Abstract
Most candidates for hematopoietic stem cell transplantation (HSCT) lack a human leukocyte antigen (HLA)-identical sibling donor. Some patients may have a related donor with whom they are mismatched at 1 antigen/allele. It is not known whether such a match is preferable to a matched unrelated donor (MUD). We evaluated the outcomes (survival, relapse, nonrelapse mortality [NRM]) of all 28 patients with a single HLA antigen/allele mismatch identified through high-resolution HLA typing at HLA-A, -B, -C, -DRB1, and -DQB1, and all 318 patients with myeloid malignancies who received transplants from a 10/10 MUD treated during the same period of time at a single institution. Overall, outcomes for patients treated from a 1-antigen/allele mismatch related donor were significantly worse than from a MUD, primarily because of increased NRM. Overall survival (OS) rates at 3 years for 1-antigen/allele mismatched related donor and MUD transplant recipients were 19% and 45% (P =.007), and NRM rates were 40% and 26% (P =.05), respectively. Patients with class I mismatches appeared to have poorer OS than did patients with class II mismatches. A higher incidence of graft rejection was identified in the mismatched related donor group (P =.02). These results indicate that transplant outcomes are better with a MUD than with a 1 antigen/allele-mismatched related donor.
Keywords: Hematopoietic stem cell transplantation, HLA matched unrelated donors, 9/10 matched related donors, Class I HLA mismatch, Class II HLA mismatch
INTRODUCTION
Most candidates for hematopoietic transplantation lack a human leukocyte antigen (HLA)-identical sibling donor. Options for these patients are alternative donor transplants using a matched unrelated donor (MUD) or a mismatched related donor [1,2]. Some transplant candidates have a related donor mismatched by only 1 antigen or allele; however, it is uncertain whether such a donor would be preferable to a MUD.
Several studies have compared the outcomes of matched related donor (MRD) and MUD transplants [3–6]. Whereas smaller studies have shown equivalent outcomes for both donor categories, larger studies have shown better outcomes with MRD, primarily related to higher rates of nonrelapse mortality (NRM) and graft-versus-host disease (GVHD) for recipients of MUD grafts [7–9]. Many studies have compared outcomes between related and unrelated donors using intermediate- and/or high-resolution HLA typing at 3 loci or 4 loci (HLA-A, -B, -C, ±-DRB1), and the results emphasize the importance of extending high-resolution HLA typing to at least 4 HLA loci [10–12]. A few studies have compared transplant outcomes for patients treated from a 7/8 MRD with 8/8 MUD (HLA-A, -B, -C, -DRB1) and reported similar outcomes [13–15]. High-resolution HLA typing is now routinely performed at 5 loci (HLA-A, -B, -C, -DRB1, and -DQB1).
We retrospectively analyzed and compared the outcomes of patients who received transplants from either a MUD or a 1-antigen/allele mismatched related donor treated at a single institution.
METHODS
Study Population
All patients with myeloid leukemias who had received their first hematopoietic transplants from 1 HLA antigen/allele mismatch related donor between 1995 and 2009 were identified in the M.D. Anderson Cancer Center (MDACC) database. This group was compared with a cohort of patients who received an MUD transplant during the same period of time. All patients had intermediate/high-resolution HLA typing at all 5 loci either prospectively for those treated after 2002, or retrospectively if treated before 2002.
All 367 patients with myeloid malignancies identified were analyzed in this study: 318 received a MUD (10/10 allele match), and 49 were identified to have a 1-antigen/allele mismatch related donor transplant by 4-loci HLA typing (7/8 antigen/allele match). Of the 49 patients treated with mismatched related donors, 28 patients (57%) had 1 antigen/allele mismatched at HLA class I or II loci (or 9/10), 18 patients (37%) had 2 alleles mismatched (or 8/10), and 3 patients (6%) had 3 alleles mismatched (or 7/10). From the 28 patients with a 1-allele mismatch, 24 had class I mismatches at either HLA-A or -B locus, and 4 had class II mismatches at either HLA-DR or -DQ locus. All patients had provided written informed consent to undergo hematopoietic stem cell transplantation (HSCT). A retrospective data review protocol and a waiver of informed consent were approved by the institutional review board of MDACC for this study.
HLA Typing
Intermediate- or high-resolution HLA typing at HLA-A, -B, -C, -DRB1, and -DQB1 was performed for all samples either prospectively or on archived samples by polymerase chain reaction (PCR) amplification and oligonucleotide hybridization using molecular methods and commercial kits from Invitrogen (Carlsbad, CA), ELPHA (Dreieich, Germany), and/or One Lambda (Canoga Park, CA) that achieved intermediate resolution. The patients were also typed for these loci by high-resolution methods (PCR amplification and nucleotide sequencing) using SEQR Sequence Based Typing Kits (Abbott Park, IL). Additional high-resolution tests for selected loci were done in the donors for whom an allele-level mismatch could not be ruled out [16].
Statistical Analysis
Time-to-event was assessed starting on the day of transplantation. Actuarial overall survival (OS) and progression-free survival (PFS) were estimated by the method of Kaplan and Meier with death from any cause, disease progression, and death in the absence of disease progression considered the outcomes of interest, respectively [17]. The incidence of disease progression, NRM, and GVHD were estimated using the cumulative incidence method to account for competing events [18]. Death in the absence of disease progression, disease progression, and death without GVHD were considered competing events for the respective outcomes. Comparison of outcomes was performed by univariate analysis using the Cox proportional hazards model [19]. Because of sample size limitations, we could not perform multivariate analyses to evaluate the independent effect of type of donor on transplantation outcome. Instead, we performed a matched analysis to adjust for the factors most commonly correlated with outcome, including diagnosis, disease status at transplantation, intensity of the conditioning regimen, and patient age (within a decade). The matching was performed manually at a ratio of 1:1 for recipients of a related and MUD. If more than 1 match was available, the 1 with the closest transplant date was selected. Statistical significance was defined as P ≤ .05. Analysis was performed using STATA software: Release 9.0. (Stata Corp, College Station, TX).
RESULTS
Outcomes for Patients Treated with a 7/8 HLA MRD
We initially evaluated outcomes in the mismatched related donor group based on 4 loci including high-resolution typing at the HLA-C locus only. Specifically, we compared outcomes of patients treated with related donors and 1-antigen/allele mismatch (or 7/8 MRD, n = 49) with outcomes of patients who received a 10/10 MUD transplant (n =318). In univariate analyses, outcomes for patients who received 7/8 MRD transplants were worse at 3 years, with an OS rate of 24% (95% confidence interval [CI] 12–38) and a PFS rate of 22% (95% CI 11–36) compared with 45% (95% CI 38–51) and 42% (95% CI 36–48) for the MUD group (hazard ratio [HR]OS = 1.7; 95% CI 1.2–2.5; P = .05 and HRPFS= 1.8; 95% CI 1.2–2.6; P = .03).
Outcomes for Patients Treated with 9/10 HLA MRD
To further assess the impact of high-resolution typing on outcomes, we performed a subset analysis on 28 of the 49 patients described above who had received a transplant from a 9/10 MRD based on 5 loci (including -DQB1) typing. Outcomes of these patients were compared with outcomes of patients who had received a MUD graft (n = 318), both in unmatched and matched analyses. Only 24 of 28 9/10 MRD patients could be matched and included in the comparison with the MUD transplant patients based on age, disease status, conditioning regimen, and source of stem cells. Characteristics and results of the matched and unmatched analyses are presented in Tables 1 and 2.
Table 1.
Patients’ Characteristics
| Unmatched Analysis
|
Matched Analysis
|
|||
|---|---|---|---|---|
| 9/10 Related (n = 28) | 10/10 MUD (n = 318) | 9/10 Related (n = 24) | 10/10 MUD (n = 24) | |
| Age | ||||
| Median (range) | 47 (12–69) | 53 (13–75) | 52 (14–69) | 51 (23–68) |
| P* | .08 | |||
| Diagnosis (number, %) | ||||
| AML/MDS | 23 (82%) | 266 (84%) | 21 (88%) | 22 (92%) |
| CML/MPD | 5 (18%) | 52 (16%) | 3 (12)% | 2 (8%) |
| P* | .5 | |||
| Disease status (number, %) | ||||
| Active disease | 16 (57%) | 187 (59%) | 14 (58%) | 14 (58%) |
| First relapse refractory | 4 | 4 | ||
| First relapse untreated | 5 | 5 | ||
| Primary induction failure | 4 | 4 | ||
| Untreated | 1 | 1 | ||
| Remission | 12 (43%) | 131 (41%) | 10 (42%) | 10 (42%) |
| First complete remission | 4 | 4 | ||
| Second complete remission | 4 | 4 | ||
| First chronic phase | 1 | 1 | ||
| Second chronic phase | 1 | 1 | ||
| P* | .90 | |||
| Conditioning (number, %) | ||||
| Ablative | 21 (75%) | 201 (63%) | 17 (71%) | 17 (71%) |
| RIC | 7 (25%) | 117 (37%) | 7 (29%) | 7 (29%) |
| P* | .20 | |||
| Stem cell source (number, %) | ||||
| Bone marrow | 20 (70%) | 185 (58%) | 17 (71%) | 17 (71%) |
| Peripheral blood | 8 (30%) | 133 (42%) | 7 (29%) | 7 (29%) |
| P* | .20 | |||
| ATG (number, %) | ||||
| Yes | 19 (68%) | 304 (96%) | 19 (79%) | 24 (100%) |
| No | 9 (32%) | 14 (4%) | 9 (32%) | 0 |
| P* | .02 | <.01 | ||
| Pentostatin (number, %) | ||||
| Yes | 3 (11%) | 45 (14%) | 3 (12%) | 3 (12%) |
| No | 25 (89%) | 273 (86%) | 21 (88%) | 21 (88%) |
| P* | .40 | |||
| Median transplant year (range) | 2004 (1996–2009) | 2006 (2001–2009) | 2004 (1999–2009) | 2005 (2001–2009) |
| P | .30 | |||
MUD indicates matched unrelated donor; RIC, reduced-intensity conditioning; ATG, antithymocyte globulin; AML, acute myeloid leukemia; MDS, myelodysplastic syndromes; CML, chronic myeloid leukemial; MPD, myeloproliferative diseases.
P is for comparison of patients characteristics of the 9/10 matched related versus 10/10 matched unrelated patients.
Table 2.
Treatment Outcomes for 346 Patients Treated from Matched Unrelated (n = 318) and 9/10 Matched Related (n = 28) Donors
| Outcomes at 3 Years Posttransplant | Unmatched Analysis
|
Matched Analysis
|
||
|---|---|---|---|---|
| 9/10 Related (n = 28) | 10/10 MUD (n = 318) | 9/10 Related (n = 24) | 10/10 MUD (n = 24) | |
| Median follow-up in survivors (months, range) | 56 (6–143) | 22 (2–93) | 32 (6–59) | 49 (10–93) |
| Graft failure | ||||
| Primary | 2 (7%) | 0 | 2 (8%) | 0 |
| Secondary | 4 (14%) | 0 | 4 (17%) | 0 |
| P† | .02 | |||
| Overall survival | ||||
| Median (months) | 6 | 18 | 6 | 18 |
| OS (95% CI) | 19% (7–35) | 45% (38–51) | 13% (3–30) | 38% (17–59) |
| Hazard ratio | 1.8 | Reference | 2.1 | Reference |
| 95% CI | (1.2–2.9) | (1.0–4.2) | ||
| P† | .007 | .04 | ||
| Progression-free survival | ||||
| Median (months) | 4 | 15 | 4 | 7 |
| PFS† (95% CI) | 19% (7–36) | 42% (36–48) | 13% (3–31) | 31% (14–51) |
| Hazard ratio | 1.8 | Reference | 1.6 | Reference |
| 95% CI | (1.2–2.9) | (0.85–3.1) | ||
| P† | .006 | .10 | ||
| Progression | ||||
| Cumulative incidence (95% CI) | 40% (25–64) | 25% (20–30) | 43% (27–69) | 39% (23–65) |
| Hazard ratio | 2.1 | Reference | 1.4 | Reference |
| 95% CI | (1.1–3.9) | (0.5–3.4) | ||
| P† | .02 | .50 | ||
| Nonrelapse mortality | ||||
| Cumulative incidence (95% CI) | 40% (25–63) | 26% (21–32) | 43% (27–68) | 25% (13–50) |
| Hazard ratio | 1.9 | Reference | 2.7 | Reference |
| 95% CI | (1.0–3.6) | (0.9–7.9) | ||
| P† | .05 | .07 | ||
| Grade II–IV acute GVHD | ||||
| 100 days cumulative incidence | 27%* (15–52) | 38% (32–44) | 23%* (10–49) | 42% (26–67) |
| Hazard ratio | 0.7 | Reference | 0.5 | Reference |
| 95% CI | (0.3–2.5) | (0.2–1.5) | ||
| P† | .40 | .10 | ||
| Causes of death | n = 24 | n = 154 | n = 22 | n = 13 |
| Graft failure | 5 (21%) | 4 (3%) | 5 (23%) | 0 (0%) |
| Infection | 3 (12.5%) | 31 (20%) | 2 (9%) | 2 (15%) |
| Relapse | 9 (37.5%) | 76 (49%) | 9 (41%) | 7 (54%) |
| GVHD | 2 (8.3%) | 23 (15%) | 2 (9%) | 3 (23%) |
| Organ failure | 2 (8.3%) | 13 (8.5%) | 1 (4.5%) | 1 (8%) |
| Other | 3 (12.5%) | 7 (4.5%) | 3 (13.6%) | — |
MUD indicates matched unrelated donor; OS, overall survival; PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; GVHD, graft-versus-host disease.
Excluding patients with primary graft failure.
P is for comparison of outcomes between the 9/10 matched related versus 10/10 matched unrelated patients.
Patient Characteristics
Unmatched analysis
Patient characteristics for the 9/10 MRD and MUD groups were comparable except for a nonsignificant younger age in the MRD group (47 years versus 53 years, P =.08) and a significantly higher proportion of patients who received antithymocyte globulin (ATG) as part of the conditioning regimen in the MUD group (96% versus 68%, P = .02).
The majority of patients in both the 9/10 MRD (82%) and MUD (84%) groups had acute myeloid leukemia/myelodysplastic syndrome with a similar proportion of patients having active disease at the time of transplantation (57% and 59%, respectively). Two-thirds of patients in both the 9/10 MRD and MUD groups had received ablative conditioning. The median year of transplantation was also similar (2004 for the 9/10 MRD group and 2006 for the MUD group). A small proportion of patients in both groups received pentostatin as part of a clinical trial aimed at preventing acute GVHD (aGVHD) (Table 1).
Matched analysis
Patients who received transplants from 9/10 MRD and MUD were matched 1:1 for the characteristics mentioned above. The only significant difference between the 2 groups was the use of ATG in the conditioning regimen, as all patients in the unrelated group had received ATG compared with 79% in the related group (P <.01) (Table 1).
Outcomes for 9/10 HLA Matched Related versus 10/10 MUD Transplants
Engraftment, NRM, aGVHD, and progression
Graft failure was more common in patients treated from 1-allele mismatch related donors than from MUD. The incidences of primary graft failure for the 9/10 MRD group in the unmatched and matched analyses were 7% and 8%, respectively, whereas none of the MUD transplant recipients had a primary (or secondary) graft failure (P = .02) (Table 2).
The incidence of NRM was also higher in the 9/10 MRD than in the MUD group, with a 3-year cumulative incidence rate of 40% versus 26% in the unmatched analysis (HR = 1.9; 95% CI 1.0–3.6; P = .05) and 43% versus 25% in the matched analysis (HR =2.7; 95% CI 0.9–7.9; P =.07; Table 2). The cumulative incidence of grade II–IV aGVHD appeared higher in MUD transplant recipients; however, this did not reach statistical significance (P = .10) (Table 2). Because ATG is used primarily to prevent aGVHD, and because all patients who had graft rejection in the 9/10 MRD group received ATG as did the great majority of the patients in the 10/10 MUD group, differences in ATG use between the 9/10 MRD and MUD groups noted in this study are unlikely to be responsible for worse outcomes seen with the use of 9/10 MUD grafts. Moreover, differences between the 2 groups could not be explained by differences in conditioning as 181/318 patients (57%) received myeloablative fludarabine and busulfan (FluBu) and 98/318 (31%) received fludarabine and melphalan (FluMel) in the MUD groups compared with 14/28 (50%) FluBu and 7/28 (25%) FluMel in the 9/10 MRD group (P = .50 for both). A nonsignificant higher rate of disease progression between the 9/10 MRD compared with the MUD group was noted in the matched analysis (P = .5) (Table 2).
Survival and causes of death
After a median follow-up of 56 months (range: 6–143) in recipients of 9/10 MRD grafts and 22 months (range: 2–93 months) in recipients of MUD grafts, a total of 24 deaths had occurred in the first group and 154 deaths in the second group. The majority of these deaths occurred within 2 years after transplantation, including 79% in the related group and 96% in the MUD group. Significantly better 3-year OS and PFS were found in the MUD than in the 9/10 MRD transplant groups in the unmatched analysis. Better OS in the MUD group was also noted in the matched analysis at 3 years; however, differences in PFS did not reach statistical significance, likely because differences between the 2 groups were related to NRM rather than relapse (Table 2). In the unmatched analysis, median survival was 6 months for the 9/10 MRD group versus 18 months for the 10/10 MUD group. Three-year OS rates were 19% and 45% in 9/10 MRD and MUD groups, respectively (HR = 1.8; 95% CI 1.2–2.9; P = .007). Similarly, 3-year PFS rates were more favorable in the MUD group, with 42% versus 19% (HR = 1.8; 95% CI 1.2–2.9; P = .006) in the unmatched analysis.
Because all but 1 of the MUD transplants included in this study (n =317, 99%) were performed after 2001 compared with only 20 out of 28 (71%) of 9/10 MRD transplants, we compared outcomes for the 2 groups transplanted after this year. Our data showed significant differences in survival, OS was 44% (38–51) in the MUD versus 22% (6–42) in the 9/10 MRD group (HR = 1.7, 95% CI 0.98–2.8; P = .06), and PFS 42% (36–49) versus 22% (7–43) (HR = 1.7; 95% CI 1.03–2.9; P = .04) for patients treated after the year 2001.
Causes of death in the study patients are presented in Table 2. In the unmatched analysis, 21% of the patients had graft failure as their primary cause of death in the MRD group versus 3% in the MUD group. This difference was even greater in the matched analysis (23% versus 0%, respectively). Taken together, these results suggest that transplant outcomes for patients treated with a 9/10 MRD are worse than those for patients treated with 10/10 MUD transplants.
However, the poorer survival in the 9/10 MRD group could be because 24 of 28 patients in this group had class I mismatches. For a subset analysis, we compared the 24 patients with class I mismatches and 11 patients with class II mismatches (4 patients with a single class II mismatch at -DRB1 and 7 patients with both -DRB1 and -DQB1 mismatches). Median OS for class I and class II allele mismatches was 5 months and 44 months, and actuarial 2-year OS rates were 29% versus 54% (P = .30). Compared with the 10/10 MUD group, patients treated with class II mismatches (HLA-DRB1 ±-DQB1) in the MRD group had similar outcomes, with an actuarial PFS at 2 years of 45% in the MRD group and 46% in MUD group (P = .80), and no differences in incidence of aGVHD, relapse, or NRM. However, outcomes for 9/10 MRD transplant patients with class I mismatches (n = 24) were significantly worse than outcomes in those with MUD transplants (Figure 1). In the unmatched analysis, the actuarial OS rate at 2 years was 27% for the 9/10 MRD group and 48% for the MUD transplant group (HR =1.9; 95% CI 1.1–3.1; P =.01). When adjusted for disease status at transplant, the difference remained statistically significant (P = .03).
Figure 1.
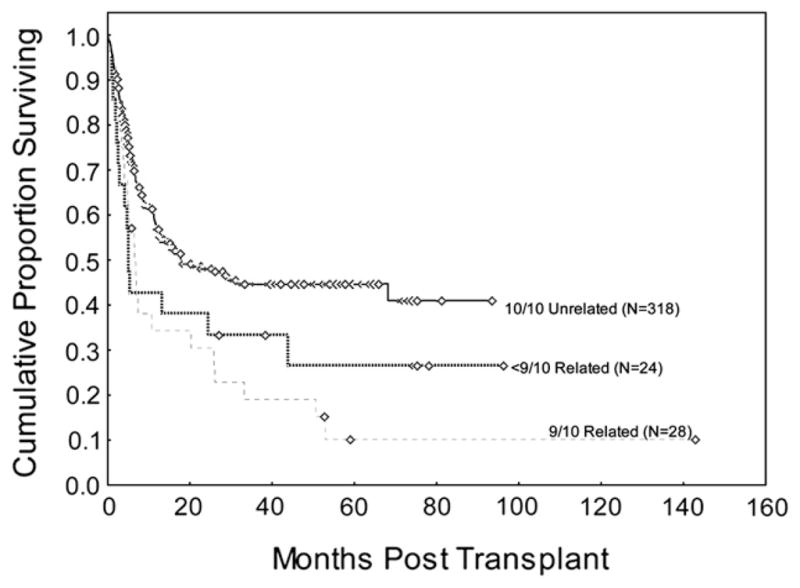
Difference in 3-year OS between matched unrelated donor (n = 318) and 1-antigen/allele mismatch related donor transplants (n = 28) at M.D. Anderson Cancer Center by high-resolution HLA typing at HLA-A, -B, -C, -DRB1, and -DQB1 (P =.04).
DISCUSSION
HLA typing has improved over time, leading to improved outcomes for patients treated with hematopoietic stem cell transplantation (HSCT). Here, we compared the outcomes of patients treated with 9/10 MRD grafts having a single mismatched antigen/allele with outcomes of those treated with MUD grafts. We have found that patients treated with mismatched related grafts fared significantly worse than those treated with MUD transplants.
HLA 1-antigen/allele mismatch related donors are relatively uncommon, and data from a small number of studies have shown similar outcomes between 7/8 MRD and 8/8 MUD groups [13–15]. Many centers have preferentially used a 9/10 related donor for transplantation because of the immediate availability and because the potentially larger number of minor antigen mismatches in unrelated individuals could negatively influence outcomes [20,21].
Previous studies have identified a higher risk of graft rejection with increasing numbers of HLA mismatches [2,9,14,22]. Leung et al. [23] suggested that a single mismatch in class I antigens (HLA-A or -B) may not be better tolerated than a disparity at HLA-DRB1. Morishima and colleagues [24] also reported that, after assessment with high-resolution HLA typing, disparities at HLA-A and -B are associated with more graft failure, GVHD, and worse survival. In unrelated donor setting, Flomenberg et al. [25] found that HLA mismatches at HLA-A, -B, -C and -DRB1 adversely affected outcomes, whereas mismatches at -DQB1 and -DPB1 did not.
Our findings suggest that differences in outcomes can be explained by the higher rates of NRM in the mismatched related group, predominately because of a higher incidence of graft rejection, because GVHD rates were not significantly different. In order to explain differences in graft rejection between the 9/10 MRD patients that engrafted and those that did not, we evaluated the incidence of different factors potentially associated with graft rejection. No significant differences in the use of ATG or in ABO blood group mismatch were found between these 2 groups. In addition, 2 of the 6 patients who had primary graft failure were tested for donor-specific anti-HLA antibodies, and they were negative [26].
Contrary to our findings, other investigators have shown equivalent outcomes between 7/8 MRD donors and 8/8 MUD grafts. This difference may result from the fact that our study contained more patients with class I mismatches, which appear to have higher NRM and worse prognosis. Conflicting data exist regarding -DRB1 mismatches. Some studies have suggested that outcomes are worse with such mismatches, whereas other studies did not confirm this finding [2,9,10,14, 23–25]. Our results suggest that mismatches at the -DRB1 locus are better tolerated than single class I mismatches. Furthermore, similarly to data from MUD setting, we have found that -DQB1 mismatches are better tolerated and not associated with worse outcomes when this mismatch is added to either a class I or -DRB1 mismatch in the 9/10 MRD patients [10].
Limitations of the present study include its retrospective nature and the relatively small number of patients. However, we identified a marked difference in outcomes between 1-antigen/allele MRD and 10/10 MUD transplants. Future studies will need to evaluate outcomes for patients with class I versus class II 1-antigen/allele mismatches using HLA typing at 5 loci, because our results suggest that patients with class II mismatches have outcomes comparable with outcomes of those having 10/10 MUD transplants and fare significantly better than those with class I mismatches.
In conclusion, our results suggest that, using the standard approach, outcomes are worse for recipients of 1-antigen/allele mismatch related donor hematopoietic stem cell transplants, and a 10/10 MUD, should be the preferred choice for patients who lack a matched-related donor. However, newer approaches to haploidentical transplantation using high-dose post-transplant cyclophosphamide have improved engraftment, decreased NRM, and improved outcomes, suggesting that patients receiving transplants from a 9/10 MRD, at least with a class I mismatch, should be treated on protocols similar to haploidentical transplants [27].
Acknowledgments
We thank Maude Veech and Dianne Hackett for excellent manuscript proofreading.
Footnotes
Financial disclosure: The authors have no relevant conflicts of interest to declare.
AUTHORSHIP STATEMENT
S.O.C. contributed to the study design, data collection, and interpretation of the results, and wrote the manuscript. R.M.S. contributed with statistical analysis, and reviewed and approved the manuscript. G.R. and P.A.P. contributed with data collection, and reviewed and approved the manuscript. F.A., P.C., and M.F.-V. contributed with HLA typing of samples, and reviewed and approved the manuscript. B.S.A., P.K., and U.P. contributed with patient accrual, and reviewed and approved the manuscript. R.E.C. contributed to the study design, and reviewed and approved the manuscript. M.D. contributed to the study design, data collection, interpretation of results, reviewed and approved the manuscript.
References
- 1.Copelan E. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Sasazuki T, Juji T, Morishima Y, et al. Effect of matching of class I HLA alleles on outcome after transplantation of hematopoietic stem cells from an unrelated donor. Japan Marrow Donor Program. N Eng J Med. 1998;339:1177–1185. doi: 10.1056/NEJM199810223391701. [DOI] [PubMed] [Google Scholar]
- 3.Kiehl MG, Kraut L, Schwerdtfeger R, et al. Outcome of allogeneic hematopoietic stem-cell transplantation in adult patients with acute lymphoblastic leukemia: no difference in related compared with unrelated transplant in first complete remission. J Clin Oncol. 2004;22:2816–2825. doi: 10.1200/JCO.2004.07.130. [DOI] [PubMed] [Google Scholar]
- 4.Yakoub-Agha I, Mesnil F, Kuentz M, et al. Allogeneic marrow stem cell transplantation from human leukocyte antigen-identical sibling versus human leukocyte antigen-allelic-matched unrelated (10/10) in patients with standard-risk hematologic malignancy: a prospective study from the French Society of Bone Marrow Transplantation and Cell Therapy. J Clin Oncol. 2006;24:5695–5702. doi: 10.1200/JCO.2006.08.0952. [DOI] [PubMed] [Google Scholar]
- 5.Moore J, Nivison-Smith I, Goh K, et al. Equivalent survival for sibling and unrelated donor allogeneic stem cell transplantation for acute myelogenous leukemia. Biol Blood Marrow Transplant. 2007;13:601–607. doi: 10.1016/j.bbmt.2007.01.073. [DOI] [PubMed] [Google Scholar]
- 6.Schetelig J, Bornhauser M, Schmid C, et al. Matched unrelated or matched sibling donors result in comparable survival after allogeneic stem-cell transplantation in elderly patients with acute myeloid leukemia: a report from the cooperative German Transplant Study Group. J Clin Oncol. 2008;26:5183–5191. doi: 10.1200/JCO.2007.15.5184. [DOI] [PubMed] [Google Scholar]
- 7.Petersdorf EW, Hansen JA, Martin PJ, et al. Major-histocompatibility-complex class I alleles and antigens in hematopoietic-cell transplantation. N Engl J Med. 2001;345:1794–1800. doi: 10.1056/NEJMoa011826. [DOI] [PubMed] [Google Scholar]
- 8.Weisdorf DJ, Anasetti C, Antin JH, et al. Allogeneic bone marrow transplantation for chronic myelogenous leukemia: comparative analysis of unrelated versus matched sibling donor transplantation. Blood. 2002;99:1971–1977. doi: 10.1182/blood.v99.6.1971. [DOI] [PubMed] [Google Scholar]
- 9.Arora M, Weisdorf DJ, Spellman SR, et al. HLA-identical sibling compared with 8/8 matched and mismatched unrelated donor bone marrow transplant for chronic phase chronic myeloid leukemia. J Clin Oncol. 2009;27:1644–1652. doi: 10.1200/JCO.2008.18.7740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–4583. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 11.Weisdorf D, Spellman S, Haagenson M, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biol Blood Marrow Transplant. 2008;14:748–758. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Szydlo R, Goldman JM, Klein JP, et al. Results of allogeneic bone marrow transplants for leukemia using donors other than HLA-identical siblings. J Clin Oncol. 1997;15:1767–1777. doi: 10.1200/JCO.1997.15.5.1767. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa W, Lipton JH, Messner HA, et al. Influence of one human leukocyte antigen mismatch on outcome of allogeneic bone marrow transplantation from related donors. Hematology. 2003;8:27–33. doi: 10.1080/1024533031000072054. [DOI] [PubMed] [Google Scholar]
- 14.Kanda Y, Chiba S, Hirai H, et al. Allogeneic hematopoietic stem cell transplantation from family members other than HLA-identical siblings over the last decade (1991–2000) Blood. 2003;102:1541–1547. doi: 10.1182/blood-2003-02-0430. [DOI] [PubMed] [Google Scholar]
- 15.Ottinger HD, Ferencik S, Beelen DW, et al. Hematopoietic stem cell transplantation: contrasting the outcome of transplantations from HLA-identical siblings, partially HLA mismatched related donors, and HLA-matched unrelated donors. Blood. 2003;102:1131–1137. doi: 10.1182/blood-2002-09-2866. [DOI] [PubMed] [Google Scholar]
- 16.Parmar S, de Lima M, Zou T, et al. Donor-recipient mismatches in MHC class I chain-related gene A in unrelated donor transplantation lead to increased incidence of acute graft-versus-host disease. Blood. 2009;114:2884–2887. doi: 10.1182/blood-2009-05-223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan EL, Meier P. Non parametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, et al. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 19.Cox RD. Regression analysis and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 20.Ottinger H, Grosse-Wilde M, Schmitz A, Grosse-Wilde H. Immunogenetic marrow donor search for 1012 patients: a retrospective analysis of strategies, outcome and costs. Bone Marrow Transplant. 1994;14:S34–38. [PubMed] [Google Scholar]
- 21.Goulmy E, Schipper R, Pool J, et al. Mismatches of minor histocompatibility antigens between HLA-identical donors and recipients and the development of graft-versus-host disease after bone marrow transplantation. N Engl J Med. 1996;334:281–285. doi: 10.1056/NEJM199602013340501. [DOI] [PubMed] [Google Scholar]
- 22.Anasetti C, Amos D, Beatty PG, et al. Effect of HLA compatibility on engraftment of bone marrow transplants in patients with leukemia or lymphoma. N Engl J Med. 1989;320:197–204. doi: 10.1056/NEJM198901263200401. [DOI] [PubMed] [Google Scholar]
- 23.Leung WH, Turner V, Richardson SL, et al. Effect of HLA class I or class II incompatibility in pediatric marrow transplantation from unrelated and related donors. Hum Immunol. 2001;62:399–407. doi: 10.1016/s0198-8859(01)00220-8. [DOI] [PubMed] [Google Scholar]
- 24.Morishima Y, Sasazuki T, Inoko H, et al. The clinical significance of human leukocyte antigen (HLA) allele compatibility in patients receiving a marrow transplant from serologically HLA-A, HLA-B and HLA-DR matched unrelated donors. Blood. 2002;99:4200–4206. doi: 10.1182/blood.v99.11.4200. [DOI] [PubMed] [Google Scholar]
- 25.Flomenberg N, Baxter-Lowe LA, Confer D, et al. Impact of HLA class I and class II high resolution matching on outcomes of unrelated donor bone marrow transplantation: HLA-C mismatching is associated with a strong adverse effect on transplantation outcome. Blood. 2004;104:1923–1930. doi: 10.1182/blood-2004-03-0803. [DOI] [PubMed] [Google Scholar]
- 26.Ciurea SO, de Lima M, Cano P, et al. High risk of graft failure with anti-HLA antibodies undergoing haploidentical stem-cell transplantation. Transplantation. 2009;88:1019–1024. doi: 10.1097/TP.0b013e3181b9d710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciurea SO, de Lima M, Kebriaei P, et al. Safety of T-cell replete haploidentical stem cell transplantation using fludarabine, melphalan and thiotepa conditioning and high-dose post transplant cyclophosphamide. Biol Blood Marrow Transplant. 2010;16:s218. (abstract #163) [Google Scholar]


