Abstract
A high risk of regimen-related toxicity with allogeneic hematopoietic stem cell transplantation (allo-HSCT) limits this potentially curative treatment for patients with a left ventricular ejection fraction (LVEF) of ≥50%. We evaluated the frequency of cardiac complications and 100-day nonrelapse mortality (NRM) in 56 patients with a LVEF of ≤45%, who received allo HCTat our institution. The results were retrospectively compared with a matched control group with LVEF of ≥50%, which received an allogeneic stem cell transplantation (allo-SCT). After a median follow-up of 29 months in the study group, grade ≥2 cardiac complications were seen in 7 of 56 (12.5%) patients and cumulative incidence of 100-day NRM was 12.5% with no deaths from cardiac causes. In contrast, after a median follow-up of 49 months in the control group, grade >2 cardiac complications were seen in 19 of 161 patients (11.8%; P = 1.00) and cumulative incidence of 100-day NRM was 14.9% (P =.82). The presence of at least 1 of the 7 pretransplant cardiac risk factors (past history of smoking, hypertension, hyperlipidemia, coronary artery disease, arrhythmia, prior myocardial infarction, and congestive heart failure) was associated with a higher cardiac complication rate in the study group (P = .03). In conclusion, selected patients with a LVEF of ≤45% can safely receive allo-HCT without a significant increase in cardiac toxicity or NRM.
Keywords: Allogeneic hematopoietic stem cell transplantation, Low ejection fraction
INTRODUCTION
High-dose chemotherapy followed by an allogeneic hematopoietic stem cell transplant (allo-HSCT) is potentially curative for various benign and malignant diseases [1]. Because of a high risk of nonrelapse mortality (NRM) and other life-threatening complications, including cardiac toxicity, only patients with adequate vital organ functions are considered for this procedure [2–6]. Accordingly, patients with a left ventricular ejection fraction (LVEF) of <50% are considered ineligible for allo-HSCT to exclude those at a higher risk of NRM from cardiac causes [2–6]. However, the validity of this pretransplant cardiac assessment as a predictor for cardiac complications and/or mortality is not established, and may deny a potentially curative treatment to patients with no alternate therapeutic options.
Bidimensional echocardiogram (2D-echo) or multi-gated acquisition cardiac (MUGA) scan are useful tools to evaluate LVEF, a surrogate marker of cardiac function [7]. Two-dimensional (2D) echocardiography allows real-time imaging of the heart and its various structures using ultrasonic waves. Estimation of the LVEF by 2D echocardiography can be done either qualitatively by visual inspection of global and regional function or quantitatively, using geometric assumptions regarding the shape of the LV cavity. 2D echocardiography has several shortcomings, including interobserver and intraobserver variability, limited diagnostic value in patients with poor acoustic windows, such as obese individuals, patients with hyperinflated lungs because of obstructive lung diseases, and patients with musculoskeletal deformities like kyphosis or pectus excavatum [7].
We performed this retrospective analysis to study the safety of allo-HSCT in patients with low LVEF (≤45%), and to assess its impact on post-allo HSCT cardiac complications and NRM. The outcomes were retrospectively compared with a matched control group undergoing an allogenic stem cell transplantation (allo-SCT) with a normal LVEF (≥50%).
MATERIALS AND METHODS
Patients
We reviewed our database for patients with a LVEF of 45% or lower, who received allo-HSCT between January of 2000 and February of 2006 at the University of Texas, M.D. Anderson Cancer Center. A total of 56 patients were eligible for this analysis. LVEF was measured within 30 days pretransplant, either by a 2D-echo or a MUGA scan. Patients provided informed consent to receive allo-HSCT in accordance with the Declaration of Helsinki. This retrospective study was approved by the institutional review board. We grouped the patients into high-, intermediate-, and low-risk categories, based on the disease status at allo-HSCT (Table 1).
Table 1.
Patient and Disease Characteristics
| Characteristic | Study Group
|
Control
|
P-Value |
|---|---|---|---|
| Number n = 56 (%) |
Number N = 161 (%) |
||
| Sex | |||
| Male | 40 (71) | 95 (59) | .11 |
| Female | 16 (29) | 66 (41) | |
| Median age (years) | 43.5 (range: 18–70) | 51 (range: 20–71) | .01 |
| Diagnosis | |||
| AML/MDS | 23 (41) | 69 (43) | .87 |
| ALL | 9 (16) | 18 (11) | |
| Lymphoma | 12 (21) | 38 (24) | |
| Hodgkin disease | 4 (7) | 12 (7) | |
| CML | 3 (5) | 11 (7) | |
| CLL | 1 (2) | 5 (3) | |
| Multiple myeloma | 2 (4) | 8 (5) | |
| Systemic sclerosis | 2 (4) | 0 (0) | |
| Disease status* | |||
| Low risk | 9 (16) | 23 (14) | .75 |
| Intermediate risk | 14 (25) | 49 (30) | |
| High risk | 33 (59) | 89 (55) | |
| Donor type | |||
| Related | 28 (50) | 91 (57) | .43 |
| HLA-matched | 23 (41) | 85 | |
| HLA-mismatched | 5 (9) | 6 | |
| Unrelated | 28 (50) | 70 (43) | |
| HLA-matched | 23 (41) | 62 | |
| HLA-mismatched | 5 (9) | 8 | |
| Preparative regimens | |||
| Myeloablative, | 21 (37) | 59 | 1.00 |
| RIC | 35 (62) | 102 | |
| Pre-allo-HSCT LVEF (%) | |||
| >50 | 0 | 161 | .0001 |
| ≤45 | 25 (45) | ||
| ≤40 | 21 (37) | ||
| ≤35 | 10 (18) | ||
| Risk factors for cardiac complications† | |||
| Yes | 35 (62.5) | 97 (60) | .87 |
| No | 21 (37.5) | 64 (40) | |
| Cyclophosphamide in preparative regimen | |||
| Yes | 17 (30) | 39 (24) | .37 |
| No | 39 (70) | 122 (76) | |
RIC indicates reduced-intensity conditioning; AML, acute myelogenous leukemia; ALL, acute lymphoblastic leukemia; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; allo-HSCT, allogeneic hematopoietic stem cell transplantation; LVEF, left ventricular ejection fraction; MDS, myelodysplastic syndromes.
Low-risk: CML chronic phase #1, CR1 for all other diseases; Intermediate-risk: CML in equal to or greater than second chronic phase, CR2 or beyond for all other diseases; High risk: CML in accelerated or blast phase; relapsed or refractory disease for all other diseases.
Risk factors include smoking, hypertension, hyperlipidemia, coronary artery disease, arrhythmia, myocardial infarction, or congestive heart failure.
Risk Factors
We studied 7 risk factors (history of smoking, hypertension, hyperlipidemia, coronary artery disease, cardiac arrhythmias, prior acute myocardial infarction, and congestive heart failure [CHF]) that may increase the incidence of posttransplant cardiac complications and mortality. Thirty-five patients (62%) in the study group had 1 or more of these risk factors prior to allo-HSCT.
Control Group
We compared their outcome of the study group with a control group of 161 patients, who received allo-SCT at the same time, and were matched for age, diagnosis, risk factors and disease status. Ninety-seven patients in the control group (60%; P = .87) had 1 or more cardiac risk factors.
Endpoints
Primary endpoints were grade 2 or higher cardiac complications and 100-day NRM. Cardiac complications were defined according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events, Version 3.0 (CTCAE v3.0) [8]. These included grade ≥2 cardiac complications under the categories of arrhythmia, CHF, and cardiac ischemia (Table 2). NRM was defined as death occurring in the absence of progression or relapse of underlying disease. The cumulative incidence method was used to estimate NRM considering death attributed to underlying disease as a competing risk; and the risk of cardiac toxicity considering death in the absence of cardiac toxicity as a competing risk. Secondary endpoint was 3-year actuarial overall survival (OS) estimated by the method of Kaplan and Meier. Impact of risk factors on the outcome was evaluated with univariate analysis using the Cox’s proportional hazards model. All P values are 2 sided. Statistical analyses were carried out using STATA 9.0 software.
Table 2.
Grading of Cardiac Complications after allo-HSCT*
| Cardiac Complication | Grade
|
||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Arrhythmia | Asymptomatic, intervention not indicated | Nonurgent medical intervention indicated | Incompletely controlled, medically or with device (eg, pacemaker) | Life-threatening (eg, associated with CHF, hypotension, syncope, shock | Death |
| Congestive Heart Failure | Asymptomatic diagnostic finding; intervention not indicated | Asymptomatic, intervention indicated | Symptomatic CHF responsive to intervention | Refractory CHF, poorly controlled; intervention such as ventricular assist device or heart transplant indicated | Death |
| Cardiac Ischemia | Asymptomatic arterial narrowing without ischemia | Asymptomatic and testing suggesting ischemia; stable angina | Symptomatic; testing consistent with ischemia; unstable angina; intervention needed | Acute myocardial infarction | Death |
CHF indicates congestive heart failure; allo-HSCT, allogeneic hematopoietic stem cell transplantation.
Adapted from Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0 (CTCAE v3.0), DCTD, NCI, NIH, DHHS.
RESULTS
Patient characteristics for the study and the control groups are summarized in Table 1. Median age was 43.5 years (range: 18–70 years) for the study group and 51 years (20–71 years) for the control group. As shown in Table 1, other than LVEF (P = .0001) there was no significant difference between the study and the control groups in terms of diagnosis, disease status, donor type, preparative regimen, or cardiac risk factors. Baseline LVEF was measured by either 2D-echo or a MUGA scan and ranged from 20% to 45% in the study group and 50% to 65% in the control group (Table 1).
Cardiac Complications
After a median follow-up of 29 months (range: 11–82) in the study group, Grade ≥2 cardiac complications were seen in 7 of 56 (12.5%) patients. These adverse events included CHF in 4 (7.%) and atrial fibrillation (AF) in 4 (7%) patients. One patient had both CHF and AF. There were no documented episodes of acute coronary ischemia or deaths directly related to cardiac events. Cardiac complications were seen in 7 of 35 patients (20%), with at least 1 of the 7 cardiac risk factors pre-allo-HSCT. In contrast, none of the 21 patients without a cardiac risk factors developed a cardiac complication (P =.03). On univariate analysis, variables such as age, diagnosis, sex, type of donor, intensity of preparative regimen (myeloablative [MA] versus reduced-intensity conditioning [RIC]), or exposure of cyclophosphamide (Cy) did not emerge as significant predictors of posttransplant cardiac complications (Table 3).
Table 3.
Impact of Prognostic factors on Outcome
| N | NRM at Day 100
|
P-Value | Risk of Cardiac Complications
|
95% CI | P- Value | ||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | HR | |||||
| Age | |||||||
| ≤43 | 28 | Ref. | Ref. | ||||
| >43 | 28 | 2.6 | 0.5–13.4 | 0.25 | 2.9 | 0.6–15.2 | .2 |
| HCT-CI | |||||||
| 1–2 | 39 | Ref | |||||
| >2 | 17 | 0.9 | 0.2–4.5 | 0.9 | 0.3 | 0.04–2.8 | 0.3 |
| Donor type | |||||||
| Matched related | 23 | 0.2 | 0.02–1.4 | 0.1 | 0.5 | ||
| Matched unrelated | 23 | Ref. | Ref. | 0.8 | |||
| Mismatched | 10 | 0.4 | 0.05–3.8 | 0.5 | can’t estimate | ||
| Pre-TP LVEF | |||||||
| ≤35 | 10 | Ref. | Ref. | ||||
| 36–40 | 21 | 1.6 | 0.2–15.1 | 0.7 | 1.6 | 0.2–16 | 0.7 |
| >40 | 25 | 1.3 | 0.1–13 | 0.8 | 1.3 | 0.1–12.5 | 0.8 |
| Smoking status | |||||||
| Smoker | 20 | 0.7 | 0.1–3.4 | 0.6 | 3.9 | 0.7–20.2 | 0.1 |
| Nonsmoker | 36 | Ref. | |||||
| Prep regimen | |||||||
| Ablative | 21 | 0.7 | 0.1–3.4 | 0.6 | 0.2 | 0.03–2.0 | 0.2 |
| Reduced intensity | 35 | Ref. | |||||
| Cyclophosphamide in prep regimen | |||||||
| Yes | 17 | 0.9 | 0.2–4.7 | 0.9 | 1.6 | 0.3–6.9 | 0.6 |
| No | 39 | Ref. | |||||
| Diagnosis | |||||||
| Acute leukemia | 32 | Ref. | Ref. | ||||
| Lymphoma | 16 | 1.5 | 0.2–8.8 | 0.7 | 1.4 | 0.2–8.4 | 0.7 |
| Others | 8 | 3 | 0.5–17.9 | 0.2 | 3.5 | 0.5–18.3 | |
NRM indicates nonrelapse mortality; HR, hazard ratio; CI, confidence interval; HCT-CI, hematopoietic stem cell transplantation comorbidity index; TP, transplant; Prep regimen, preparative regimen.
Median follow-up in the control group was 49 months (range: 1–98 months). Grade >2 cardiac complications were seen in 19 of 161 patients (11.8%; P = 1.00). Cardiac complications were seen in 15 of 97 patients with at least 1 of the 7 cardiac risk factors pre-allo-HSCT and in 4 of 64 patients without any cardiac risk factors (P = .08). These adverse events included CHF in 8 patients, arrythmias (mainly AF) in 15, and acute ischemic episodes in 5 patients. Seven patients had a combination of 2 or 3 cardiac events.
100-Day NRM
Overall, 7 patients died of nonrelapse causes within the first 100 days in the study group, with a cumulative NRM of 12.5%. None of the deaths were directly related to cardiac complications. The causes of death were as follows: multiorgan failure/sepsis (n = 4), acute graft-versus-host disease (aGVHD; n = 2), and diffuse alveolar hemorrhage (DAH; n = 1). NRM at 100 days was 10.0%, 14.3%, and 12.0% in patients with pre-allo-HSCT LVEF of ≤35%, ≤40%, and ≤45%, respectively. On univariate analysis, variables such as age, sex, type of donor, intensity of preparative regimen (MA versus RIC), or the underlying disease did not emerge as significant predictors of NRM (Table 3).
In the control group, 24 patients died of nonrelapse causes within the first 100 days (14.9%; P = .82). The causes of death were as follows: multiorgan failure/sepsis (7), aGVHD (9), idiopathic pneumonia syndrome (4), graft failure (1), and other (3). As in the study group, no deaths were directly attributable to cardiac causes.
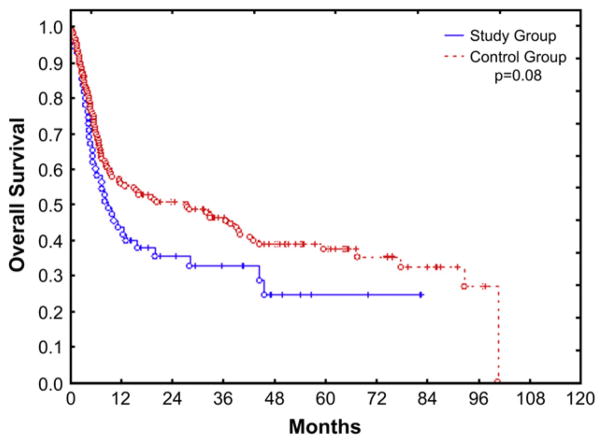
Survival
Kaplan-Meier estimate of 3-year OS was 32% for the study group and 45% for the control group (P = .08) (Figure 1). Donor type was the only significant predictor of OS, with recipients of a matched related graft having a significantly lower mortality rate (hazard ratio [HR] = 0.4, P = .02). At the time of last follow-up, 39 patients in the study group had died, 18 because of disease progression, and 21 because of nonrelapse causes, with aGVHD or chronic GVHD (cGVHD) being the most common cause of NRM (6 patients: 10%).
Figure 1.
Kaplan-Meier estimate of OS in study and control groups.
DISCUSSION
Our results suggest that patients with an LVEF of <45% can safely undergo an allo-HSCT. We observed a cardiac complication rate of 12.5% in 56 patients who received allo-HSCT and had a low LVEF (≤45%). The 100-day NRM and 3-year OS were 12.5% of 32%, respectively, with no early mortality related to cardiac causes. These rates were comparable to a control group of 161 patients that matched the study group in almost all parameters, except for an older age and a normal LVEF (50%–65%). Both NRM and OS in the study group are acceptable given the high-risk patient population, where 33 (59%) patients had relapsed or refractory disease and 14 (25%) were in second remission or beyond [3,9,10].
Cardiac toxicity in the immediate post-alllo-HCT period is reported in 0.9% to 43% of patients [2,11–17]. These complications include cardiomyopathy, arrhythmias, ischemic events, CHF, pericarditis, tamponade, and death because of cardiac compromise. Many factors, including Cy, anthracyclines, total body irradiation (TBI), prior mediastinal radiotherapy, and transfusion associated iron overload increase the risk of post-alllo-HCT cardiac complications [4,5,18–23]. Murdych et al. [14] reported a serious cardiac complication rate of only 0.9% in a large cohort of patients (n = 2821). A higher incidence is reported by others with varying definitions of cardiac toxicity. Incidentally, the majority of patients in these studies had a pretransplant LVEF of ≥50%, considered an acceptable range for allo-HSCT [21]. Other groups have also reported cardiac complication rate in patients with low LVEF (cutoffs varying from ≤55% to ≤40%). However, the number of patients in those reports was smaller than 56 patients reported by us: Yoshimi et al. [6] 6.7% (1 of 15); Sakata-Yanagimoto et al. [4] 11.1% (2 of 18); Bearman et al. [2] 20.0% (2 of 10); and Fujimaki et al. [3] 42.9% (3 of 7).
We analyzed the impact of factors other than LVEF that may predispose a patient to post-al-lo-HSCT cardiac complications, including smoking, hypertension, hyperlipidemia, coronary artery disease, arrhythmia, prior infarction, and CHF We found that patients with at least 1 of these 7 risk factors pre-allo-HSCT were significantly more likely to develop cardiac complications when compared to patients without them. These risk factors may be incorporated in a prognostic model to predict the risk of cardiac complications in patients with low LVEF. If validated, this approach may help in identifying a high-risk population that may benefit from therapeutic intervention to reduce the risk of cardiac toxicity.
We evaluated the impact of various prognostic factors on cardiac toxicity and NRM in a univariate analysis. None of these, including high-dose Cy, age, diagnosis, comorbidity index (HCT-CI), as reported by Sorror et al. [24,25], emerged as significant predictors of outcome. That may be because of the inherent limitations of a retrospective analysis, including the variability in therapeutic agents, doses, and their potential for cardio-toxicity, small sample size, and heterogeneity of diagnosis and treatments.
In summary, selected patients with LVEF ≤45% can safely undergo allo-HSCT with acceptable risk of cardiac complications, NRM, and OS.
Footnotes
Financial disclosure: The authors have nothing to disclose.
AUTHORSHIP
Contribution: M.H.Q. designed the study, collected, analyzed, and interpreted the data; and finalized the manuscript. A.I.A. assisted in literature search, manuscript preparation, and meticulous review. S.Q, Z.U.K., and F.J.B assisted in data collection and manuscript preparation. R.M.S performed the statistical analysis. J.J.M, C.H, S.A.G, M.J.D., and U.R.P made valuable contributions in designing the study and patient care. S.W.Y interpreted the cardiac function studies and contributed in study design. J.E.C is the Department Chair, and provided support in study design, data collection, and final organization of manuscript.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Bearman SI, Petersen FB, Schor RA, et al. Radionuclide ejection fractions in the evaluation of patients being considered for bone marrow transplantation: risk for cardiac toxicity. Bone Marrow Transplant. 1990;5:173–177. [PubMed] [Google Scholar]
- 3.Fujimaki K, Maruta A, Yoshida M, et al. Severe cardiac toxicity in hematological stem cell transplantation: predictive value of reduced left ventricular ejection fraction. Bone Marrow Transplant. 2001;27:307–310. doi: 10.1038/sj.bmt.1702783. [DOI] [PubMed] [Google Scholar]
- 4.Sakata-Yanagimoto M, Kanda Y, Nakagawa M, et al. Predictors for severe cardiac complications after hematopoietic stem cell transplantation. Bone Marrow Transplant. 2004;33:1043–1047. doi: 10.1038/sj.bmt.1704487. [DOI] [PubMed] [Google Scholar]
- 5.Tichelli A, Bhatia S, Socie G. Cardiac and cardiovascular consequences after haematopoietic stem cell transplantation. Br J Haematol. 2008;142:11–26. doi: 10.1111/j.1365-2141.2008.07165.x. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimi A, Nannya Y, Sakata-Yanagimoto M, et al. A myeloablative conditioning regimen for patients with impaired cardiac function undergoing allogeneic stem cell transplantation: reduced cyclophosphamide combined with etoposide and total body irradiation. Am J Hematol. 2008;83:635–639. doi: 10.1002/ajh.21208. [DOI] [PubMed] [Google Scholar]
- 7.Gopal AS, Shen Z, Sapin PM, et al. Assessment of cardiac function by three-dimensional echocardiography compared with conventional noninvasive methods. Circulation. 1995;92:842–853. doi: 10.1161/01.cir.92.4.842. [DOI] [PubMed] [Google Scholar]
- 8.Common Terminology Criteria for Adverse Events, Version 3.0 (CTCAE v3.0) DCTD, NCI, NIH, DHHS; 2006. [Google Scholar]
- 9.Akahori M, Nakamae H, Hino M, et al. Electrocardiogram is very useful for predicting acute heart failure following myeloablative chemotherapy with hematopoietic stem cell transplantation rescue. Bone Marrow Transplant. 2003;31:585–590. doi: 10.1038/sj.bmt.1703890. [DOI] [PubMed] [Google Scholar]
- 10.Zangari M, Henzlova MJ, Ahmad S, et al. Predictive value of left ventricular ejection fraction in stem cell transplantation. Bone Marrow Transplant. 1999;23:917–920. doi: 10.1038/sj.bmt.1701734. [DOI] [PubMed] [Google Scholar]
- 11.Cazin B, Gorin NC, Laporte JP, et al. Cardiac complications after bone marrow transplantation. A report on a series of 63 consecutive transplantations. Cancer. 1986;57:2061–2069. doi: 10.1002/1097-0142(19860515)57:10<2061::aid-cncr2820571031>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 12.Hertenstein B, Stefanic M, Schmeiser T, et al. Cardiac toxicity of bone marrow transplantation: predictive value of cardiologic evaluation before transplant. J Clin Oncol. 1994;12:998–1004. doi: 10.1200/JCO.1994.12.5.998. [DOI] [PubMed] [Google Scholar]
- 13.Kupari M, Volin L, Suokas A, Timonen T, Hekali P, Ruutu T. Cardiac involvement in bone marrow transplantation: electrocardiographic changes, arrhythmias, heart failure and autopsy findings. Bone Marrow Transplant. 1990;5:91–98. [PubMed] [Google Scholar]
- 14.Murdych T, Weisdorf DJ. Serious cardiac complications during bone marrow transplantation at the University of Minnesota, 1977–1997. Bone Marrow Transplant. 2001;28:283–287. doi: 10.1038/sj.bmt.1703133. [DOI] [PubMed] [Google Scholar]
- 15.Sucak GT, Ozkurt ZN, Aki Z, Yagci M, Cengel A, Haznedar R. Cardiac systolic function in patients receiving hematopoetic stem cell transplantation: risk factors for posttransplantation cardiac toxicity. Transplant Proc. 2008;40:1586–1590. doi: 10.1016/j.transproceed.2007.11.077. [DOI] [PubMed] [Google Scholar]
- 16.Buja LM, Ferrans VJ, Graw RG., Jr Cardiac pathologic findings in patients treated with bone marrow transplantation. Hum Pathol. 1976;7:17–45. doi: 10.1016/s0046-8177(76)80004-4. [DOI] [PubMed] [Google Scholar]
- 17.Trigg ME, Finlay JL, Bozdech M, Gilbert E. Fatal cardiac toxicity in bone marrow transplant patients receiving cytosine arabinoside, cyclophosphamide, and total body irradiation. Cancer. 1987;59:38–42. doi: 10.1002/1097-0142(19870101)59:1<38::aid-cncr2820590112>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 18.Baello EB, Ensberg ME, Ferguson DW, et al. Effect of high-dose cyclophosphamide and total-body irradiation on left ventricular function in adult patients with leukemia undergoing allogeneic bone marrow transplantation. Cancer Treat Rep. 1986;70:1187–1193. [PubMed] [Google Scholar]
- 19.Benoff LJ, Schweitzer P. Radiation therapy-induced cardiac injury. Am Heart J. 1995;129:1193–1196. doi: 10.1016/0002-8703(95)90403-4. [DOI] [PubMed] [Google Scholar]
- 20.Braverman AC, Antin JH, Plappert MT, Cook EF, Lee RT. Cyclophosphamide cardiotoxicity in bone marrow transplantation: a prospective evaluation of new dosing regimens. J Clin Oncol. 1991;9:1215–1223. doi: 10.1200/JCO.1991.9.7.1215. [DOI] [PubMed] [Google Scholar]
- 21.Coghlan JG, Handler CE, Kottaridis PD. Cardiac assessment of patients for haematopoietic stem cell transplantation. Best Pract Res Clin Haematol. 2007;20:247–263. doi: 10.1016/j.beha.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Jimenez J, Cervero C, Munoz A, et al. Cardiovascular toxicities related to the infusion of cryopreserved grafts: results of a controlled study. Bone Marrow Transplant. 1994;13:789–793. [PubMed] [Google Scholar]
- 23.Shan K, Lincoff AM, Young JB. Anthracycline-induced cardiotoxicity. Ann Intern Med. 1996;125:47–58. doi: 10.7326/0003-4819-125-1-199607010-00008. [DOI] [PubMed] [Google Scholar]
- 24.Sorror ML, Giralt S, Sandmaier BM, et al. Hematopoietic cell transplantation specific comorbidity index as an outcome predictor for patients with acute myeloid leukemia in first remission: combined FHCRC and MDACC experiences. Blood. 2007;110:4606–4613. doi: 10.1182/blood-2007-06-096966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]