Abstract
Brain abscess is an unusual complication of uncontrolled diabetes. A solitary thalamic abscess is an uncommon type of brain abscess. We report a case of thalamic abscess, whereupon diabetes mellitus and periodontitis were diagnosed. The diagnosis and management of thalamic abscess, and the interplay of type 2 diabetes and periodontitis are discussed. A 56-year-old, Caucasian, man with no medical or travel history, presented with 5-day symptoms of meningeal irritation. Body mass index 30.6 kg/m2. CT demonstrated a solitary midline lesion with neoplasia as a differential diagnosis. It was biopsied and cultures grew Streptococcus milleri. He was treated by stereotactic puncture, external drainage and targeted intrathecal and systemic antibiotic therapy. HIV negative but glycated haemoglobin (HbA1c) 10.7% (93 mmol/mol). Dental examination revealed a small molar abscess. Radiological resolution of the thalamic abscess occurred within 2 months. Diabetes improved with 7 weeks of insulin, and maintained on metformin, HbA1c 6.9% (51 mmol/mol). There was no residual neurological disability.
Background
A thalamic abscess is a rare infection that necessitates urgent management as it can lead to death or permanent neurological damage. Features on imaging may be non-specific and a high index of suspicion is required to avoid diagnostic errors and inappropriate and/or delayed treatment.
We describe the case of a 56-year-old man presenting with a thalamic abscess developing on the background of previously undiagnosed diabetes mellitus and silent, chronic, dental infection. New guidelines as to the screening and management of periodontal disease in diabetes are discussed, with relevance to this case.
Case presentation
A 56-year-old Caucasian man presented with a 5-day history of persistent, frontal headache radiating to the occiput, malaise and fever. There was no history of weight loss, nausea or vomiting. He was previously fit and well, with no recent infection, trauma or surgery. He took no regular medications.
On presentation he was drowsy but rousable, with Glasgow Coma Scale score of 14/15. Body mass index 30.6 kg/m2. He was haemodynamically stable and non-feverish. Cardiorespiratory, cranial and peripheral nervous system examinations were normal and there was no nuchal rigidity or papilloedema. White cell count was 8.9×109/L (4.0–11.0×109/L) with 79% neutrophil predominance, C reactive protein 22.4 mg/L (<5 mg/L), calcium 2.3 mmol/L (2.15–2.60 mmol/L), creatinine 74 μmol/L (45–120 μmol/L), urea 4.3 mmol/l (3.3–6.7 mmol/L) and liver function tests were normal.
Contrast CT demonstrated a solitary 28 mm low-density area centred on the right basal ganglia with surrounding oedema and 4 mm midline shift (figure 1). MRI with gadolinium contrast subsequently showed a ring-enhancing 30 mm mass in the right thalamus (figures 2 and 3). The imaging findings were suggestive of a brain neoplasm and the patient was counselled about the likely diagnosis of primary brain tumour and oncology follow-up was organised. He received two doses of dexamethasone 8 mg but this was not continued as a lymphoma secondary to immunosuppression was being considered.
Figure 1.
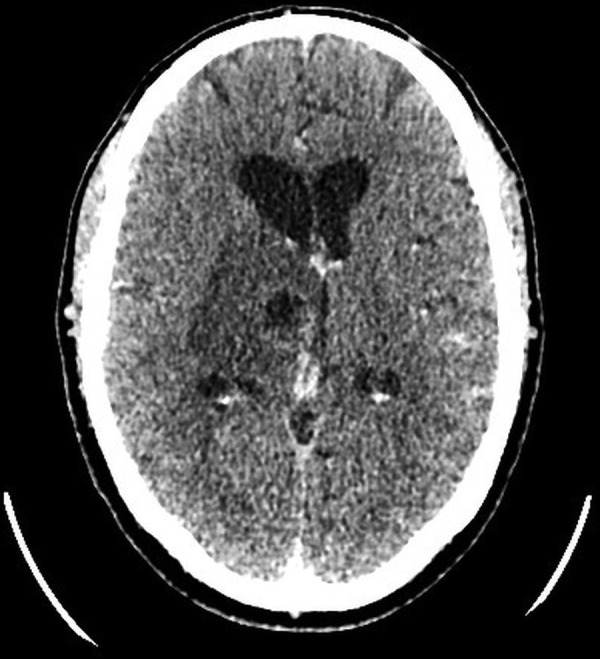
Postcontrast axial CT showing a 28 mm low-density lesion within the right thalamus. There is surrounding oedema and 4 mm midline shift with distortion of the third ventricle.
Figure 2.

Coronal T1-weighted MRI showing a 3 cm ring-enhancing lesion in the right thalamus with a surrounding mass effect.
Figure 3.
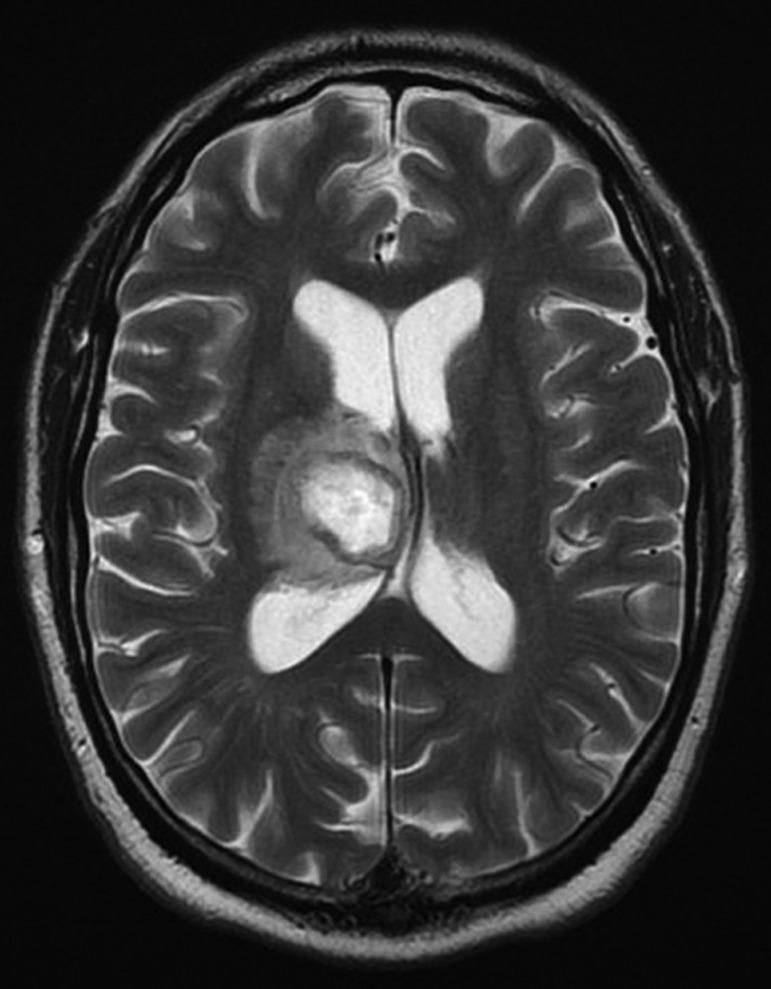
Axial T2-weighted MRI showing the right-sided thalamic ring-enhancing lesion and midline shift.
He represented 5 days later feeling unsteady on his feet, feverish and with new, abrupt, jerky, involuntary movements of the left upper limb. There was no history of loss of consciousness. Glasgow Coma Scale score was 15/15. Left-sided past-pointing and intention tremor were evident. Muscle power was not affected. Cardiac examination was normal (no cardiac murmurs evident). Repeat CT imaging showed an increase in the size of the right-sided thalamic space-occupying lesion with compression of Monroe’s foramen, hydrocephalus (figure 4) plus a small left-sided cerebellar infarct (not shown).
Figure 4.
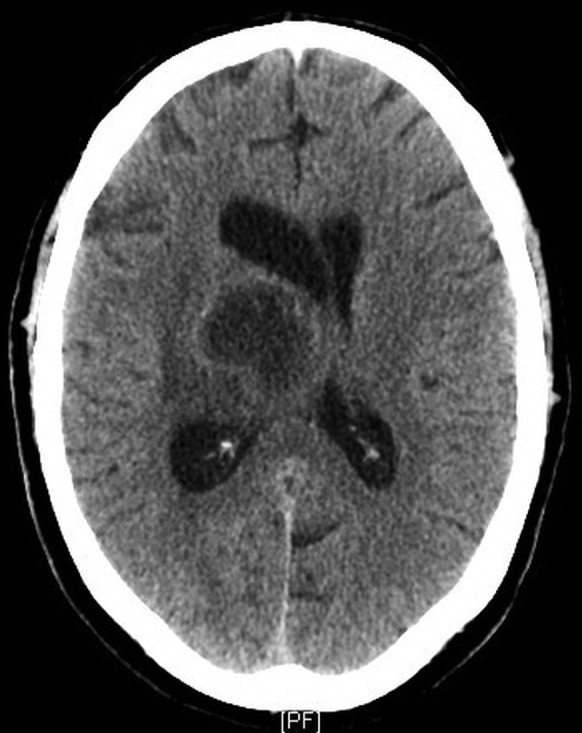
Postcontrast axial CT showing an increase in size and mass effect of the right thalamic lesion and further dilation of the right lateral ventricle.
Treatment
The presence of fever and the progression of neurological and imaging findings led to a revision of his earlier diagnosis of cerebral neoplasm to that of a thalamic abscess. This diagnosis was confirmed by an urgent neuronavigation-guided aspiration that obtained 20 mL of dark brown pus. A right frontal Ommaya reservoir was inserted in order to deliver intraventricular antibiotics and for the management of obstructive hydrocephalus. At surgery, it was evident that the abscess was complicated by intraventricular rupture with spread of pus to the cerebrospinal fluid (CSF). A CT-guided redo-aspiration was necessary 7 days later due to pus re-accumulation in the abscess cavity. Initial antimicrobial therapy was with ceftriaxone, metronidazole and rifampicin. After 48 h, pus culture isolated Streptococcus milleri. CSF and blood cultures (×4) were negative and there was no growth of acid-fast bacilli. The cerebellar infarct was considered to be a septic embolus. Bacterial endocarditis was considered unlikely in view of negative blood cultures and lack of a cardiac murmur or peripheral stigmata of endocarditis. A transthoracic ECG was also normal. His antibiotic regime was continued during the 4 weeks of his hospitalisation and was augmented by intraventricular vancomycin for 3 weeks. Intravenous ceftriaxone was continued in the community for an additional 3 weeks via a peripherally inserted central catheter (PICC).
Investigation for predisposing factors did not reveal any chest, sinusitic or otogenic infection. HIV serology was negative. Random glucose was normal (in the context of illness-induced anorexia), but a new diagnosis of diabetes mellitus was made based on a glycated haemoglobin (HbA1c) of 10.7% (93 mmol/mol). To achieve rapid glucose control and thereby improve recovery from sepsis, he started basal-bolus insulin treatment. Although there was no history of dental disease or symptoms, a dental consultation was made. From this, radiography showed a small abscess related to the upper-left last standing molar with concomitant apical radiolucency and decreased alveolar bone height. Dental extraction was arranged 3 months later, following full neurosurgical recovery. No material suitable for culture was available at the time of dental extraction.
Outcome and follow-up
The patient was discharged after 1 month without any residual neurological deficit. After 7 weeks of treatment with insulin his HbA1c had improved to 6.9% (51 mmol/mol). Insulin treatment was then stopped and replaced by metformin. Complete radiological resolution of the thalamic abscess was evident 2 months after hospital discharge (figure 5).
Figure 5.
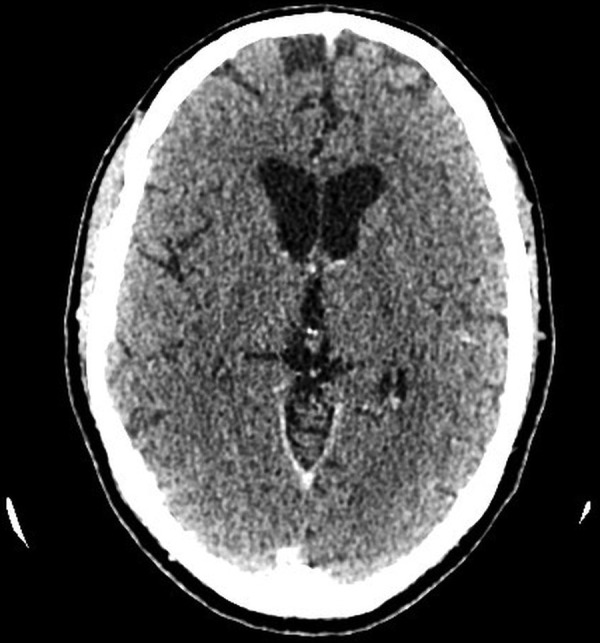
Axial CT postcontrast after 2 months showing complete resolution.
Discussion
A bacterial brain abscess is an uncommon life-threatening intracranial infection with an incidence of 0.3–1.3/100 000 persons per year.1 Thalamic abscess accounts for 2.2–4.5% of all reported brain abscesses.2 3
The mortality rate from a thalamic abscess is 9–14%,2 4 rising to 80% with the occurrence of intraventricular rupture.3 Survivors are at risk of permanent neurological damage: in published series, totalling 24 cases, 5 cases of minimal or moderate neurological damage and 1 case of severe disability were reported.3 4
The diagnosis of a thalamic abscess is challenging, even with the contribution of neuroimaging. The typical ring-enhancing lesion cannot always be differentiated on CT or MRI from other lesions of the area such as cystic glial or metastatic tumours, fungal, nocardial or tuberculous abscess, neurocysticercosis, toxoplasmosis, resolving haematoma, infarct, lymphoma or radionecrosis.5 Diagnostic errors have been reported leading to inappropriate and delayed treatment. Often a tissue diagnosis is necessary,3 4 although the use of diffusion-weighted MRI (DWI) may add to the diagnostic potential of imaging.6 Given radiological uncertainty, the diagnosis of thalamic abscess is often made by the presence of predisposing conditions, which include: metastatic infection—secondary to congenital heart disease or chest sepsis: local infection arising from skull bone, ear, tooth or sinuses: diabetes mellitus or other causes for immunosuppression.2–4 7
Among the various microorganisms involved, the S. milleri group is a recognised cause of brain abscess,8 particularly so in the presence of diabetes mellitus.9 10
Treatment options are (1) access to the abscess cavity with evacuation of the pus and installation of intracavitary antibiotics, (2) craniotomy with excision of the abscess or (3) non-surgical management with systemic antibiotics.2 Techniques used to access the abscess cavity include: aspiration guided by stereotaxy or neuronavigation, with or without continuous drainage; repeated aspiration; freehand aspiration through a burr hole; stereo-endoscopic aspiration; ultrasound-guided aspiration.
Although contentious, type 2 diabetes and periodontal disease have been proposed as inter-related chronic diseases. Both are of high prevalence in the population and so it cannot be proven whether they were directly responsible for the thalamic abscess in this case, or were an epiphenomenon. Diabetes mellitus may predispose to dental and periodontal infection, while severe periodontitis is thought to adversely affect glycaemic control and to be an independent risk factor for the development of diabetes.11 12 There is also some evidence of a direct relationship between periodontitis severity and diabetes complications, including macroalbuminuria, end-stage renal disease and cardiorenal mortality.11 12
A meta-analysis of nine studies comprising 775 patients, published in 2013, indicated a −0.36% reduction in HbA1c after periodontal therapy compared to no treatment.13
The inter-relationship between periodontal inflammation, glycaemic status and the complications of diabetes has been debated over the past 15–20 years. Guidelines for the management of diabetes with periodontitis were published more recently: in 2009 (International Diabetes Federation/World Dental Federation) and in 2013 (European Federation of Periodontology/American Academy of Periodontology).11 12 These guidelines promote the importance of educating patients with diabetes of the need for oral healthcare. Medical and dental health professionals are also advised to screen the diabetic population (or patients at risk for diabetes) for periodontal disease, promote regular dental examination and promptly treat periodontal disease. However, there remains a requirement for well-powered prospective trials to clearly determine the relevance of periodontal treatment on metabolic control.
Learning points.
Thalamic abscess is rare and may share clinical and radiological features of a brain neoplasm.
Diabetes mellitus is a risk factor for thalamic abscess.
Periodontal disease often coexists with diabetes mellitus and may remain clinically silent.
Periodontal disease is an independent risk factor for brain abscess.
Footnotes
Contributors: IK wrote the manuscript. CC was the neurosurgeon involved in the case and reviewed the manuscript. MBW was the diabetologist involved in the case and reviewed the manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Beckham JD, Tyler KL. Neuro-intensive care of patients with acute CNS infections. Neurotherapeutics 2012;9:124–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutz TW, Landolt H, Wasner M, et al. Diagnosis and management of abscesses in the basal ganglia and thalamus: a survey. Acta Neurochir 1994;127:91–8 [DOI] [PubMed] [Google Scholar]
- 3.Callovini GM, Gammone V, Pertella G. First-line stereotactic treatment of thalamic abscesses: report of three cases and review of the literature. Cent Eur Neurosurg 2009;70:143–8 [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Hagiwara S, Umebara Y, et al. Solitary pyogenic thalamic abscess—two case reports. Neurol Med Chir 1993;33:630–3 [DOI] [PubMed] [Google Scholar]
- 5.Apuzzo MLJ, Chandrasoma P, Zelman V, et al. Computed tomographic guidance stereotaxis in the management of lesions of the third ventricular region. Neurosurgery 1984;15:502–8 [DOI] [PubMed] [Google Scholar]
- 6.Chang SC, Lai PH, Chen WL, et al. Diffusion-weighted MRI features of brain abscess and cystic or necrotic brain tumors: comparison with conventional MRI. Clin Imaging 2002;26:227–36 [DOI] [PubMed] [Google Scholar]
- 7.Yoritaka A, Sugira K, Hiramatu M, et al. A case of solitary thalamic abscess treated by sterotactic aspiration. No To Shinkei 1990;42:749–53 [PubMed] [Google Scholar]
- 8.Bert F, Bariou-Lancelin M, Lambert-Zechovsky N. Clinical significance of bacteremia involving the “Streptococcus milleri” group: 51 cases and review. Clin Infec Dis 1998;27:385–7 [DOI] [PubMed] [Google Scholar]
- 9.Skogberg K, Simonen H, Renkonen OV, et al. Beta-haemolytic group A, B, C and G streptococcal septicaemia: a clinical study. Scand J Infec Dis 1988;20:119–25 [DOI] [PubMed] [Google Scholar]
- 10.Salata RA, Lerner PI, Shlaes DM, et al. Infections due to Lancefield Group C streptococci. Medicine (Baltimore) 1989;68:225–39 [DOI] [PubMed] [Google Scholar]
- 11. Guideline on oral health for people with diabetes 2009. International Diabetes Federation. http://www.idf.org/webdata/docs/OralHealth_EN_RTP.pdf.
- 12.Chapple IL, Genco R; Working Group 2 of the Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the joint EFP/AAP workshop on periodontitis and systemic diseases. J Periodontol 2013;84(Suppl 4):S106–12 [DOI] [PubMed] [Google Scholar]
- 13.Engebretson S, Kocher T. Evidence that periodontal treatment improves diabetes outcomes: a systematic review and meta-analysis. J Clin Periodontal 2013;40(Suppl 14):S153–63 [DOI] [PubMed] [Google Scholar]


