Abstract
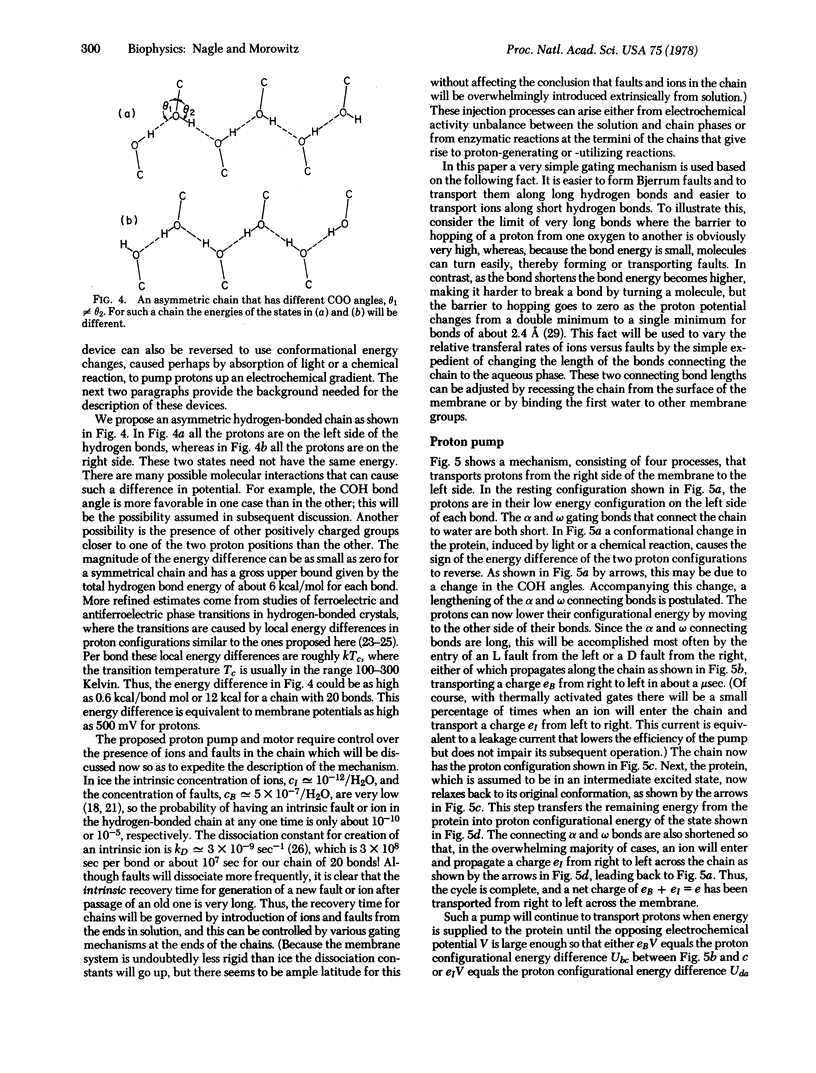
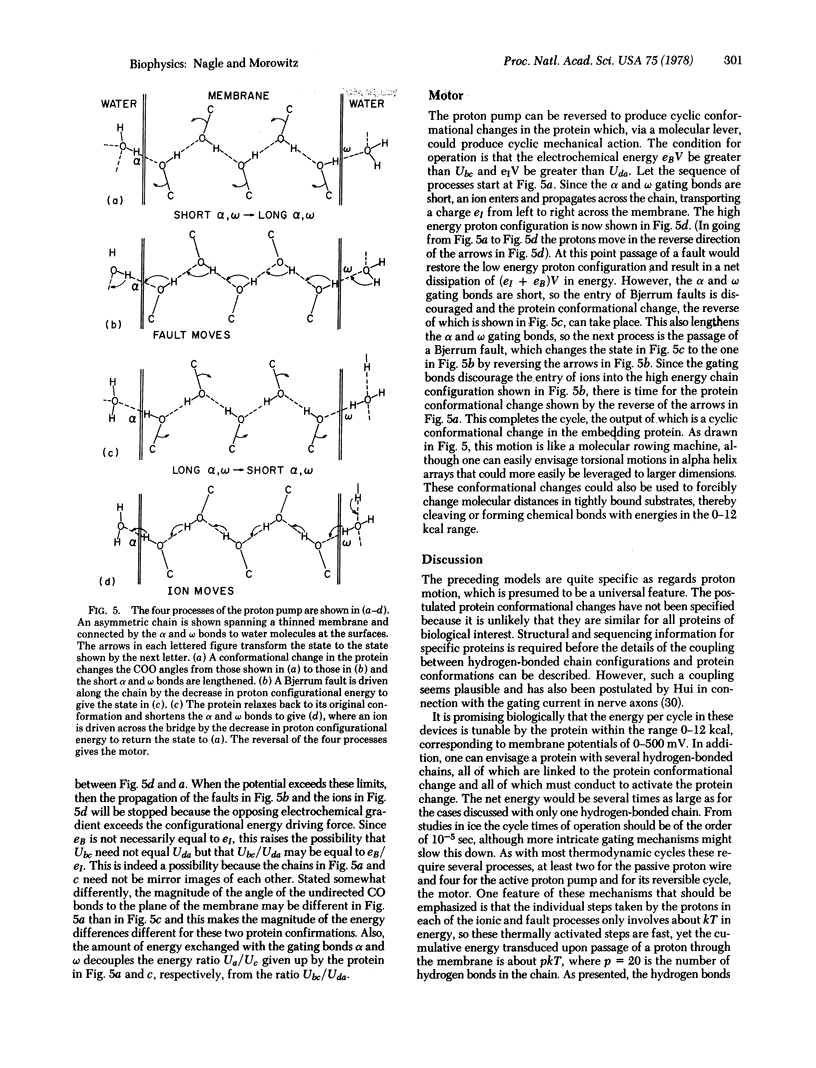
Likely mechanisms for proton transport through biomembranes are explored. The fundamental structural element is assumed to be continuous chains of hydrogen bonds formed from the protein side groups, and a molecular example is presented. From studies in ice, such chains are predicted to have low impedance and can function as proton wires. In addition, conformational changes in the protein may be linked to the proton conduction. If this possibility is allowed, a simple proton pump can be described that can be reversed into a molecular motor driven by an electrochemical potential across the membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Drachev L. A., Frolov V. N., Kaulen A. D., Liberman E. A., Ostroumov S. A., Plakunova V. G., Semenov A. Y., Skulachev V. P. Reconstitution of Biological Molecular generators of electric current. Bacteriorhodopsin. J Biol Chem. 1976 Nov 25;251(22):7059–7065. [PubMed] [Google Scholar]
- Gibor A. Acetabularia: a useful giant cell. Sci Am. 1966 Nov;215(5):118–124. doi: 10.1038/scientificamerican1166-118. [DOI] [PubMed] [Google Scholar]
- Green D. E. The structure of biological membranes in relation to the principle of energy coupling. J Theor Biol. 1976 Oct 21;62(2):271–285. doi: 10.1016/0022-5193(76)90120-x. [DOI] [PubMed] [Google Scholar]
- Henderson R. The purple membrane from Halobacterium halobium. Annu Rev Biophys Bioeng. 1977;6:87–109. doi: 10.1146/annurev.bb.06.060177.000511. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Hui C. S. Possible origin of gating current in nerve membrane. Biosystems. 1977 Apr;8(4):207–212. doi: 10.1016/0303-2647(77)90042-9. [DOI] [PubMed] [Google Scholar]
- Jagendorf A. T., Uribe E. ATP formation caused by acid-base transition of spinach chloroplasts. Proc Natl Acad Sci U S A. 1966 Jan;55(1):170–177. doi: 10.1073/pnas.55.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MITCHELL P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961 Jul 8;191:144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- Onsager L. The motion of ions: principles and concepts. Science. 1969 Dec 12;166(3911):1359–1364. doi: 10.1126/science.166.3911.1359. [DOI] [PubMed] [Google Scholar]
- Papa S. Proton translocation reactions in the respiratory chains. Biochim Biophys Acta. 1976 Apr 30;456(1):39–84. doi: 10.1016/0304-4173(76)90008-2. [DOI] [PubMed] [Google Scholar]
- Sone N., Yoshida M., Hirata H., Okamoto H., Kagawa Y. Electrochemical potential of protons in vesicles reconstituted from purified, proton-translocating adenosine triphosphatase. J Membr Biol. 1976 Dec 28;30(2):121–134. doi: 10.1007/BF01869663. [DOI] [PubMed] [Google Scholar]


