Abstract
OBJECTIVE
To determine the role of hypoxia-inducible factor-2α (HIF2α) on the sensitivity of renal cell carcinoma (RCC) cell lines to ionizing radiation and to determine if the mTOR antagonist, rapamycin, could decrease HIF2α protein levels.
MATERIALS AND METHODS
Cell lines expressing stable short-hairpin RNAs (shRNAs) encoding HIF2α shRNAs or an empty vector were transfected with a hypoxia responsive element (HRE)-driven firefly luciferase reporter gene. Two separate paired cell lines were assayed for their response to increasing doses of ionizing radiation. Proliferation and cell cycle kinetics were compared for cell lines expressing HIF2α shRNAs and empty vectors. The effect of an mTOR antagonist, rapamycin on HIF1α and HIF2α proteins levels was also assessed.
RESULTS
We confirmed that the 786-O RCC lines with stably integrated shRNAs against HIF2α had decreased activation of a plasmid with a HRE-driven firefly luciferase reporter gene. Lines from two separate cell clones with decreased HIF2α levels showed a significant increase in radiation sensitivity and an increase in G2 cell cycle arrest. Rapamycin, while effective in decreasing HIF1α protein levels, did not affect HIF2α levels in either of the RCC cell lines.
CONCLUSIONS
These results show that decreasing levels of HIF2α leads to an increased sensitivity to ionizing radiation. This finding may explain in part, the known resistance of RCC to radiation therapy. Although mTOR antagonists are approved for the treatment of RCC, these agents do not decrease HIF2α levels and therefore might not be effective in enhancing the radio-sensitivity of these tumours.
Keywords: renal cancer, radiation, hypoxia, hypoxia-inducible factor
INTRODUCTION
A substantial percentage of RCCs are associated with the loss of the von Hippel-Lindau tumour-suppressor gene (VHL) [1]. One of the functions of VHL is to target the α subunits of the hypoxia-inducible factors (HIF1α and HIF2α) for degradation by the proteasome after hydroxylation of specific proline residues in the presence of oxygen [2]. Thus, a characteristic of RCCs with loss of VHL is high levels of the α subunit of either HIF1 or HIF2 due to their prolonged intracellular half-life [2].
The HIF1α and HIF2α subunits heterodimerize with the ubiquitously expressed HIF1β and HIF2β subunits to form the HIF1 and HIF2 transcription factors [1,2]. Expression of HIF1α has been associated with several features of tumorigenesis including increased neovasculature and altered metabolism [3]. For this reason, high levels of HIF1α and HIF2α in tumour cells have been considered oncogenic [3–5]. Because the levels of the α subunits are relatively low in normal, normoxic cells, it is their levels, rather than the stable levels of HIF1β and HIF2β that affect the capacity of HIF1 and HIF2 to act as transcription factors [6].
Resistance to radiation therapy is well known to be enhanced by hypoxia [7]. It has been hypothesized that radiation resistance might be related to HIFα expression as these protein levels are dramatically increased under hypoxic conditions. Multiple lines of evidence have shown that expression of HIF1α is associated with radiation resistance [8,9]. Although the precise mechanisms for how HIF1α mediates radiation resistance are not known, it has been shown that resistance may result from an effect on either tumour cells [10] or the tumour vasculature [11] and may be dependent on cell cycle kinetics [12].
Despite the fact that HIF2α is the predominant HIFα subunit expressed in VHL-deficient RCC and is preferentially expressed in tumour cells compared with normal parts of the kidney [1,6], there have been few studies of the role of this protein on radiation resistance. We assessed the role of HIF2α in mediating radiation sensitivity using clonogenic cell lines in which HIF2α levels were reduced by stably integrated short-hairpin RNAs (shRNAs) to determine if this protein is a potential therapeutic target. It has already been shown that inhibition of mTOR signalling leads to decreased levels of HIF1α [13,14] and an mTOR antagonist, temsirolimus, has recently been approved for the treatment of RCC. Therefore, if decreased levels of HIF2α are associated with enhanced radiation sensitivity, and if mTOR antagonists decrease HIF2α levels, a potential therapeutic strategy for treating RCC would be apparent.
MATERIALS AND METHODS
The parental RCC 786-O cell line is VHL−/− on the basis of an inactivating mutation in one VHL allele and a deletion in the second allele. It expresses high levels of the HIF2α but not HIF1α [5]. The lines established from 786-O with high and low HIF2α expression have been described previously [5]. The pTR1 and pSR1 lines contain a vector encoding shRNA for HIF2α and the pTV1 and pSV1 cell lines contain a vector encoding the same plasmids but devoid of the shRNA segment [5]. RCC4 is a RCC line that expresses both HIF1α and HIF2α (kind gift of Celeste Simon). All cell lines were maintained in RPMI 1640 media with 10% fetal bovine serum and cultured under normoxic conditions with 5% CO2. The pTR1/pTV1 and pSR1/pSV1 cell lines were also maintained in 10 μg/mL blasticidin.
Plasmids containing a hypoxia-responsive element (HRE) upstream of firefly luceriferase were co-transfected with plasmids containing a SV40 promoter upstream of Renilla in the paired pTR1/pTV1 and pSR1/pSV1 cell lines. Cells at 30% confluence were transfected by lipofection under conditions described by the manufacturer (Fugene 6, Roche Diagnostics, Mannheim, Germany). After 48 h cells were lysed and luceriferase activity determined and normalized for Renilla levels.
For irradiation of pTR1/pTV1 and pSR1/pSV1 cell lines, cells at 60% confluence were irradiated via a Gammacell-40 ionizing radiation chamber (Atomic Energy of Canada), which contains a 137Cs source and delivers a dose rate of 114 Rad/min. Plates were then re-incubated at 37 °C (5% CO2) for 5 h to allow for potentially lethal damage repair, trypsinized, and serially diluted into 24-well tissue culture plates. Surviving cells were counted on Day 5 using a haemocytometer to monitor trypan blue excluding cells or subjected to a tetrazolium salt (MTS)-based colorimetric assay (CellTiter, Promega, Madison, WI, USA).
For flow cytometry analysis, Subconfluent pSV1 and pTV1 cells were treated with or with no radiation 48 h before harvesting. Cells were stained using 10 μg/mL propidium iodine (Sigma, St. Louis, MO, USA) in the presence of 250 μg/mL RNAase A in PBS. Samples were analysed on FACScan (BD Bioscience, Mountain View, CA, USA) and the cell cycle data was analysed using ModFit 3.0 (Verity, Topsham, CA, USA).
For Western blot analysis, cellular lysates were obtained using RIPA buffer and protein content analysed by Bradford assay (Bio-Rad Laboratories, Richmond, CA, USA). Proteins were separated by SDS-PAGE with a standard reducing protocol before being electroblotted to a nitrocellulose membrane. The blots were blocked using standard methods. Immunoblotting was performed with designated antibodies (HIF1α, BD Transduction Laboratories, San Jose, CA, USA; Phospho-S6-kinase (Ser235/236), Cell Signalling, Beverly, MA, USA; beta-tubulin, Santa Cruz Biotechnologies, Santa Cruz, CA, USA; HIF2α, Novus, Littleton, CO, USA); and visualized with an enhanced chemiluminescence detection system (Pierce, Rockford, IL, USA).
For quantification of the immunoblots, autoradiographs were scanned as TIFF files and analysed for densitometric quantification using the Biogen 4.5 program (Bio-Rad Software, Quantity One 4.5.0). In each of three separate experiments the density of HIFα bands was compared with glyceraldehyde-3-phosphate dehydrogenase bands for normalization.
ANOVA and subsequent Bonferroni tests were used to determine if there was a statistically significant difference in mean cell counts and MTS values for the effect of radiation on RCC cell lines.
RESULTS
EFFECT OF SHRNA TREATMENT ON ACTIVATION OF AN HRE-LUCIFERASE REPORTER PLASMID
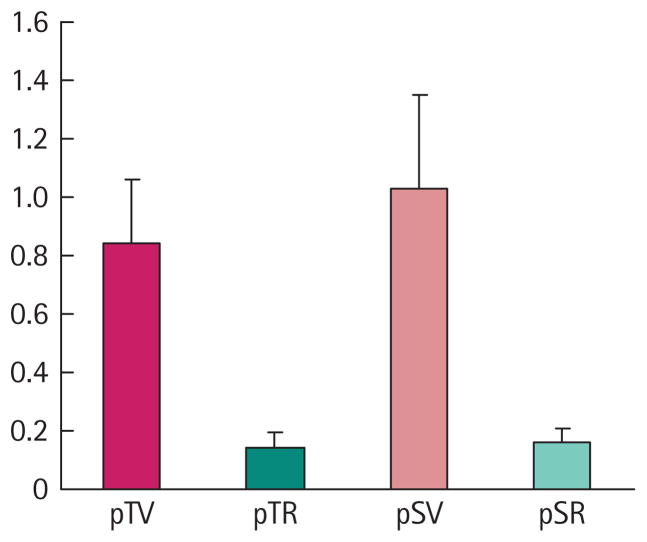
The 786-O cells used in these experiments were stably transfected with shRNAs that decreased protein levels of HIF2α (pTV1 and pSV1) or empty vectors that did not contain these shRNAs (pTR1 and pSR1). Previously it has been reported that the clonally derived cell lines expressing shRNAs against HIF2α have consistently shown dramatic reductions in HIF2α protein levels [5]. Although these cells were maintained under selection conditions, we wanted to assure their phenotypic status before assessing their sensitivity to ionizing radiation. Figure 1 shows the results of six separate transfections. The pTR1 cell line containing the shRNA for HIF2α has only 17% of the capacity to transactivate the HRE-containing plasmid compared with the pTV1 cell line with the empty vector. The results were similar for the other pair of clonally derived lines, in that the pSR1 (shRNA against HIF2α) cell line was only 15% as effective in transactivating the HRE-containing plasmid compared with the pSV1 (empty vector) cell line. Also, as previously reported [5], both cell lines with the shRNAs directed against HIF2α had a >80% reduction in HIF2α protein levels on Western immunoblots compared with their paired cell lines with empty vectors (data not shown).
FIG. 1.
HRE-directed reporter expression in pTR1/pTV1 and pSR1/pSV1 cell lines. Expression of firefly luceriferase and Renilla was measured following transient transfection of pTR1 (PTR)/pTV1 (PTV) and pSR1 (PSR)/pTV1 (PSV) cell lines with HRE and SV40-containing reporter plasmids. The median ratios of luceriferase to Renilla levels are shown for six separate transfections. Error bars represent the SD in these experiments.
HIF2α INHIBITION INCREASES CELL DEATH AND DECREASES CELL PROLIFERATION UPON RADIATION TREATMENT
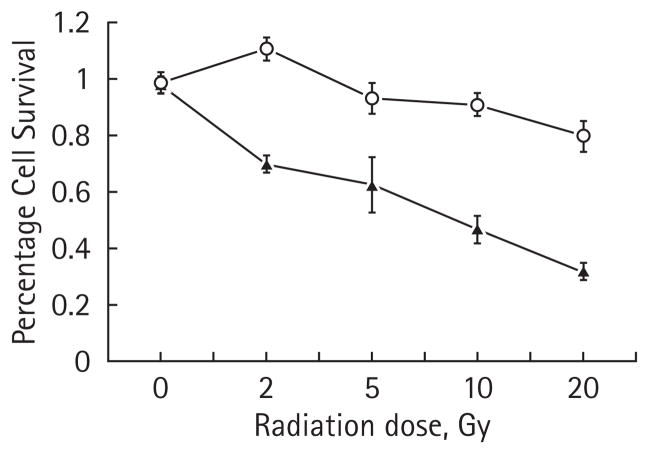
The 786-O cells expressing an empty vector (pTV1) or an shRNA encoding HIF2α (pTR1) were treated with increasing single dose radiation exposures. At 7 days after radiation treatment of 2, 5, 10, 20 and 60 Gy, cell proliferation was assessed by an MTS assay (Fig. 2). The pTV1 cells (with high levels of HIF2α) are markedly resistant to radiation, even at a dose of 60 Gy. By contrast, as the radiation dose increased, the pTR1 cells with markedly lower HIF2α levels exhibited a significant decrease in MTS uptake consistent with a marked decrease in cell proliferation at all radiation doses of >2 Gy.
FIG. 2.
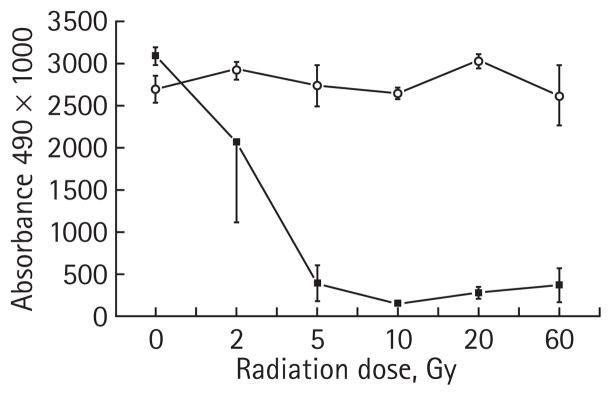
Radiation sensitivity of human 786-O cell line with and with no HIF2α shRNA. 786-O RCC cell lines with stably transfected vector containing HIF2α RNAi (pTR1, –■–) or an empty vector (pTV1, –○–) were irradiated and cell proliferation was assayed using a tetrazolium MTS-based colourimetric assay. The experiment was performed in triplicate and the mean of each result is displayed, with error bars representing the SD. The results with 5, 10, 20 and 60 Gy are significantly different comparing the two lines (P < 0.001).
To extend these findings, we assessed the effects of radiation treatment on a second set of clonally derived 786-O cell lines using trypan blue exclusion to assay cell death. At radiation doses of ≥2 Gy, there is a statistically significant difference in survival between the two lines with the pSR1 cell line showing a marked degree of radiation sensitivity (Fig. 3). These data also exhibit a dose–response effect. The pSR1/pSV1 cell lines also showed similar results to the MTS assay as the paired pTV1/pTR1 lines discussed above (data not shown).
FIG. 3.
Radiation sensitivity of human 786-O cell line with and with no HIF2α shRNA. 786-O RCC cell lines with stably transfected vector containing HIF2α RNAi (pSR1, –▲–) or an empty vector (pSV1, –○–) were irradiated and cells were counted based on their ability to exclude trypan blue. The experiment was performed in triplicate and the mean of each result is displayed, with error bars representing the SD. The results with 5, 10 and 20 Gy are significantly different comparing the two cell lines (P < 0.001).
HIF2α INHIBITION INCREASES RADIATION-INDUCED G2 ARREST
Experiments in Figs 2 and 3 show that doses as low as 2 Gy are associated with a significant enhancement in radiation sensitivity for cell lines in which HIF2α levels are decreased. Radiation therapy is known to induce G2 arrest [12]. Thus, the effects of 2 Gy and 5 Gy of radiation treatment on cell cycle progression were therefore analysed in the pTR1 and pTV1 cells 48 h after treatment. The pTV1 cell line (expressing an empty vector) showed an increase of 6% of cells in G2 for the 2 Gy treatment and 42% over baseline for the 5 Gy treatment. By contrast, treatment with 2 Gy of radiation to the pTR1 cell line (expressing the HIF2α shRNA) increased the percentage of cells in G2 by 31% and treatment with 5 Gy to this cell line increased this population by 70% above baseline (Table 1). Figure 4 shows the differences in the cell cycle profiles for the pTR1 cell line and the pTV1 cell line 48 h after 5 Gy of radiation exposure. This figure shows that the proportion of cells from the pTR1 cell line in G2 is dramatically higher than cells from the pTV1 cell line. In contrast, there is an increased number of cells in G0/G1 from the pTV1 cell line compared with the pTR1 cell line. There was an insignificant number of apoptotic cells at 48 h even after 5 Gy exposure. Similar studies using pSV1/pSR1 yielded comparable results (data not shown).
TABLE 1.
Effect of radiation on cell cycle kinetics
| 0 Gy | 2 Gy | 5 Gy | |
|---|---|---|---|
| % (SD*): | |||
| pTV1 | |||
| G1 | 43.9 (0.9) | 46.9 (2.6) | 26.6 (0.2) |
| S | 33.1 (1.2) | 23.3 (2.9) | 9.4 (0.1) |
| G2 | 23.2 (0.4) | 29.4 (0.5) | 64.0 (0.2) |
| pTR1 | |||
| G1 | 33.8 (0.2) | 23.0 (1.4) | 2.9 (0.5) |
| S | 42.1 (1.6) | 20.6 (0.1) | 2.3 (0.6) |
| G2 | 25.0 (1.8) | 56.3 (1.4) | 94.8 (0.2) |
Subconfluent pTV1 and pTR1 cells were treated with 0, 2 or 5 Gy 48 h before harvesting cells for cell cycle analysis;
obtained from three separate experiments.
FIG. 4.
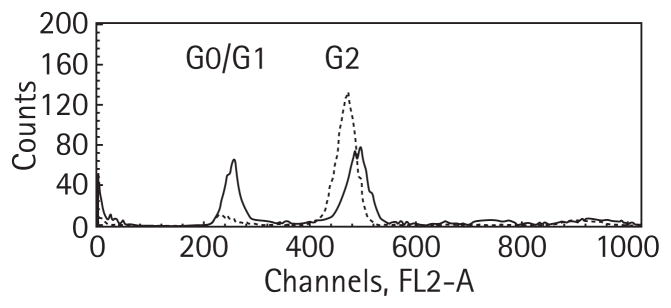
The effect of 5 Gy of radiation on cell cycle kinetics. Subconfluent pTV1 cells (solid line) and pTR1 cells (dashed line) were treated with 5 Gy 48 h before harvesting cells for cell cycle analysis. The peak from 200 nm to 280 nm represents cells in the G0/G1 phase of the cell cycle and the peak from 400 nm to 560 nm represents the G2 phase of the cell cycle. The ordinate is labelled for flow-sorted cells.
HIF2α AND HIF1α PROTEIN LEVELS IN RESPONSE TO RAPAMYCIN
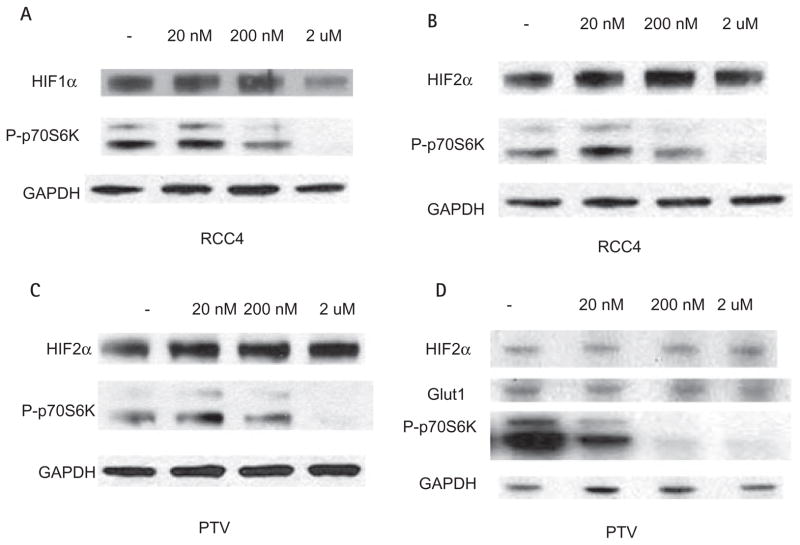
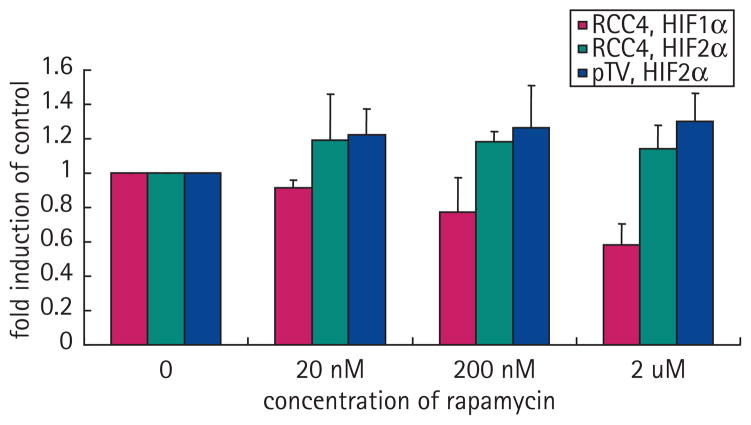
It has been previously shown that several mTOR antagonists such as rapamycin and rapamycin analogs can decrease the expression of HIF1α [13,14]. Treatment of the pTV1 cell line (786-O cell lines transfected with the empty vector) with rapamycin did not affect the protein levels of HIF2α (Fig. 5C). To determine if the lack of effect of rapamycin on decreasing HIF2α levels was specific to 786-O cells, we investigated the effect of this compound on HIF1α and HIF2α levels in RCC4 cells, an RCC cell line that expresses both HIFα factors. In this case rapamycin reduced expression of HIF1α protein but not of HIF2α (Fig. 5A,B). To confirm the activity of rapamycin in these cells, we assessed its effects on the expression of phosphorylated p70S6 kinase, as treatment with mTOR antagonists inhibits the phosphorylation of this protein as one of its downstream targets [13] (Fig. 5A–D). To further support that rapamycin did not affect the expression of HIF2α, we measured the expression of Glut1, a downstream target of HIF2α [15]. Increasing doses of rapamycin, while able to decrease the expression of p70S6 kinase, did not affect levels of HIF2α or Glut1 (Fig. 5D). The results of three separate Western experiments were quantified using laser densitometry (Fig. 6).
FIG. 5.
Effect of rapamycin on HIF protein levels. Western analysis of cell lysates from RCC4 cells or pTV cells treated with rapamycin for 7 h is shown. HIF1α levels decrease at high concentrations of rapamycin as does phospho-P70S6 in RCC4 cells (A). By contrast, despite lowering of phospho-P70S6, rapamycin does not decrease HIF2α levels in RCC4 cells (B) or PTV (pTV1) cells (C). Similar to HIF2α, Glut1 does not decrease with rapamycin treatment in PTV cells (D). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
FIG. 6.
Western analysis quantification. Western autoradiographs were scanned and quantified using Microsoft Excel. HIF1α (solid bar) decreases with therapy but HIF2α, either in the RCC4 or the PTV (pTV1) lines, did not decrease (checked and light stripes, respectively). Means of three separate experiments are shown. Error bars represent SD.
DISCUSSION
HIF2α is often the only HIFα subunit expressed in RCC [1]. Therefore it is important to determine if its expression affects radiation resistance and is therefore a target for therapy. There are two novel features of the present study. This is the first report that HIF2α mediates radiation resistance. We also show that inhibition of the mTOR pathway does not decrease HIF2α levels, despite findings that the same treatment will reduce HIF1α levels. These data have potential implications for treating RCC with radiation therapy.
The effect of HIF2α on radiation resistance was shown by using paired clonally derived cell lines containing a stably integrated plasmid with a shRNA targeted to HIF2α or the same cell line with a stably integrated empty vector [5]. Two separate clonally derived cell lines with presumably different sites of integration of the shRNA HIF2α plasmids demonstrated the same results. Cell lines with shRNAs against HIF2α showed dramatic increases in radiation sensitivity compared with paired cell lines containing empty vectors. These data suggest that HIF2α levels, as has been shown for HIF1α levels [10], are associated with resistance to ionizing radiation. We also show, for the first time, that decreasing HIF2α levels dramatically increases the percentage of cells arrested in the G2 phase of the cell cycle after radiation. These findings lend greater credibility to the present results, as it is known that ionizing radiation is known to arrest cell growth in the G2 phase of the cell cycle [12].
Prior to these studies, the effects of HIF2α on radiation resistance were ambiguous and somewhat conflicting. A clinical study of radiation treatment for head and neck cancer reported that levels of both HIF1α and HIF2α correlated with radiation resistance [9]. However, in experiments by Palayoor et al. [16], transfection of VHL into RCC cells lacking this gene did not alter radiation sensitivity, despite leading to an increase in protein levels for both HIF1α and HIF2α. One possible reason for the difference in the present results and that obtained by Palayoor et al. may be due to the pleiomorphic effects of VHL on gene expression. Restoration of VHL function in VHL-deficient lines decreases the half-life of HIFα proteins, but also alters the levels of many other proteins [17]. It may be that changes in the levels of one or more of these proteins counteract the effects of decreased HIF1α and HIF2α.
Based on the extensive homology between the proteins and previous data regarding HIF1α and radiation sensitivity, it may not be surprising that HIF2α can also mediate radiation resistance. The proteins are likely to have overlapping functions and gene targets as transcription factors. For example, replacing HIF1α with HIF2α in teratocarcinoma cells has a comparable effect on tumour progression [18]. The similarity between the function of these genes led us to postulate that mTOR antagonists might inhibit HIF2α as has shown to be possible for HIF1α [13,14]. Part of the motivation behind these experiments is that the mTOR antagonist, temsirolimus, is now approved for the treatment of patients with RCC.
In these experiments, we show that inhibition of the mTOR pathway has no effect on decreasing HIF2α protein levels in 786-O cells, an RCC line that exclusively expresses HIF2α. We confirmed this result using a second line, RCC4 a unique RCC line that expresses HIF1α and HIF2α. Using this cell line, we showed that only HIF1α protein levels are reduced by rapamycin treatment but HIF2α levels are unchanged. As further validation of the present findings, we determined that mTOR inhibition did not decrease expression of Glut1, a target of both HIF1α and HIF2α [13,19]. Although not previously well appreciated, Blancher et al. [20] reported in breast cancer cells that the phosphatidylinositol-3-kinase antagonist, LY294002, inhibited HIF1α expression, but not HIF2α expression. Although it is expected that a PI3 kinase antagonist would inhibit proteins in the mTOR pathway, the present results extend these findings and include renal cell lines.
The present results may have important ramifications. While mTOR antagonists have been shown to regulate HIF1α at the transcriptional level [13,14], this is not the case for HIF2α. These results suggest there is likely to be differences in the 5′ regulatory regions and transcription factors that affect expression of HIF1α and HIF2α. This may not be surprising based on the differential expression of these proteins in different tissues. For example, HIF1α is often expressed in breast cancer and other solid tumours [18] whereas HIF2α is expressed in RCC [1]. The clinical implication of the present finding is that mTOR inhibitors may not improve radiation sensitivity for tumours that express HIF2α such as RCC and it may be important to identify therapies that inhibit or decrease levels of HIF2α in the future.
Acknowledgments
This research was possible through the generous support of the Hershey Family Foundation for Prostate Cancer Research, the DF/HCC Renal SPORE and Prostate SPORE Programs (Renal SPORE # 5 P50 CA101942-04, Prostate SPORE # 2POCA90381). We also thank Celeste Simon (University of Pennsylvania, Philadelphia, PA, USA) for providing RCC4 cell lines and acknowledge the technical assistance of Sonia Sinha.
R.S.B. supported by NIH 5 P50 CA101942-04 and G.J.B. supported by NIH 2POCA90381.
Abbreviations
- HIF
hypoxia-inducible factor
- shRNA
short-hairpin RNA
- HRE
hypoxia responsive element
- VHL
von Hippel-Lindau tumour-suppressor gene
Footnotes
CONFLICT OF INTEREST
None declared.
References
- 1.Turner KJ, Moore JW, Jones A, et al. Expression of hypoxia-inducible factors in human renal cancer: relationship to angiogenesis and to the von Hippel-Lindau gene mutation. Cancer Res. 2002;62:2957–61. [PubMed] [Google Scholar]
- 2.Kaelin WG., Jr The von Hippel-Lindau protein, HIF hydroxylation, and oxygen sensing. Biochem Biophys Res Commun. 2005;338:627–38. doi: 10.1016/j.bbrc.2005.08.165. [DOI] [PubMed] [Google Scholar]
- 3.Ryan HE, Lo J, Johnson RS. HIF-1 alpha is required for solid tumor formation and embryonic vascularization. EMBO J. 1998;17:3005–15. doi: 10.1093/emboj/17.11.3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kondo K, Klco J, Nakamura E, Lechpammer M, Kaelin WG., Jr Inhibition of HIF is necessary for tumor suppression by the von Hippel-Lindau protein. Cancer Cell. 2002;1:237–46. doi: 10.1016/s1535-6108(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 5.Zimmer M, Doucette D, Siddiqui N, Iliopoulos O. Inhibition of hypoxia-inducible factor is sufficient for growth suppression of VHL−/− tumors. Mol Cancer Res. 2004;2:89–95. [PubMed] [Google Scholar]
- 6.Krieg M, Haas R, Brauch H, Acker T, Flamme I, Plate KH. Up-regulation of hypoxia-inducible factors HIF-1α and HIF-2α under normoxic conditions in renal carcinoma cells by von Hippel-Lindau tumor suppressor gene loss of function. Oncogene. 2000;19:5435–43. doi: 10.1038/sj.onc.1203938. [DOI] [PubMed] [Google Scholar]
- 7.Harrison LB, Chadha M, Hill RJ, Hu K, Shasha D. Impact of tumor hypoxia and anemia on radiation therapy outcomes. Oncologist. 2002;7:492–508. doi: 10.1634/theoncologist.7-6-492. [DOI] [PubMed] [Google Scholar]
- 8.Ishikawa H, Sakurai H, Hasegawa M, et al. Expression of hypoxic-inducible factor 1α predicts metastasis-free survival after radiation therapy alone in stage IIIB cervical squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;60:513–21. doi: 10.1016/j.ijrobp.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Koukourakis MI, Giatromanolaki A, Sivridis E, et al. Hypoxia-inducible factor (HIF1A and HIF2A), angiogenesis, and chemoradiotherapy outcome of squamous cell head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2002;53:1192–202. doi: 10.1016/s0360-3016(02)02848-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Kon T, Wang H, et al. Enhancement of hypoxia-induced tumor cell death in vitro and radiation therapy in vivo by use of small interfering RNA targeted to hypoxia-inducible factor-1α. Cancer Res. 2004;64:8139–42. doi: 10.1158/0008-5472.CAN-03-2301. [DOI] [PubMed] [Google Scholar]
- 11.Moeller BJ, Dreher MR, Rabbani ZN, et al. Pleiotropic effects of HIF-1 blockade on tumor radiosensitivity. Cancer Cell. 2005;8:99–110. doi: 10.1016/j.ccr.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 12.Cordes N, van Beuningen D. Arrest of human lung fibroblasts in G2 phase after irradiation is regulated by converging phospatidyinositol-3 kinase and β1-integrin signaling in vitro. Int J Radiat Oncol Biol Phys. 2004;58:453–62. doi: 10.1016/j.ijrobp.2003.09.069. [DOI] [PubMed] [Google Scholar]
- 13.Majumder PK, Febbo PG, Bikoff R, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 14.Hudson CC, Liu M, Chiang GG, et al. Regulation of hypoxia-inducible factor 1α expression and function by the mammalian target of rapamycin. Mol Cell Biol. 2002;22:7004–14. doi: 10.1128/MCB.22.20.7004-7014.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maranchie JK, Zhan Y. Nox4 is critical for hypoxia-inducible factor 2α transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65:9190–3. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palayoor ST, Burgos MA, Shoaibi A, Tofilon PJ, Coleman CN. Effect of radiation and ibuprofen on normoxic renal carcinoma cells overexpressing hypoxia-inducible factors by loss of von Hippel-Lindau tumor suppressor gene function. Clin Cancer Res. 2004;10:4158–64. doi: 10.1158/1078-0432.CCR-04-0005. [DOI] [PubMed] [Google Scholar]
- 17.Nakamura E, Abreu-e-Lima P, Awakura Y, et al. Clusterin is a secreted marker for a hypoxia-inducible factor-independent function of the von Hippel-Lindau tumor suppressor protein. Am J Pathol. 2006;168:574–84. doi: 10.2353/ajpath.2006.050867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Covello KL, Simon MC, Keith B. Targeted replacement of hypoxia-inducible factor-1α by a hypoxia-inducible factor-2α knock-in allele promotes tumor growth. Cancer Res. 2005;65:2277–86. doi: 10.1158/0008-5472.CAN-04-3246. [DOI] [PubMed] [Google Scholar]
- 19.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1α (HIF-1α) and HIF-2α in hypoxic gene regulation. Mol Cell Biol. 2003;23:9361–74. doi: 10.1128/MCB.23.24.9361-9374.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blancher C, Moore JW, Robertson N, Harris AL. Effects of ras and von Hippel-Lindau (VHL) gene mutations on hypoxia-inducible factor (HIF)-1α, HIF-2α, and vascular endothelial growth factor expression and their regulation by the phosphatidylinositol 3′-kinase/Akt signaling pathway. Cancer Res. 2001;61:7349–55. [PubMed] [Google Scholar]