Abstract
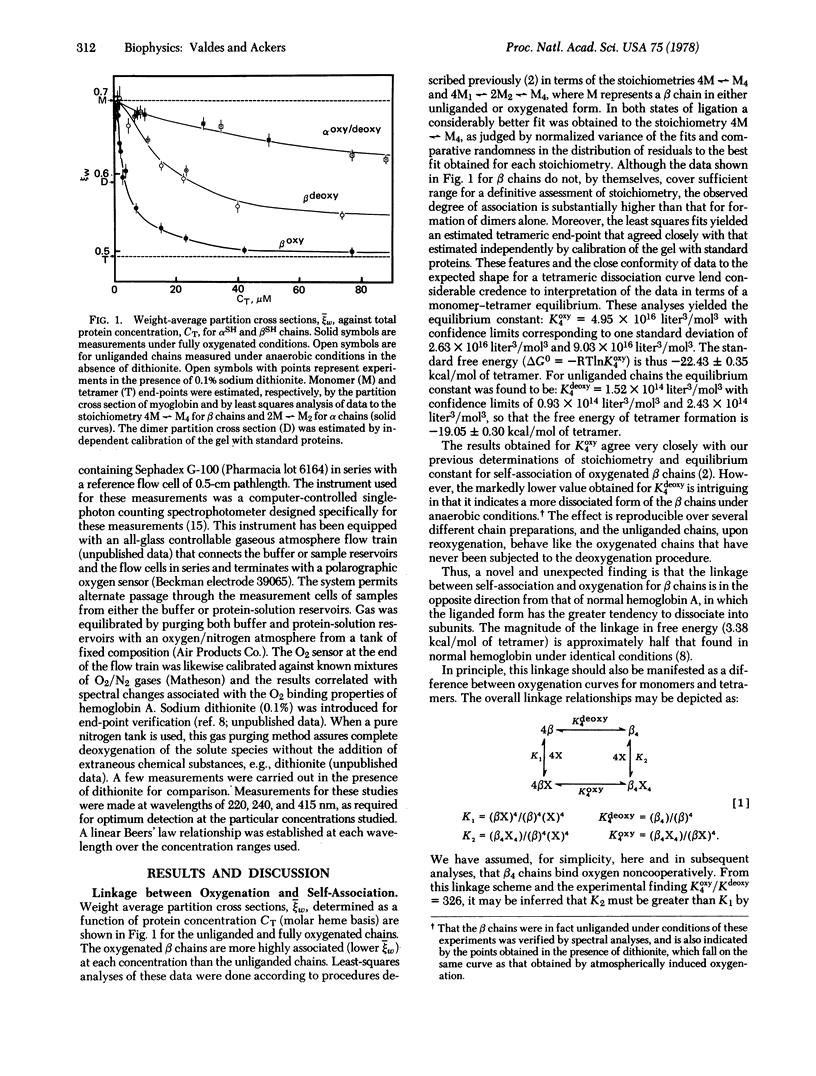
Self-association of unliganded βSH chains into tetramers (4 β1 [unk] β4) is experimentally found to be energetically less favorable (ΔG0 = -19.05 ± 0.30 kcal) than the corresponding oligomerization of fully oxygenated chains (4 β1X [unk] β4X4; ΔG0 = -22.45 ± 0.35 kcal). Hence the tetramers must bind oxygen with a higher affinity than that of dissociated chains. Calculations are presented showing why this affinity difference is not easily detected. The linkage is in a direction opposite to that exhibited by normal hemoglobin A, in which oligomerization of high-affinity unliganded dimers (2 αβ [unk] α2β2) leads to tetramers with decreased oxygen affinity. In contrast, the oligomerization of high-affinity, unliganded βSH chains leads to tetramers with even higher affinity. The results imply the existence of at least two conformational states for β chains.
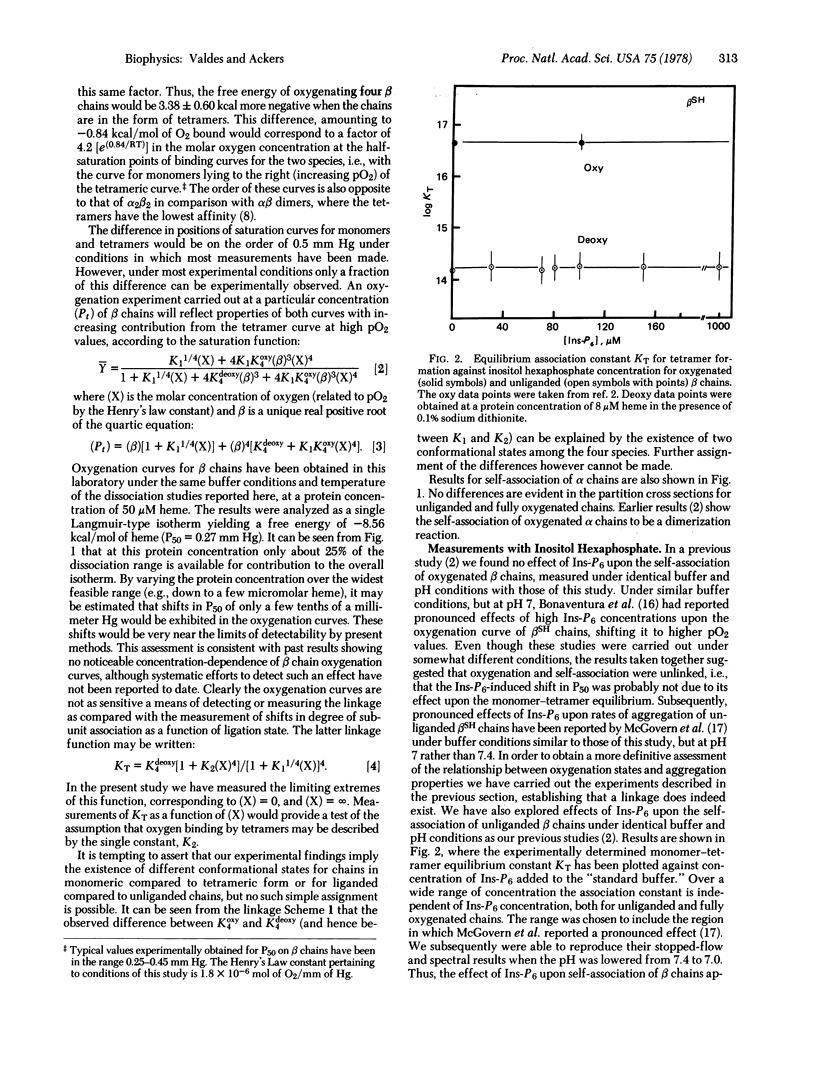
Effects of inositol hexaphosphate on β chain association were investigated. Inositol hexaphosphate was found to have no measurable effect at pH 7.4, in contrast to pH 7 where very pronounced effects have been observed. Some theoretical aspects of the linkages are presented and the relationship of the findings to concepts of structural transition and allosteric regulation is discussed.
In contrast to the β chains, self-association of α chains into dimers was found to occur with the same free energy in both unliganded and fully oxygenated states. Thus, the self-association of α chains is not linked to oxygenation.
Keywords: protein association, ligand binding, thermodynamics, equilibrium gel permeation
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackers G. K., Brumbaugh E. E., Ip S. H., Halvorson H. R. Equilibrium gel permeation: a single-photon counting spectrophotometer for studies of protein interaction. Biophys Chem. 1976 Mar;4(2):171–179. doi: 10.1016/0301-4622(76)85007-7. [DOI] [PubMed] [Google Scholar]
- Andersen M. E., Moffat J. K., Gibson Q. H. The kinetics of ligand binding and of the association-dissociation reactions of human hemoglobin. Properties of deoxyhemoglobin dimers. J Biol Chem. 1971 May 10;246(9):2796–2807. [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. Homos and heteros among the hemos. Science. 1974 Sep 13;185(4155):905–908. doi: 10.1126/science.185.4155.905. [DOI] [PubMed] [Google Scholar]
- Bonaventura J., Bonaventura C., Amiconi G., Tentori L., Brunori M., Antonini E. Allosteric interactions in non-alpha chains isolated from normal human hemoglobin, fetal hemoglobin, and hemoglobin Abruzzo (beta143 (H21) His replaced by Arg). J Biol Chem. 1975 Aug 25;250(16):6278–6281. [PubMed] [Google Scholar]
- De Renzo E. C., Ioppolo C., Amiconi G., Antonini E., Wyman J. Properties of the alpha and beta chains of hemoglobin prepared from their mercuribenzoate derivatives by treatment with 1-dodecanethiol. J Biol Chem. 1967 Nov 10;242(21):4850–4853. [PubMed] [Google Scholar]
- Hewitt J. A., Kilmartin J. V., Eyck L. F., Perutz M. F. Noncooperativity of the dimer in the reaction of hemoglobin with oxygen (human-dissociation-equilibrium-sulfhydryl-absorption-x-ray analysis). Proc Natl Acad Sci U S A. 1972 Jan;69(1):203–207. doi: 10.1073/pnas.69.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ip S. H., Ackers G. K. Thermodynamic studies on subunit assembly in human hemoglobin. Temperature dependence of the dimer-tetramer association constants for oxygenated and unliganded hemoglobins. J Biol Chem. 1977 Jan 10;252(1):82–87. [PubMed] [Google Scholar]
- Kellett G. L. Ligand-free haemoglobin dimers. Nat New Biol. 1971 Dec 8;234(49):189–191. doi: 10.1038/newbio234189a0. [DOI] [PubMed] [Google Scholar]
- McGovern P., Reisberg P., Olson J. S. Aggregation of deoxyhemoglobin subunits. J Biol Chem. 1976 Dec 25;251(24):7871–7879. [PubMed] [Google Scholar]
- Mills F. C., Johnson M. L., Ackers G. K. Oxygenation-linked subunit interactions in human hemoglobin: experimental studies on the concentration dependence of oxygenation curves. Biochemistry. 1976 Nov 30;15(24):5350–5362. doi: 10.1021/bi00669a023. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Edelstein S. J. Observation of the dissociation of unliganded hemoglobin. J Biol Chem. 1972 Dec 25;247(24):7870–7874. [PubMed] [Google Scholar]
- Valdes R., Jr, Ackers G. K. Thermodynamic studies on subunit assembly in human hemoglobin. Calorimetric measurements on the reconstitution of oxyhemoglobin from isolated chains. J Biol Chem. 1977 Jan 10;252(1):88–91. [PubMed] [Google Scholar]
- Valdes R., Jr, Ackers G. K. Thermodynamic studies on subunit assembly in human hemoglobin. Self-association of oxygenated chains (alphaSH and betaSH): determination of stoichiometries and equilibrium constants as a function of temperature. J Biol Chem. 1977 Jan 10;252(1):74–81. [PubMed] [Google Scholar]



