Abstract
Arsenic trioxide (ATO) is synergistic with ascorbic acid (AA) and melphalan against myeloma both in vitro and in vivo. The aim of this randomized phase II trial was to determine the safety and efficacy of a combination of ATO, melphalan, and AA as preparative regimen in 48 patients undergoing autologous hematopoietic stem cell transplantation (ASCT) for multiple myeloma (MM). Forty-eight patients received melphalan 200 mg/m2 i.v. over 2 days and AA 1000 mg i.v. over 7 days in 3 treatment arms: no ATO (arm 1), ATO 0.15 mg/kg i.v. × 7 days (arm 2), and ATO 0.25 mg/kg i.v. × 7 days (arm 3). No dose-limiting toxicity, engraftment failure, or non-relapse mortality (NRM) was seen in the first 100 days post-ASCT. Complete responses (CR) were seen in 12 of 48 patients (25%), with an overall response rate (ORR = CR + PR) of 85%. Median progression-free survival (PFS) was 25 months; median overall survival (OS) has not yet been reached. There was no significant difference in CR, PFS, or OS among the 3 treatment arms, and no adverse effect of ATO on melphalan pharmacokinetics. Addition of ATO +AA to high-dose melphalan is safe and well tolerated as a preparative regimen for MM.
Keywords: Myeloma, Arsnic trioxide, Autologous Introduction
INTRODUCTION
Multiple myeloma (MM) is a clonal disorder of plasma cells affecting approximately 50, 000 patients in the United States, with an annual incidence of about 20,000 new cases [1,2]. Over the last 20 years high-dose therapy (HDT) and autologous hematopoietic stem cell transplantation (ASCT) have evolved into a safe and effective therapeutic approach for patients with MM. When compared to standard chemotherapy, intensified chemotherapy followed by ASCT has been shown to prolong both event-free and overall survival (OS) in selected previously untreated patients with myeloma. One comparative study and 2 randomized trials have shown survival benefits in favor of ASCT of approximately 12 months [3–5]. Two other randomized trials, however, failed to show this survival benefit [6,7]. However, this approach is not curative, and most patients undergoing ASCT for MM eventually develop disease recurrence [8]. We and others have shown that salvage ASCT can be safely performed at relapse, with median remission duration between 6 and 12 months and an OS approaching 3 years [9,10].
Melphalan 200 mg/m2 i.v. is the standard conditioning regimen used for ASCT in myeloma, with dose reductions based on age and renal function [11–13]. In nonrandomized studies and a registry analysis, use of more intensive preparative regimens, such as busulfan with melphalan, thiotepa, busulfan, and cyclophosphamide, or high-dose idarubicin, cyclophosphamide, and melphalan, did not result in better outcomes than melphalan at a dose of 200 mg/m2 [14–16]. Arsenic trioxide (ATO) is an antineoplastic chemotherapeutic agent approved for the treatment of relapsed or refractory acute promyelocytic leukemia [17,18]. The use of ATO to treat MM is supported by preclinical studies where it was shown to inhibit growth, reduce viability, and induce apoptosis in several MM cell lines at concentrations that can be safely achieved in patients [19–21]. The antitumor activity of ATO is dependent upon the generation of reactive oxygen species (ROS) that damage mitochondria. In addition, critical intracellular antioxidant, free glutathione (GSH) is directly conjugated to ATO and subsequently expelled out of the cell by multidrug resistance efflux pumps. Agents such as ascorbic acid (AA) that deplete GSH sensitize cells to ATO-induced apoptosis and are expected to enhance the anti-MM effects of ATO [22].
The combination of ATO with the cytotoxic agent melphalan helps to overcome the resistance to melphalan both in vitro and in severe combined immunodeficient-human (scid-hu) murine models of human myeloma [23]. Adding AA to this combination regimen enhances anti-MM effects of the melphalan/ ATO combination both in vitro and in vivo [22,23].
Early clinical studies of ATO for patients with advanced refractory MM have demonstrated significant, albeit minor, responses in approximately one-third of patients with daily dosing schedules [24,25]. A phase II trial for the safety and efficacy of melphalan, ATO and AA (MAC) combination therapy was performed in patients with MM who failed more than 2 different prior regimens. Objective responses were seen in 31 of 65 (48%) patients, including 2 complete remission (CR), 15 partial remission (PR), and 14 minor responses. Median pregression-free suvival (PFS) and OS were 7 and 19 months, respectively. Specific grade 3 or 4 hematologic (3%) or cardiac adverse events occurred infrequently. This steroid-free regimen was effective and well tolerated in this heavily pretreated group, making it a potential therapeutic option for patients with relapsed or refractory MM [26].
We conducted a randomized phase II trial with a combination of high-dose melphalan, ATO, and AA to evaluate its impact on safety, engraftment, response rate, and survival. We also assessed the impact of ATO on melphalan PK. We decided to use the doses of ATO that were safely used in combination with standard-dose melphalan and AA [26]. AA and ATO were administered for 5 days before melphalan to deplete GSH and generation of ROS, which in turn, are expected to enhance the antimyeloma activity of melphalan [20,21,24].
METHODS
Patients
Forty-eight patients were randomized to 3 treatment arms and received a single ASCT between April 2004 and August 2005 ClinicalTrials.gov Identifier: NCT00661544). At study entry, 29 (60%) patients had a partial response to induction therapy and had received a median of 2 different induction regimens (range: 1–3). Nineteen (40%) patients had relapsed refractory disease and had received a median of 4 prior regimens (range: 2–9).
Patient inclusion criteria were age <70 years, Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, left ventricular ejection fraction (LVEF) of >40%, pulmonary diffusing capacity (DLCO) of >40%; serum concentration of aspartate aminotransferase (AST) or alanine amino-transferase (ALT) no higher than 4 times the upper limit of normal; serum total bilirubin concentration no greater than twice the upper limit of normal; corrected QT interval on electrocardiogram <500 milliseconds. Patients had to have measurable serum or urine paraprotein. Patients agreed to use contraception, and a confirmed negative pregnancy test before enrollment was required for women. All patients gave written informed consent before entering the study, which was obtained in accordance with the Declaration of Helsinki, under the auspices of protocols approved by the institutional review board.
Study Design and Treatment
Peripheral blood stem cells (PBSC) were mobilized and collected following granulocyte colony-stimulating factor (G-CSF) alone or chemotherapy + G-CSF. All 48 patients received high-dose Melphalan at 100 mg/m2 i.v. on days −4 and −3, and AA 1000 mg i.v. daily on days −9 to −3. Patients in arm 1 did not receive ATO; patients in arm 2 received ATO 0.15 mg/kg i.v. from days −9 to −3; patients in arm 3 received ATO at 0.25 mg/kg from days −9 to −3 (Table 1). Melphalan was infused over 30 minutes in 2 divided doses over 2 consecutive days. Unmanipulated autologous stem cells were infused 48 hours later. All patients received G-CSF, 5 μg/kg/day from day +1 until the absolute neutrophil count (ANC) was 0.5 × 109/L for 2 consecutive days, in accordance with our departmental guidelines. Oral levofloxacin, acyclovir, and fluconazole were given for the duration of neutropenia. Blood products were given for hemoglobin ≤8 g/dL and platelets <20 × 109/L.
Table 1.
Treatment Schema
| Arm 1 | Arm 2 | Arm 3 |
|---|---|---|
| Melphalan | Melphalan | Melphalan |
| 100 mg/m2 i.v. × days | 100 mg/m2 i.v. | 100 mg/m2 i.v. |
| −4, −3 | Days −4, −3 | Days −4, −3 |
| Ascorbic acid | Ascorbic acid | Ascorbic acid |
| 1000 mg i.v. | 1000 mg i.v. | 1000 mg i.v. |
| Days −9 to −3 | Days −9 to −3 | Days −9 to −3 |
| Arsenic trioxide | Arsenic trioxide | Arsenic trioxide |
| None | 0.15 mg/kg Days −9 to −3 |
0.25 mg/kg Days −9 to −3 |
Statistical Methods
This is a randomized phase II trial of ATO-containing preparative regimen for ASCT in MM. All patients received a fixed dose of Melphalan (200 mg/m2) and AA (1000 mg). A maximum of 48 patients were randomized fairly between no ATO, 0.15 mg/kg/day ATO or 0.25 mg/kg/day ATO for 7 days prior to ASCT. A Bayesian model and decision rules were used to monitor the outcomes continuously throughout the trial. Actuarial rates of OS and PFS were estimated by the Kaplan-Meier method [27]. Prognostic factors for survival were evaluated using Cox’s proportional hazards model for univariate analyses. Statistical significance was defined at the .05 level. Analysis was performed using STATA 7.0 (StataCorp., 2001, Stata Statistical Software: Release 7.0. College Station, TX).
Response Criteria
The primary endpoints were toxicity of the regimen and engraftment. The secondary endpoints were response rates, PFS, and OS. Additional secondary endpoint was the impact of ATO on melphalan PK. Toxicity was graded according to National Cancer Institute Common Terminology Criteria (version 3.1; Bethesda, MD). Engraftment was defined as ANC of >0.5 × 109/L for 2 consecutive days. Response or progression was assessed according to the criteria of the European Group for Blood and Marrow Transplantation (EBMT) [28]. PFS was time from the day of ASCT to progression or time last known alive. OS was time from the day of ASCT to death or time last known alive.
Pharmacokinetics
For melphalan PK, including area under the curve (AUC) measurements, a series of timed blood specimens, 5 mL per tube, were drawn on the days of melphalan administration (days −4 and −3) and placed on ice. Plasma was separated by centrifugation at 4°C and stored at −70°C until analysis. After deproteinization with perchloric acid, melphalan plasma levels were determined by high-performance liquid chromatography (HPLC) using dansyl-proline as internal standard. Pharmacokinetic modeling employed WinNonlin 3.0 (Pharsight Corporation, Mountain View, CA) [29].
Samples for ATO blood level were drawn on day 0, approximately 30 minutes prior to the stem cell infusion. Measurement of elemental arsenic was performed by inductively coupled mass spectrometry (ICP-MS) [29].
Comorbidity Indices
Charlson comorbidity index (CCI) and Seattle’s hematopoietic cell transplantation-specific comorbidity index (HCT-CI) were used to score the comorbidities [30,31].
RESULTS
Patients
From April 2004 to August 2005, 48 patients were randomly assigned to 3 treatment arms. Sixteen patients were randomized to arm 1, 17 to arm 2 (ATO 0.15 mg/kg), and 15 patients were randomized to arm 3 (ATO 0.25 mg/kg). Table 2 summarizes the characteristics of the 48 patients treated on this study. Median interval from diagnosis to ASCT was 14 months (3–93). The median age was 54 years (range: 35–70). According to International Staging System (ISS) for MM [32], 17 (35%) patients had stage I, 17 (35%) had stage II, and 8 (16%) patients had stage III disease. Nine patients (19%) had a clonal cytogenetic abnormality by conventional karyotypic analysis, either at baseline or prior to ASCT. Four patients (8%) had elevated serum creatinine (>1.5 mg/dL) at the time of transplant.
Table 2.
Patient Characteristics
| Total | Arm 1 (N = 16) | Arm 2 (N = 17) | Arm 3 (N = 15) | P Value | |
|---|---|---|---|---|---|
| Median age (range) | 54 (35–70) | 58 (49–69) | 54 | 52 | .4 |
| Median interval Diagnosis—ASCT (months) | 13.7 | 13.7 | 14.2 | 13.3 | .5 |
| IgG | 22 | 8 | 8 | 6 | |
| IgA | 16 | 5 | 5 | 6 | |
| Light chain only | 10 | 3 | 4 | 3 | |
| Chromosomal abnormalities | 9 | 4 | 3 | 2 | .5 |
| β2 M >3 mg/L | 14 | 2 | 6 | 6 | .2 |
| LDH >618 IU/L | 15 | 5 | 8 | 2 | .13 |
| Albumin <3.5 g/dL | 5 | 1 | 2 | 2 | .9 |
| Creatinine >1.5 mg/dL | 4 | 1 | 3 | 0 | .3 |
| HCT CI ≥3 | 15 | 4 | 7 | 4 | .8 |
| Prior ASCT | 12 | 2 | 4 | 6 | .2 |
| Relapsed at ASCT | 19 | 3 | 8 | 8 | .05 |
β2 M indicates beta 2 microglobulin; LDH, lactic dehydrogenase; ASCT, autologous hematopoietic stem cell transplantation; HCT CI, hematopoietic stem cell transplantation comorbidity index.
Stem Cell Mobilization and Engraftment
Forty-two (87%) patients were mobilized with either G-CSF 10 μg/kg/day or pegylated G-CSF alone, given subcutaneously. Six (12%) patients with high tumor burden received chemotherapy + G-CSF 10 μg/ kg/day, with modified cyclophosphamide, vincristine, adriamycin, and dexamethasone (CVAD) regimen to achieve cytoreduction and mobilization [33]. The median CD34+cell dose infused was 4.6 × 106/kg (range: 2.7–10.1). Median time to ANC ≥500/dL was 9 days, with no engraftment failures or delays in either the control or ATO arms. Median time to platelet count of ≥20 × 109/l was 10 days (range: 8–21).
Treatment-Related Toxicity
No transplant-related mortality or engraftment failure was seen in the first 100 days after ASCT. Toxicity was limited to grade 1 or 2 nausea, vomiting, and diarrhea and was comparable in all 3 arms (Table 3). There was no significant difference in toxicities between the 3 arms. Grade 2 cardiac toxicities consisting of pedal edema, hypertension, and venous thromboembolism was seen in 10% of patients. Eight patients have died; 6 because of progressive disease, 1 from sepsis, and 1 patient died of pancreatic cancer that was unrelated to therapy.
Table 3.
Adverse Events
| Adverse Events | Overall (n = 48) | Arm 1 (n = 16) | Arm 2 (n = 17) | Arm 3 (n = 15) | P Value | |
|---|---|---|---|---|---|---|
| Cardiac* | Grade I | 2 | 0 | 1 | 1 | .8 |
| Grade II | 1 | 0 | 1 | 0 | .9 | |
| GI: diarrhea | Grade I | 24 | 7 | 8 | 9 | .7 |
| Grade II | 1 | 0 | 0 | 0 | .6 | |
| GI: vomiting | Grade I | 16 | 4 | 7 | 5 | .6 |
| Grade II | 2 | 1 | 0 | 1 | .5 | |
| GI: nausea | Grade I | 24 | 8 | 10 | 6 | .6 |
| Grade II | 12 | 1 | 4 | 7 | .03 | |
| Grade III | 2 | 1 | 1 | 0 | .9 | |
| GI: stomatitis | Grade I | 7 | 2 | 3 | 2 | .9 |
| Grade II | 5 | 2 | 1 | 2 | .7 | |
| Grade III | 1 | 0 | 0 | 1 | .3 | |
| Hepatic† | Grade I | 1 | 0 | 1 | 0 | .9 |
| Renal‡ | Grade I | 1 | 0 | 1 | 0 | .9 |
| Grade II | 2 | 0 | 2 | 0 | .3 | |
| Skin rash | Grade I | 2 | 0 | 2 | 0 | .3 |
| Fever/infections | Grade III | 11 | 3 | 4 | 4 | .9 |
Pedal edema, hypertension, venous thromboembolism.
Increase in AST, ALT.
Increase in serum creatinine.
Response Rates
Twelve of the 48 patients (25%) achieved a CR. There was no significant difference between the 3 arms in terms of CR (25%, 23%, and 27%, respectively; P = .9). Overall response rate (CR + PR) was 85%, with no significant differences between the 3 arms (87%, 70%, and 86%, respectively). Among patients receiving this regimen as consolidation of a first remission the CR rates were 28%, 30%, and 22%, respectively. Because of its small sample size (48 patients) and randomization to 3 arms, this study was not statistically empowered to detect the differences in response between the 3 treatment arms.
Patients receiving CVAD +G-CSF for mobilization
These 6 patients were evenly distributed between 3 treatment arms (2 in each arm). Prior to ASCT, 3 patients had a partial remission, whereas other 3 had primary refractory or relapsed disease. One patient achieved a CR, 4 achieved a PR, and 1 patient had stable disease. The PFS and OS of these patients were not significantly different from those mobilized with G-CSF alone.
Survival and Prognostic Factors
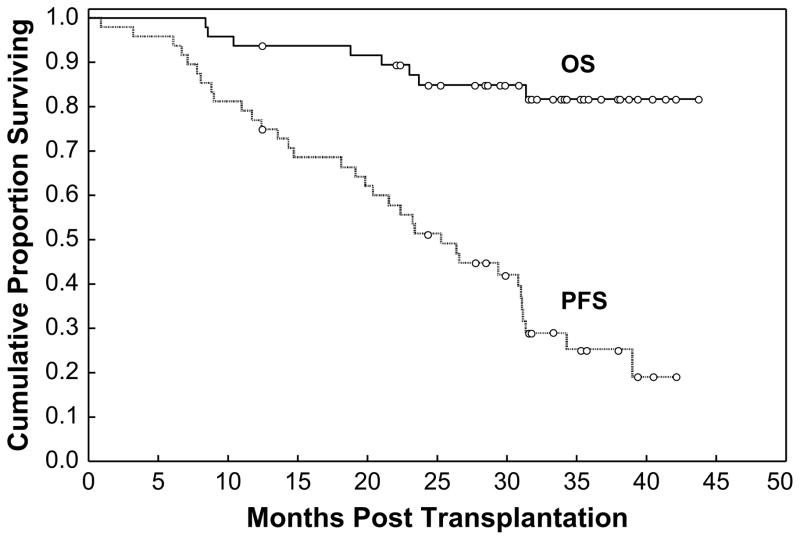
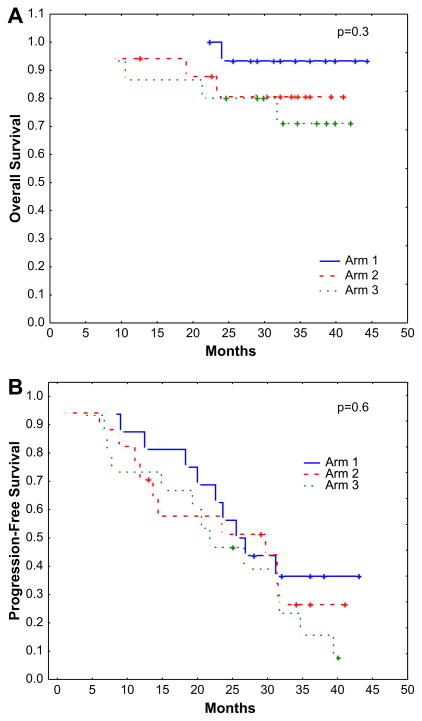
The median follow-up among surviving patients was 33 months (range: 13–44). The 3-year PFS and OS were projected at 25% and 82%, respectively. Median PFS was 25 months, whereas median OS has not been reached (Figure 1). There was no significant difference in PFS or OS between the 3 treatment arms (P = 6 and .3, respectively). (Figure 2A and B). Because of its small sample size (48 patients) and randomization to 3 arms, this study was not statistically empowered to detect the differences in PFS and OS among the 3 treatment arms.
Figure 1.
Kaplan-Meier estimates for OS and PFS probability for all 48 patients treated on the protocol.
Figure 2.
(A) Kaplan-Meier estimates for OS probability according to randomization to 3 treatment arms (arm 1 = no ATO, arm 2 = ATO 0.15 mg/kg, arm 3 = ATO 0.25 mg/kg). (B) Kaplan-Meier estimates for PFS probability according to randomization to 3 treatment arms (arm 1 = no ATO, arm 2 = ATO 0.15 mg/kg, arm 3 = ATO 0.25 mg/kg).
We analyzed the impact of a number of prognostic factors on 3-year PFS. We were unable to detect any significant impact of the serum albumin, β2 microglobulin or lactic dehydrogenase (LDH) level, disease status, chromosomal abnormalities, prior ASCT, or the HCT-CI index on the outcome.
Patients undergoing a salvage ASCT
We analyzed the outcome of patients undergoing a second ASCT for relapsed disease. In 12 patients with salvage ASCT, 2 achieved a CR (16%), 6 achieved a PR (50%) with an overall relative risk (ORR) of 66%. Median PFS was 12 months, and median OS has not been reached.
Pharmacokinetics
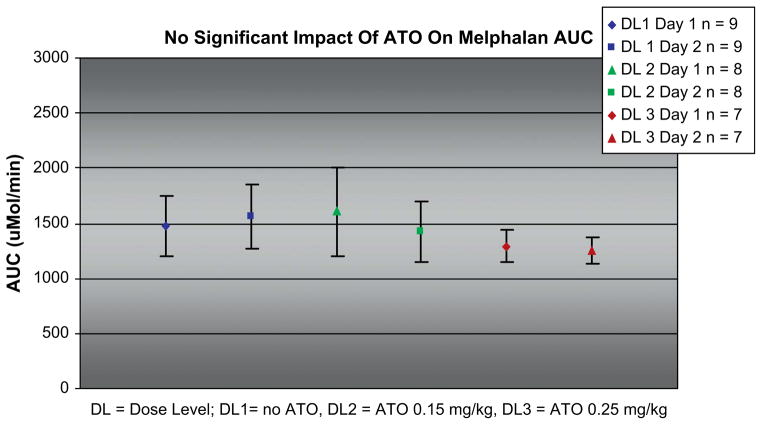
Melphalan pharmacokinetics was performed on 24 patients (arm 1: 9, arm 2: 8, arm 3: 7) for whom adequate samples were collected. As shown in Figure 3, the melphalan AUC remained unchanged regardless of the presence of ATO, or its dose level. In arm 1, the median AUC was 1525 μmol/min (range: 1200–1850); in arm 2 median AUC was 1500 μmol/min (range: 1250–2000), and in arm 3 it was 1275 μmol/ min (range: 1190–1475) (P = .6).
Figure 3.
Impact of ATO dose on melphalan AUC on the days of melphalan administration. AUC =area under the curve; DL1 = dose level 1/no ATO; DL2 = dose level 2/ATO 0.15 mg/kg; DL3 = dose level 3/ATO 0.25 mg/kg.
Median serum concentrations of dimethylarsonic acid (DMA) on day 0 were 0.2, 26.3, and 46.2 ng/mL in arms 1, 2, and 3, respectively, consistent with the ATO dose in that arm.
DISCUSSION
Disease progression that is generally resistant to chemotherapy is the most common cause of treatment failure in MM. A number of approaches are being studied to prevent the emergence of resistance and achieve durable remission. These approaches include the combination of existing and novel antimyeloma agents in conventional doses [34], tandem ASCT [35,36], maintenance therapy [37], and sequential autologous and allogeneic transplantation [38]. Furthermore, rationally designed preparative regimens for ASCT should also be explored. In this phase II randomized trial, we tested 1 such combination, consisting of ATO, high-dose melphalan, and AA. We hypothesized that this combination would be safe, and would enhance antimyeloma activity of melphalan without exacerbating toxicity [23]. As there are no data to support the benefit of combining melphalan and AA, we used this combination as control. The dose of ATO was chosen based on documented safety of with 0.15 mg/kg i.v. in acute promyelocytic leukemia (APL) [16] and 0.25 mg/kg i.v. in MM [24]. AA in a dose of 1000 mg/day i.v. has been reported to be safe and effective in combination with ATO in MM [24].
The regimen was well tolerated. The inclusion of ATO with HD melphalan was associated with acceptable toxicity. QT interval prolongation or torsades de pointes were not seen in the ATO-containing arms [39]. We did not encounter leukocytosis or APL differentiation syndrome in this trial [18]. That could be explained by an abbreviated course of ATO (7 days only), the presence of high-dose melphalan in the regimen, and perhaps the fact that this syndrome is only seen in patients with APL [18]. There were no engraftment delays or failures in ATO-containing arms. There were no treatment-related deaths within the first 100 days. That was significant because patients up to age 70 were treated and 12 patients received a salvage ASCT.
We did not detect any interaction between ATO with melphalan PK at either dose level of ATO. This important observation was not unexpected, as both drugs follow different PK pathways. ATO is not protein bound, and is methylated in the liver to its major metabolites monomethylarsonic acid and dimethylarsonic acid, which are mostly excreted into the urine. In contrast, Melphalan is extensively protein bound and undergoes spontaneous plasma hydrolysis to dechlorinated inert products.
At the time of this analysis, there was no significant difference in PFS and OS between the 3 treatment arms. The median PFS was 25 months, which is similar to our historic data [10,40,41], although it may be noted that approximately 40% of patients had relapsed disease at transplant, and 25% had failed a prior ASCT. We observed that patients with a prior ASCT had a median PFS of 12 months, and the median OS has not yet been reached at the last follow-up (median: 33 months). Because of a small number of patients and events in each arm, the study was not sufficiently powered to detect the difference in PFS and OS between the 3 treatment arms.
Of note, 16 of the 19 patients with relapsed disease were randomized to ATO-containing arms (P = .05), but their PFS and OS were comparable to patients treated on the control arm.
In summary, addition of ATO and AA to high-dose melphalan is safe and well tolerated as a preparative regimen for ASCT in patients with MM, including patients with relapsed and refractory disease. There was no adverse impact of ATO on engraftment. Longer follow-up is needed to assess the efficacy of this combination.
Footnotes
Financial disclosure: Dr. Qazilbash had an educational grant from Cephalon and is on the speakers’ bureau for Cephalon. The remaining authors have nothing to disclose.
Conception and design: Muzaffar H. Qazilbash, Rima Saliba, Sergio Giralt, Richard Champlin. Collection and assembly of data: Muzaffar H. Qazilbash, Gaurav Parikh, Matteo Pelosini, Floralyn Mendoza, Suhail R Queshi, Fatima B Khan. Data analysis and interpretation: Rima M Saliba, Muzaffar H. Qazilbash. Manuscript writing: Muzaffar H. Qazilbash, Richard Champlin, Sergio Giralt. Manuscript review: Yago Nieto, Chitra hosing, Donna M Weber, Michael Wang, Amin Alousi, Uday Popat, Sergio Giralt. Melphalan pharmacokinetics: Yago Nieto, Roy B Jones.
References
- 1.Hari P, Pasquini MC, Vesole DH. Cure of multiple myeloma— more hype, less reality. Bone Marrow Transplant. 2006;37:1–18. doi: 10.1038/sj.bmt.1705194. [DOI] [PubMed] [Google Scholar]
- 2.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1860–1873. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 3.Barlogie B, Jagannath S, Vesole DH, et al. Superiority of tandem autologous transplantation over standard therapy for previously untreated multiple myeloma. Blood. 1997;89:789–793. [PubMed] [Google Scholar]
- 4.Attal M, Harousseau JL, Stoppa AM, et al. A prospective, randomized trial of autologous bone marrow transplantation and chemotherapy in multiple myeloma. Intergroupe Francais du Myelome. N Engl J Med. 1996;335:91–97. doi: 10.1056/NEJM199607113350204. [DOI] [PubMed] [Google Scholar]
- 5.Child JA, Morgan GJ, Davies FE, et al. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N Engl J Med. 2003;348:1875–1883. doi: 10.1056/NEJMoa022340. [DOI] [PubMed] [Google Scholar]
- 6.Fermand JP, Katsahian S, Divine M, et al. High-dose therapy and autologous blood stem-cell transplantation compared with conventional treatment in myeloma patients aged 55 to 65 years: long-term results of a randomized control trial from the Group Myelome-Autogreffe. J Clin Oncol. 2005;23:9227–9233. doi: 10.1200/JCO.2005.03.0551. [DOI] [PubMed] [Google Scholar]
- 7.Blade J, Rosinol L, Sureda A, et al. High-dose therapy intensification compared with continued standard chemotherapy inmultiple myeloma patients responding to the initial chemotherapy: long-term results from a prospective randomized trial from the Spanish cooperative group PETHEMA. Blood. 2005;106:3755–3759. doi: 10.1182/blood-2005-03-1301. [DOI] [PubMed] [Google Scholar]
- 8.Fassas A, Tricot G. Results of high-dose treatment with autologous stem cell support in patients with multiple myeloma. Semin Hematol. 2001;38:231–242. doi: 10.1016/s0037-1963(01)90015-0. [DOI] [PubMed] [Google Scholar]
- 9.Mehta J, Tricot G, Jagannath S, et al. Salvage autologous or allogeneic transplantation for multiple myeloma refractory to or relapsing after a first-line autograft? Bone Marrow Transplant. 1998;21:887–892. doi: 10.1038/sj.bmt.1701208. [DOI] [PubMed] [Google Scholar]
- 10.Qazilbash MH, Saliba R, DeLima M, et al. Second autologous or allogeneic transplantation after the failure of first autograft in patients with multiple myeloma. Cancer. 2006;106:1084–1089. doi: 10.1002/cncr.21700. [DOI] [PubMed] [Google Scholar]
- 11.Badros A, Barlogie B, Siegel E, et al. Results of autologous stem cell transplant in multiple myeloma patients with renal failure. Br J Haematol. 2001;114:822–829. doi: 10.1046/j.1365-2141.2001.03033.x. [DOI] [PubMed] [Google Scholar]
- 12.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myelome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 13.Badros A, Barlogie B, Siegel E, et al. Autologous stem cell transplantation in elderly multiple myeloma patients over the age of 70 years. Br J Haematol. 2001;114:600–607. doi: 10.1046/j.1365-2141.2001.02976.x. [DOI] [PubMed] [Google Scholar]
- 14.Lahuerta JJ, Martinez-Lopez J, Grande C, et al. Conditioning regimens in autologous stem cell transplantation for multiple myeloma: a comparative study of efficacy and toxicity from the Spanish Registry for Transplantation in Multiple Myeloma. Br J Haematol. 2000;109:138–147. doi: 10.1046/j.1365-2141.2000.01979.x. [DOI] [PubMed] [Google Scholar]
- 15.Anagnostopoulos A, Aleman A, Ayers G, et al. Comparison of high-dose melphalan with a more intensive regimen of thiotepa, busulfan, and cyclophosphamide for patients with multiple myeloma. Cancer. 2004;100:2607–2612. doi: 10.1002/cncr.20294. [DOI] [PubMed] [Google Scholar]
- 16.Fenk R, Schneider P, Kropff M, et al. High-dose idarubicin, cyclophosphamide and melphalan as conditioning for autologous stem cell transplantation increases treatment-related mortality in patients with multiple myeloma: results of a randomised study. Br J Haematol. 2005;130:588–594. doi: 10.1111/j.1365-2141.2005.05641.x. [DOI] [PubMed] [Google Scholar]
- 17.Niu C, Yan H, Yu T, et al. Studies on treatment of acute promyelocytic leukemia with arsenic trioxide: remission induction, follow-up, and molecular monitoring in 11 newly diagnosed and 47 relapsed acute promyelocytic leukemia patients. Blood. 1999;94:3315–3324. [PubMed] [Google Scholar]
- 18.Soignet SL, Frankel SR, Douer D, et al. United States multicenter study of arsenic trioxide in relapsed acute promyelocytic leukemia. J Clin Oncol. 2001;19:3852–3860. doi: 10.1200/JCO.2001.19.18.3852. [DOI] [PubMed] [Google Scholar]
- 19.Park WH, Seol JG, Kim ES, et al. Arsenic trioxide-mediated growth inhibition in MC/CAR myeloma cells via cell cycle arrest in association with induction of cyclin-dependent kinase inhibitor, p21, and apoptosis. Cancer Res. 2000;60:3065–3071. [PubMed] [Google Scholar]
- 20.Perkins C, Kim CN, Fang G, Bhalla KN. Arsenic induces apoptosis of multidrug-resistant human myeloid leukemia cells that express Bcr-Abl or overexpress MDR, MRP, Bcl-2, or Bcl-x(L) Blood. 2000;95:1014–1022. [PubMed] [Google Scholar]
- 21.Hideshima T, Chauhan D, Richardson P, et al. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277:16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 22.Bahlis NJ, McCafferty-Grad J, Jordan-McMurry I, et al. Feasibility and correlates of arsenic trioxide combined with ascorbic acid-mediated depletion of intracellular glutathione for the treatment of relapsed/refractory multiple myeloma. Clin Cancer Res. 2002;8:3658–3668. [PubMed] [Google Scholar]
- 23.Campbell RA, Sanchez E, Steinberg JA, et al. Antimyeloma effects of arsenic trioxide are enhanced by melphalan, bortezomib and ascorbic acid. Br J Haematol. 2007;138:467–478. doi: 10.1111/j.1365-2141.2007.06675.x. [DOI] [PubMed] [Google Scholar]
- 24.Munshi NC, Tricot G, Desikan R, et al. Clinical activity of arsenic trioxide for the treatment of multiple myeloma. Leukemia. 2002;16:1835–1837. doi: 10.1038/sj.leu.2402599. [DOI] [PubMed] [Google Scholar]
- 25.Hussein MA, Saleh M, Ravandi F, Mason J, Rifkin RM, Ellison R. Phase 2 study of arsenic trioxide in patients with relapsed or refractory multiple myeloma. Br J Haematol. 2004;125:470–476. doi: 10.1111/j.1365-2141.2004.04941.x. [DOI] [PubMed] [Google Scholar]
- 26.Berenson JR, Boccia R, Siegel D, et al. Efficacy and safety of melphalan, arsenic trioxide and ascorbic acid combination therapy in patients with relapsed or refractory multiple myeloma: a prospective, multicentre, phase II, single-arm study. Br J Haematol. 2006;135:174–183. doi: 10.1111/j.1365-2141.2006.06280.x. [DOI] [PubMed] [Google Scholar]
- 27.Kaplan EL, Meier P. Nonparametric estimator from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 28.Blade J, Samson D, Reece D, et al. Criteria for evaluating disease response and progression in patients with multiple myeloma treated by high-dose therapy and haemopoietic stem cell transplantation. Myeloma Subcommittee of the EBMT. European Group for Blood and Marrow Transplant. Br J Haematol. 1998;102:1115–1123. doi: 10.1046/j.1365-2141.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 29.Fox E, Razzouk BI, Widemann BC, et al. Phase 1 trial and pharmacokinetic study of arsenic trioxide in children and adolescents with refractory or relapsed acute leukemia, including acute promyelocytic leukemia or lymphoma. Blood. 2008;111:566–573. doi: 10.1182/blood-2007-08-107839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol. 1994;47:1245–1251. doi: 10.1016/0895-4356(94)90129-5. [DOI] [PubMed] [Google Scholar]
- 31.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–2919. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greipp PR, San Miguel J, Durie BG, et al. International staging system for multiple myeloma. J Clin Oncol. 2005;23:3412–3420. doi: 10.1200/JCO.2005.04.242. [DOI] [PubMed] [Google Scholar]
- 33.Dimopoulos MA, Weber D, Kantarjian H, Delasalle KB, Alexanian R. Hyper CVAD for VAD-resistant multiple myeloma. Am J Hematol. 1996;52:77–81. doi: 10.1002/(SICI)1096-8652(199606)52:2<77::AID-AJH2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Palumbo A, Bringhen S, Caravita T, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367:825–831. doi: 10.1016/S0140-6736(06)68338-4. [DOI] [PubMed] [Google Scholar]
- 35.Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003;349:2495–2502. doi: 10.1056/NEJMoa032290. [DOI] [PubMed] [Google Scholar]
- 36.Cavo M, Tosi P, Zamagni E, et al. Prospective, randomized study of single compared with double autologous stem-cell transplantation for multiple myeloma: Bologna 96 clinical study. J Clin Oncol. 2007;25:2434–2441. doi: 10.1200/JCO.2006.10.2509. [DOI] [PubMed] [Google Scholar]
- 37.Attal M, Harousseau JL, Leyvraz S, et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108:3289–3294. doi: 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 38.Bruno B, Rotta M, Patriarca F, et al. A comparison of allografting with autografting for newly diagnosed myeloma. N Engl J Med. 2007;356:1110–1120. doi: 10.1056/NEJMoa065464. [DOI] [PubMed] [Google Scholar]
- 39.Barbey JT, Pezzullo JC, Soignet SL. Effect of arsenic trioxide on QT interval in patients with advanced malignancies. J Clin Oncol. 2003;21:3609–3615. doi: 10.1200/JCO.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Qazilbash MH, Saliba RM, Aleman A, et al. Risk factors for relapse after complete remission with high-dose therapy for multiple myeloma. Leuk Lymphoma. 2006;47:1360–1364. doi: 10.1080/10428190500520806. [DOI] [PubMed] [Google Scholar]
- 41.Qazilbash MH, Saliba RM, Hosing C, et al. Autologous stem cell transplantation is safe and feasible in elderly patients with multiple myeloma. Bone Marrow Transplant. 2007;39:279–283. doi: 10.1038/sj.bmt.1705580. [DOI] [PubMed] [Google Scholar]