusin-2 deficiency in female rats and humans with pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;187:865–878. doi: 10.1164/rccm.201209-1687OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.