Abstract
Background
Chronic lung disease of prematurity (CLDP) is a frequent complication of premature birth. Infants and children with CLDP are often prescribed complex medication regimens, which can be difficult for families to manage.
Objective
We sought to determine whether non-adherence was associated with increased CLDP-related morbidities and to identify predictors of adherence.
Methods
Recruited caregivers of 194 children with CLDP completed questionnaires regarding self-reported adherence, respiratory outcomes, and quality of life (January 2008 – June 2010). Adherence data were available for 176 subjects, of whom 143 had self-reported data only, and 33 had prescription claims data, which were used to calculate a medication possession ratio (MPR). Participants in the Prescription Claims Sample (n=33) were more likely to have public insurance (p<0.001).
Results
Self-reported adherence substantially overestimated medication possession; the mean MPR was 38.8% (n=33) and was not associated with self-reported adherence (p=0.71; n=26). In a small sample, higher MPR was associated with decreased odds ratios of visiting the emergency department (OR=0.75 for a 10% increase in MPR [95%CI: 0.58, 0.97]; p=0.03; n=74 questionnaires from 28 participants), activity limitations (OR=0.71 [95%CI: 0.53, 0.95]; p=0.02; n=70 questionnaires from 28 participants), and rescue medication use (OR=0.84 [95%CI: 0.73-0.98]; p=0.03; n=70 questionnaires from 28 participants). Increasing caregiver worries regarding medication efficacy and side effects were associated with lower MPR (p=0.04; p=0.02, respectively; n=62 questionnaires from 27 participants). Socio-demographic and clinical risk factors were not predictors of MPR (n=33).
Conclusions
We found that non-adherence with respiratory medications was common in premature infants and children with CLDP. Using multiple timepoints in a small sample, non-adherence was associated with a higher likelihood of respiratory morbidities. Although self-reported adherence and demographic characteristics did not predict MPR, concerns about medications did. We suggest that addressing caregiver concerns about medications may improve adherence and ultimately decrease CLDP-related morbidities. Larger, prospective studies are needed to confirm these findings and determine which factors predict non-adherence.
Keywords: Bronchopulmonary dysplasia, chronic lung disease, preterm, prematurity, adherence, compliance, health beliefs
INTRODUCTION
In 2007 premature births accounted for 12.7% of live births within the United States.1 A frequent complication of prematurity is chronic lung disease of prematurity (CLDP), which occurs more commonly among premature infants and those born with very low birth weights. It has been estimated that as many as 44-77% of infants born with a birth weight of <1000g and <32 weeks gestation are afflicted with the precursor to CLDP, bronchopulmonary dysplasia.2 The development of CLDP is associated with significant morbidity as up to 50% of infants with CLDP are re-hospitalized during the first year of life, frequently for respiratory causes.3, 4 Infants and children with CLDP often experience chronic respiratory symptoms such as tachypnea, hypoxia, coughing, and wheezing.5 Furthermore, recurrent respiratory infections and other injuries during a crucial period of postnatal catch-up lung growth may influence long-term pulmonary outcomes.5
After discharge from the neonatal intensive care unit (NICU) caregivers of infants with CLDP are often burdened with complex home treatment regimens, including multiple medications, home supplemental oxygen, cardio-respiratory monitoring, and/or frequent primary care and subspecialty outpatient visits. We hypothesized that non-adherence to respiratory medications would increase morbidity, similar to other chronic respiratory diseases such as asthma6, 7 and cystic fibrosis.8 Surprisingly, in a number of chronic diseases adherence remains low despite the risk of serious complications. In pediatric asthma and cystic fibrosis, adherence with medications has been estimated to be at best 50%.9, 10
In terms of improving health outcomes for any chronic disease, improving medication adherence is an attractive target as it does not involve developing new therapies, but rather using existing ones more effectively. Adherence is related to a patient’s health beliefs, namely the perceived benefits (e.g., effectiveness) of a medication must outweigh its perceived risks (e.g., side effects) for a patient.11, 12 Concerns about medications have been shown to be an important, perhaps the most significant, predictor of medication adherence in parents of children with asthma.12, 13 Unfortunately, discussing non-medical issues, such as health beliefs, in detail may be difficult in a time-limited physician-parent encounter.12 We hypothesized that sociodemographic factors and standardized questions about medication concerns would be predictive of non-adherence in the CLDP population, and thus provide an easier means for assessing potential adherence for busy clinicians.
Little is known of medication adherence in infants and children with CLDP, therefore we sought to determine what the mean level of medication adherence was, using an objective measure of prescription claims for respiratory medications in this population. Secondary aims of our study included: (i) whether increased non-adherence is associated with the increased respiratory morbidities that are seen with CLDP, and (ii) what predictors can be easily assessed by clinicians to determine adherence. Our data was derived from questionnaires, clinic charts, and prescription claims data obtained from participants recruited through a subspecialty CLDP outpatient clinic.
METHODS
Recruitment and Data Collection
Participants for this study were recruited from patients attending the Johns Hopkins Chronic Lung Disease of Prematurity (CLDP) Clinic between January 2008 and June 2010 (Figure 1). The study protocol was approved by the Johns Hopkins University Institutional Review Board. Inclusion criteria included (i) a diagnosis of CLDP by the staffing pulmonologist, (ii) born at ≤34 weeks gestation, and (iii) provision of informed consent by a legal guardian(s), who was predominantly the primary caregiver accompanying the infant to clinic, to participate in the study. During each clinic visit, caregivers completed questionnaires regarding socio-demographics, adherence to prescribed medications, acute health care use, symptom frequency, and quality of life. For the prescription claims data, all insurance carriers were contacted and pursued until data were received or the corporate privacy office explicitly refused to release the data, despite having written caregiver informed consent.
Figure 1.
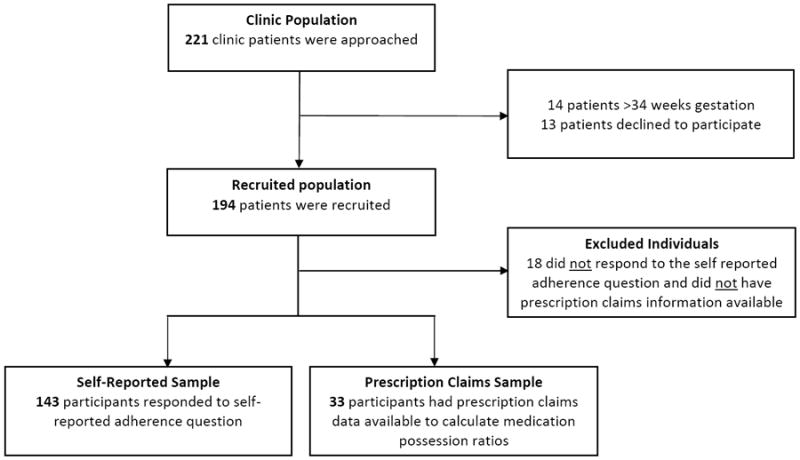
Study Population Derivation
Socio-demographics
Socio-economic factors examined were household income, primary caregiver education level, and insurance status. Median household income was derived from 2000 U.S. Census tract data, and primary caregiver education level was obtained via questionnaire. Insurance coverage (private vs. public) was obtained from billing records; the 8 participants who carried both types of coverage were classified as “private.”
Self-Reported Adherence
Self-reported medication adherence without a specified timeframe was obtained through the survey question, “How often does your child miss getting their daily breathing medications when he/she feels fine?” Caregivers who responded “none of the time” for their children were considered to have a self-report of 100% adherence. Those who responded “some of the time,” “most of the time,” or “all of the time” were considered to have a self-report of partial/non-adherence. This stratification of 100% adherence vs. partial/non-adherence was used as it was hypothesized that any non-adherence was potentially detrimental, and that as caregiver report is likely biased, any report of non-adherence was taken to reflect true non-adherence. For participants who had more than one complete questionnaire during the study period, self-reported adherence was categorized as the maximum degree of non-adherence ever reported, as again, any self-reported non-adherence was thought to be detrimental.
Medication Possession Ratio
A Medication Possession Ratio (MPR) for an individual medication was defined as the days supply of medication dispensed by a pharmacy (obtained by prescription claims data) divided by the number of days the patient was expected to be receiving the medication (obtained by clinic chart review). Participants had to have at least 90 calendar days of prescription claims data available for analysis (Mean ± SD: 300 ± 72 days; Range: 154-365); if more than 365 days of data were available for a participant only the most recent 365 days of data were used for analysis. Ultimately, the prescription claims data were available for 30 participants by a managed care organization authorized by the state of Maryland and for an additional 3 by a pharmacy benefit manager of a preferred provider organization. CLDP medications tracked were limited to chronic respiratory medications, including diuretics (spironolactone, spironolactone + hydrochlorothiazide, chlorothiazide, and furosemide), inhaled corticosteroids (fluticasone and budesonide), and montelukast. The expected number of days that a medication was prescribed was calculated as the interval between the date that the medication was prescribed and the date that the medication was discontinued in clinic. MPRs were calculated for each of the targeted medications individually with values truncated to 100% as individuals with an excess supply of a medication was considered to have a dispensing pattern consistent with 100% adherence for that medication. The MPRs of each medication were averaged across all medications to obtain the Composite MPR. If an individual was prescribed only one drug, then the composite MPR would be equal to his or her MPR for that individual drug.
Respiratory Outcomes
The respiratory morbidity outcomes were obtained through questionnaires. Acute care use outcomes included any reported emergency department (ED) visits, hospitalizations, systemic steroid use, and antibiotic use since the previous clinic visit. Symptomatic outcomes included any reported difficulty breathing, rescue medication use, activity limitation, and nighttime symptoms within the past week. All outcomes were treated as dichotomous variables.
Medication Concerns
Concerns about medications were assessed through questionnaires. Caregivers were asked to respond to the following statements: “I worry about whether or not my child’s medical treatments are working” and “I worry about the side effects of my child’s medications/medical treatments.” The caregivers ranked their frequency of worry on a scale from 0-4 (0=never; 1=almost never; 2=sometimes; 3=often; 4=almost always). As different caregivers with different levels of concern may have completed questionnaires at different clinic visits, all questionnaires were used in analyses, clustered by patient.
Statistical analyses
Descriptive frequencies of socio-demographic characteristics and risk factors were generated using means and proportions as appropriate. Statistical comparisons between samples were made using 2-tailed t tests, Fisher’s exact tests, and chi-square tests. Multivariable logistic and linear regression models were constructed to assess the relationships between MPRs and outcomes as well as between MPRs and potential predictors. Models for respiratory morbidity outcomes were adjusted for age at the time of questionnaire completion and disease severity as measured by gestational age and oxygen use at the first clinic visit. Any regressions which were based on multiple clinical visits/questionnaires per participant were adjusted using Generalized Estimating Equations (GEE) methodology clustered by subject to account for participants who completed more than one questionnaire during the course of the study.14 All odds ratios are reported as the change in likelihood of an outcome with an arbitrary 10% increase in MPR. STATA 10 (StataCorp LP, College Station, TX) was used for all statistical analyses. P-values <0.05 were considered statistically significant.
RESULTS
Demographics
Of the 194 recruited individuals, 18 were excluded due to a lack of either self-reported adherence or prescription claims data, leaving a total of 176 participants in the study population. The study population consisted of 143 participants with self-reported adherence data only and 33 patients whose insurance carriers released at least 90 days of prescription claims data to calculate adherence. The recruited population (n=194) consisted of 59.8% males and 32.0% Non-Hispanic Whites (Table 1). Excluded individuals (n=18) were more likely to be covered by public insurance (p=0.02) and were less likely to be on supplemental oxygen at the first clinic visit (p=0.02) than the study population (n=176). Participants in the Prescription Claims Sample (n=33) were more likely to have public insurance (p<0.001), a lower estimated household income (p=0.03), and a lower caregiver education level (p=0.02) than those in the Self Reported Sample (n=143), reflecting the majority of the patients being fully covered by Medicaid in the Prescription Claims Sample (28 out of 33).
Table 1.
Study Demographics
| Variable Mean ± SD [Range] | Recruited Population* (n=194) | Excluded Individuals (n=18) | Self-Reported Sample (n=143) | Prescription Claims Sample (n=33) | ||
|---|---|---|---|---|---|---|
| Demographics | Sex (% Male) | 59.8 | 72.2 | 58.0 | 60.6 | |
|
| ||||||
| Race/Ethnicity (% Non-Hispanic White) | 32.0 | 27.8 | 35.0 | 21.2 | ||
|
| ||||||
| Gestation (Weeks) | 26.4 ± 2.3 [22.7-34.0] | 26.9 ± 2.6 [22.7-32.0] | 26.5 ± 2.3 [23.0-34.0] | 25.7 ± 1.9 [23.0-31.0] | ||
|
| ||||||
| Age at discharge (Months) | 4.1 ± 2.7 [0.2-24.5] (n=193) | 4.1 ± 3.3 [1.7-15.7] | 4.0 ± 2.6 [0.2-24.5] (n=142) | 4.3 ± 2.5 [0.6-14.8] | ||
|
| ||||||
| Age at first clinic visit (Months) | 7.2 ± 5.1 [1.9-41.5] | 6.6 ± 3.5 [2.9-18.2] | 7.3 ± 5.7 [1.9-41.5] | 7.0 ± 3.1 [3.1-17.3] | ||
|
| ||||||
| Socio-Economic Status | Median household income ($’000) | 43.8 ± 17.9 [11.1-101.4] (n=193) | 38.2 ± 16.2 [11.5-65.7] | 45.7 ± 18.3 [11.1-101.4] (n=142) | 38.3 ± 15.1‡ [11.1-81.9] | |
|
| ||||||
| Education Primary caregiver (%) | <High School | 7.0 | 25.0 | 4.1 | 12.5‡ | |
| H.S. Grad. | 25.6 | 50.0 | 25.8 | 16.7 | ||
| Some College | 34.9 | 25.0 | 29.9 | 58.3 | ||
| College Grad. | 14.0 | 0.0 | 17.5 | 4.2 | ||
| Adv. Degree | 18.6 (n=129) | 0.0 (n=8) | 22.7 (n=97) | 8.3 (n=24) | ||
|
| ||||||
| Insurance status (% Public) | 57.7 | 83.3† | 48.3 | 84.9‡ | ||
|
| ||||||
| Clinical Descriptors and Exposures | Secondhand smoke (% Ever exposed) | 28.5 (n=165) | No data available | 27.0 (n=137) | 35.7 (n=28) | |
|
| ||||||
| Daycare (% Ever attended) | 23.8 (n=160) | No data available | 26.1 (n=134) | 11.5 (n=26) | ||
|
| ||||||
| Oxygen (% On oxygen at first visit) | 36.1 | 11.1† | 40.6 | 30.3 | ||
|
| ||||||
| Gastronomy tube (% On supplemental enteral feeds at first visit) | 16.5 | 22.2 | 16.8 | 12.1 | ||
|
| ||||||
| Ventilator (% On ventilator at first visit) | 1.0 | 0.0 | 1.4 | 0.0 | ||
The recruited population (n = 194) includes individuals without self-reported or prescription claims data (n = 18), individuals with only self-reported data (n = 143), and individuals with prescription claims data with or without self-reported data (n = 33) (Figure 1).
P value <0.05 compared with Self-Reported + Prescription Claims Sample.
P value <0.05 compared with Self-Reported Sample.
Self-Reported Adherence was Low
48.9% of caregivers (n=176) reported they were non-adherent “none of the time”, while 29.5% acknowledged missing doses of medication, specifically “some of the time” (19.3%), “most of the time” (2.8%), and “all of the time” (7.4%)(Table 2). Almost one out of five (18.8%) reported their child was not prescribed daily breathing medication, and 2.8% had a missing response. For the 18.8% of caregivers (n=33) who responded that their child was not on a daily breathing medication, clinical chart review indicated that 12 (36.4%) were discordant with the clinic chart; 9 participants were prescribed diuretics as their only respiratory medication, 2 were prescribed only an inhaled corticosteroid, while the remaining participant was prescribed both. The distribution of responses on the adherence item did not differ between the Self-Reported Sample and the Prescription Claims Sample (Fischer’s exact p=0.32).
Table 2.
Self-Reported Adherence
| “How often does your child miss getting their daily breathing medications when he/she feels fine?”* | Self-Reported + Prescription Claims Samples (n = 176) | Self-Reported Sample (n=143) | Prescription Claims Sample (n=33)† |
|---|---|---|---|
| None of the time (%) | 48.9 | 49.7 | 45.5 |
| Some of the time (%) | 19.3 | 18.2 | 24.2 |
| Most of the time (%) | 2.8 | 2.8 | 3.0 |
| All of the time (%) | 7.4 | 7.7 | 6.1 |
| My child is not supposed to be on a daily breathing medication (%) | 18.8 | 21.7 | 6.1 |
| Missing (%) | 2.8 | - | 15.2 |
For participants with multiple visits, self-reported non-adherence was categorized as the maximal degree of nonadherence ever reported.
Fischer’s exact test p=0.32 for Prescription Claims Sample vs. Self-Reported Sample for non-missing responses.
Self-reported Adherence was not Associated with Medication Possession Ratios
The mean MPR for the Prescription Claims Sample was 38.8 ± 27.5% (n=33; Range: 0.0, 95.6%). Caregivers who self-reported 100% adherence had an average MPR of 43.3 ± 28.8% (n=15), which did not differ from an average MPR of 39.1 ± 27.8% (n=11) for caregivers who self-reported partial/total non-adherence (p=0.71). Self-reported adherence was not available for 7 of the 33 caregivers in the Prescription Claims Sample, 2 of whom reported not being on a daily breathing medication, and the remaining 5 for whom self-reported response data were missing; the mean MPR for these 7 participants was 28.8 ± 25.3%.
Lower Medication Possession Ratios are Associated with Emergency Department Visits, Rescue Medication Use, and Activity Limitations
In the Prescription Claims Sample (n=72 questionnaires among 28 participants), a higher MPR was associated with decreased odds ratios of visiting the emergency department (OR=0.75; p=0.03)(Table 3: All odds ratios are reported as the change in likelihood of an outcome with a 10% increase in MPR). Based on these odds ratios, for a 10% increase in the MPR, the likelihood of visiting the ED would be reduced by 25%. The MPR was not associated with the acute care outcomes of steroid and antibiotic use; however, a decreased odds ratio of hospitalization with an increased MPR trended towards significance (OR=0.71; p=0.10; n=74 questionnaires among 28 participants). A higher MPR was associated with decreased odds ratios of any rescue medication use in the past week (OR=0.84; p=0.03; n=70 questionnaires among 28 participants) and experiencing any activity limitations in the past week (OR=0.71; p=0.02; n=70 questionnaires among 28 participants). In contrast to MPRs, self reported adherence was not associated with the respiratory morbidities of emergency department visits (p=0.33; n=70 questionnaires among 28 participants), rescue medication use (p=0.52; n=68 questionnaires among 28 participants), or activity limitations (p=0.64; n=70 questionnaires among 28 participants) using the same logistic regression modeling that was used for MPRs, thus implying that self-reported adherence does not predict outcomes.
Table 3.
Medication Possession Ratios and Respiratory Morbidities
| Odds ratios are calculated for a 10% increase in MPR (Adherence) | Odds Ratio [95% C.I.]* | P value | Total number of responses (n=28 participants)† | |
|---|---|---|---|---|
| Acute Care Use | Emergency Department | 0.75 [0.58, 0.97] | 0.03 | 72 |
| Hospitalization | 0.71 [0.47, 1.06] | 0.10 | 74 | |
| Steroid use | 0.99 [0.84, 1.16] | 0.88 | 73 | |
| Antibiotic use | 1.01 [0.88, 1.17] | 0.85 | 74 | |
| Symptomatic Outcomes | Days with trouble breathing | 1.12 [0.92, 1.36] | 0.25 | 70 |
| Rescue medication use | 0.84 [0.73, 0.98] | 0.03 | 70 | |
| Activity limitations | 0.71 [0.53, 0.95] | 0.02 | 70 | |
| Caregiver awake at night | 0.78 [0.53, 1.14] | 0.20 | 70 | |
Logistic regressions clustered by participant (GEE Methodology) and adjusted for age in years at the time of visit and disease severity (gestational age and the use of oxygen as recorded at the first clinic visit).
Participants (n=28) had a mean (±SD) of 2.6 ± 1.7 responses at separate clinic visits. Out of 33 individuals in the Prescription Claims Sample, 5 individuals did not complete questionnaires covering these outcomes.
Demographic Factors do not Predict Medication Possession Ratios
In the Prescription Claims Sample (n=33), no associations were found between sex, race, gestation, age at discharge, or age at first clinic visit and refill adherence (Table 4). There was no association between socio-economic status or other risk factors and MPR.
Table 4.
Demographic Predictors of Medication Possession Ratios in the Prescription Claims Sample
| Predictor Variable | Coefficient* [95% CI] (n=33) | P value | |
|---|---|---|---|
| Demographics | Sex | -1.4 [-21.7, 18.8] | 0.89 |
| 0=Female | |||
| 1=Male | |||
|
| |||
| Race/Ethnicity | -10.3 [-34.2, 13.7] | 0.39 | |
| 0=White | |||
| 1=Nonwhite | |||
|
| |||
| Gestation (weeks) | 3.4 [-1.8, 8.5] | 0.2 | |
|
| |||
| Age at discharge (months) | -1.1 [-5.1, 2.8] | 0.57 | |
|
| |||
| Age at first clinic visit (months) | -1.3 [-4.5, 1.9] | 0.41 | |
|
| |||
| Socio-economic Status | Median household income ($’000) | 0.16 [-0.51, 0.82] | 0.63 |
|
| |||
| Education | 3.2 [-8.3, 14.7] (n=24) | 0.57 | |
| 0= <High School | |||
| 1=H.S. grad | |||
| 2=Some College | |||
| 3=College Grad | |||
| 4=Adv. Degree | |||
|
| |||
| Insurance status | -3.7 [-31.3, 23.9] | 0.79 | |
| 0=Private | |||
| 1=Public | |||
|
| |||
| Risk Factors | Secondhand smoke | 0.41 [-22.8, 23.6] (n=28) | 0.97 |
| 0=Never exposed | |||
| 1=Ever exposed | |||
|
| |||
| Daycare | 5.8 [-29.2, 40.9] (n=26) | 0.73 | |
| 0=Never attended | |||
| 1=Ever attended | |||
|
| |||
| Oxygen | 13.9 [-7.1, 34.8] | 0.19 | |
| 0=Not on oxygen at first visit | |||
| 1=On oxygen at first visit | |||
|
| |||
| Gastronomy tube | 10.6 [-19.5, 40.7] | 0.48 | |
| 0=Not on supplemental enteral feeds at first visit | |||
| 1=On supplemental enteral feeds at first visit | |||
Unadjusted linear regressions.
Concerns about Medications are Associated with Medication Possession Ratios
Caregiver worries about the efficacy of medical treatments and their side effects were associated with decreased MPRs (Table 5). For each 1 point increase in the level of worry about whether or not a child’s medical treatments were working, the MPR decreased by 7.2% (p=0.04; n=62 questionnaires among 27 participants). Similarly, for each 1 point increase in the level of worry about medication side effects, the MPR decreased by 8.3% (p=0.02; n=62 questionnaires among 27 participants). Responses to these two questions were correlated (r=0.55). In a sub-analysis using GEE modeling, our data did not show any association between concerns about medications and race (Non-Hispanic Whites vs. Other; p=0.50 regarding efficacy, p=0.70 regarding side effects; n=62 questionnaires among 27 participants).
Table 5.
Health Beliefs as a Predictor of Medication Possession Ratios
| (n=27 patients, 62 responses) | Coefficient* [95% CI] | P value | R |
|---|---|---|---|
| “I worry about whether or not my child’s medical treatments are working.”† | -7.2 [-13.9, -0.4] | 0.04 | 0.26 |
| “I worry about the side effects of my child’s medications/medical treatments.”† | -8.3 [-14.8, -1.7] | 0.02 | 0.35 |
Linear regressions clustered by participant (GEE Methodology). Out of 33 individuals in the Prescription Claims Sample, 6 individuals did not complete questionnaires covering these predictors.
Reponses include: 0=Never, 1=Almost Never, 2=Sometimes, 3=Often, 4=Almost Always.
DISCUSSION
Despite the extensive training caregivers often receive over several months in neonatal intensive care unit and subacute facility settings prior to discharge and the fragile nature of these infants, we found that even in this vulnerable population non-adherence (as measured by medication possession ratios) was observed to be common, with a mean MPR for respiratory medications of 38.8% (n=33). This is similar to what has been observed in other chronic diseases affecting children.6-8 Furthermore, in a small sample (n=28) we found that reduced medication possession was associated with higher risk of emergency department visits, daily activity limitations and rescue medication use, and a trend of increased risk of hospitalization.
Given that up to 50% of CLDP patients are re-hospitalized in the first year of life,3, 4 it is crucial to identify and counsel families who are having difficulties adhering to medication regimens. However, it may be challenging for clinicians to easily identify families with barriers to adherence. We found that self-report does not predict medication possession as there was no difference in the mean MPR between those who self-reported 100% adherence and those who did not (n=26). Additionally, self-report does not predict the same adverse outcomes as does the MPR, although our sample for this analysis is limited to 26 individuals. Thus, our data echoes what has been seen in other chronic pediatric respiratory diseases that self-reported adherence does not correlate with objective measurements of adherence and over-estimates adherence as suggested by medication dispensing rates.9, 10, 15, 16
In examining predictors of non-adherence, we found that demographics, including socioeconomic status, and potential clinical risk factors did not predict medication possession (n=33). However, we did find that concerns about medications were associated with decreased medication possession. Thus, beliefs are barrier to adherence that clinicians can potentially modify by providing more counsel on efficacy and side effects on medications. For every increase in worry regarding medication efficacy and side effects, MPRs were observed to drop 7.2% and 8.3%, respectively (n=27). Our finding is similar to previous findings in pediatric asthma patients that caregivers who have low treatment expectations and are concerned about medication side effects have lower adherence rates.17-19 Some pediatric asthma studies have demonstrated that Non-Whites/Hispanic patients are more likely to be concerned about their medications than Non-Hispanic White patients,17, 19 but our data did not show any association between concerns about medications and race/ethnicity. Lastly, although we assumed that health beliefs influence adherence, an alternative hypothesis is that certain classes of medications prescribed for CLDP may not be effective leading to decreased adherence; certainly, there are no evidence-based guidelines for which medications should be used and how for long to prevent poor outcomes.
Our study has several limitations, the largest being small sample size as prescription claims data was only available for 33 participants in our study population. Thus, our observations should be taken specifically in the context of informing future research design, particularly given large confidence intervals of our estimates of association. Our sample size did not permit us to stratify by disease severity or medication class. In addition, 85% of the Prescriptions Claims Sample was fully covered by Medicaid; this more uniform subpopulation may make it more difficult to detect demographic differences that lead to poor adherence and result in selection bias. Another limitation of our study is its retrospective nature which does not permit us to determine causality between medication adherence and health beliefs or poor outcomes. Recall biases may also be present owing to retrospective data collection via questionnaires. In terms of objective data collection, although prescription claims data is several steps removed from direct observation of ingestion or inhalation of medications, other studies have shown correlations between this commonly used method of calculating adherence and other objective measures such as serum drug levels, physiologic drug effect, and pill counts.20 Nevertheless, the limited amount of pharmacy refill data (300 ± 72 days) did not permit us to assess whether adherence changes over time (e.g. perhaps decreased adherence with increased time from NICU discharge.). Finally, we recognize that there may be unmeasured confounders of adherence (e.g., maternal depression21) that may affect our results.
Our study provides a survey of adherence in a sample of CLDP patients, unfortunately demonstrating that poor adherence to respiratory medications may be common in this population. Larger studies are needed to verify that poor adherence to chronic respiratory medications in this population does indeed lead to worse outcomes. Although it may be difficult to identify non-adherent patients based on their self-reported adherence or demographic characteristics, open dialogue between health care providers and parents about their concerns regarding their children’s medications and treatments may lead to improved adherence and ultimately decreased CLDP-related morbidities.
Acknowledgments
The authors wish to thank the families who participated in the Johns Hopkins Pediatric Pulmonary Registry, its research coordinator Beth Stewart, the research assistants who interacted with the families, SeEun Jennifer Choi and Tim Ryan for compiling socio-demographic data, and Drs. Brian McGinley and Maureen Lefton-Greif for their helpful comments. This work was supported by the Thomas Wilson Foundation (S.A.M. & J.M.C.), NIH HL089410 (S.O.O.), and the University of Pittsburgh Medical School Dean’s Summer Research Program (A.J.K.).
Funding Sources: Thomas Wilson Foundation; NIH, Number HL089410; University of Pittsburgh Medical School Dean’s Summer Research Program.
The funding sources have no role in the analysis or drafting of this manuscript.
Footnotes
Disclosure: All authors disclose that they have no financial interests in the subject of this manuscript.
Reference List
- 1.Heron M, Sutton PD, Xu J, Ventura SJ, Strobino DM, Guyer B. Annual summary of vital statistics: 2007. Pediatrics. 2010;125(1):4–15. doi: 10.1542/peds.2009-2416. [DOI] [PubMed] [Google Scholar]
- 2.Ehrenkranz RA, Walsh MC, Vohr BR, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353–1360. doi: 10.1542/peds.2005-0249. [DOI] [PubMed] [Google Scholar]
- 3.Lamarche-Vadel A, Blondel B, Truffer P, et al. Re-hospitalization in infants younger than 29 weeks’ gestation in the EPIPAGE cohort. Acta Paediatr. 2004;93(10):1340–1345. doi: 10.1080/08035250410032926. [DOI] [PubMed] [Google Scholar]
- 4.Smith VC, Zupancic JA, McCormick MC, et al. Rehospitalization in the first year of life among infants with bronchopulmonary dysplasia. J Pediatr. 2004;144(6):799–803. doi: 10.1016/j.jpeds.2004.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med. 2007;357(19):1946–1955. doi: 10.1056/NEJMra067279. [DOI] [PubMed] [Google Scholar]
- 6.Bauman LJ, Wright E, Leickly FE, et al. Relationship of adherence to pediatric asthma morbidity among inner-city children. Pediatrics. 2002;110(1 Pt 1):e6. doi: 10.1542/peds.110.1.e6. [DOI] [PubMed] [Google Scholar]
- 7.Kunkov S, Crain EF. Adherence and morbidity following emergency department care among inner-city children with asthma. J Asthma. 2010;47(5):545–550. doi: 10.3109/02770901003795323. [DOI] [PubMed] [Google Scholar]
- 8.Patterson JM, Wall M, Berge J, Milla C. Associations of psychosocial factors with health outcomes among youth with cystic fibrosis. Pediatr Pulmonol. 2009;44(1):46–53. doi: 10.1002/ppul.20925. [DOI] [PubMed] [Google Scholar]
- 9.Modi AC, Lim CS, Yu N, Geller D, Wagner MH, Quittner AL. A multi-method assessment of treatment adherence for children with cystic fibrosis. J Cyst Fibros. 2006;5(3):177–185. doi: 10.1016/j.jcf.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Bender B, Wamboldt FS, O’Connor SL, et al. Measurement of children’s asthma medication adherence by self report, mother report, canister weight, and Doser CT. Ann Allergy Asthma Immunol. 2000;85(5):416–421. doi: 10.1016/s1081-1206(10)62557-4. [DOI] [PubMed] [Google Scholar]
- 11.Apter AJ, Boston RC, George M, et al. Modifiable barriers to adherence to inhaled steroids among adults with asthma: it’s not just black and white. J Allergy Clin Immunol. 2003;111(6):1219–1226. doi: 10.1067/mai.2003.1479. [DOI] [PubMed] [Google Scholar]
- 12.Riekert KA, Butz AM, Eggleston PA, Huss K, Winkelstein M, Rand CS. Caregiver-physician medication concordance and undertreatment of asthma among inner-city children. Pediatrics. 2003;111(3):e214–e220. doi: 10.1542/peds.111.3.e214. [DOI] [PubMed] [Google Scholar]
- 13.Steiner JF. Can we identify clinical predictors of medication adherence… and should we? Med Care. 2010;48(3):193–195. doi: 10.1097/MLR.0b013e3181d51ddf. [DOI] [PubMed] [Google Scholar]
- 14.Liang KY, Zeger SL. Longitudinal Data-Analysis Using Generalized Linear-Models. Biometrika. 1986;73(1):13–22. [Google Scholar]
- 15.Burgess SW, Sly PD, Morawska A, Devadason SG. Assessing adherence and factors associated with adherence in young children with asthma. Respirology. 2008;13(4):559–563. doi: 10.1111/j.1440-1843.2008.01292.x. [DOI] [PubMed] [Google Scholar]
- 16.Marcus CL, Rosen G, Ward SL, et al. Adherence to and effectiveness of positive airway pressure therapy in children with obstructive sleep apnea. Pediatrics. 2006;117(3):e442–e451. doi: 10.1542/peds.2005-1634. [DOI] [PubMed] [Google Scholar]
- 17.Conn KM, Halterman JS, Lynch K, Cabana MD. The impact of parents’ medication beliefs on asthma management. Pediatrics. 2007;120(3):e521–e526. doi: 10.1542/peds.2006-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ponieman D, Wisnivesky JP, Leventhal H, Musumeci-Szabo TJ, Halm EA. Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Ann Allergy Asthma Immunol. 2009;103(1):38–42. doi: 10.1016/S1081-1206(10)60141-X. [DOI] [PubMed] [Google Scholar]
- 19.Yoos HL, Kitzman H, McMullen A. Barriers to anti-inflammatory medication use in childhood asthma. Ambul Pediatr. 2003;3(4):181–190. doi: 10.1367/1539-4409(2003)003<0181:btamui>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 21.Bartlett SJ, Krishnan JA, Riekert KA, Butz AM, Malveaux FJ, Rand CS. Maternal depressive symptoms and adherence to therapy in inner-city children with asthma. Pediatrics. 2004;113(2):229–237. doi: 10.1542/peds.113.2.229. [DOI] [PubMed] [Google Scholar]


