Abstract
Pancreatic cancer is one of the most lethal cancers worldwide. No effective screening methods exist and available treatment modalities do not effectively treat the disease. Inflammatory conditions such as pancreatitis represent a well-known risk for pancreatic cancer development. Yet only in the past two decades has pancreatic cancer been recognized as an inflammation-driven cancer, and the precise mechanisms underlying the pathogenic role of inflammation are beginning to be explored in detail. A substantial amount of preclinical and clinical evidence suggests that bacteria are likely to influence this process by activating immune receptors and perpetuating cancer-associated inflammation. The recent explosion of investigations into the human microbiome have highlighted how perturbations of commensal bacterial populations can promote inflammation and promote disease processes, including carcinogenesis. The elucidation of the interplay between inflammation and microbiome in the context of pancreatic carcinogenesis will provide novel targets for intervention in order to both prevent and treat pancreatic cancer more efficiently. Further studies towards this direction are urgently needed.
Keywords: Pancreatic cancer, inflammation, NF-κB, microbiome, bacteria, toll-like receptors, inflammasomes, dysbiosis
Introduction
The immune system is one of the main defense mechanisms of the human organism against carcinogenesis. Immune cells continuously patrol almost every site of the human body and destroycells that display features of incipient transformation. Conversely, cancer cells in advanced tumors find ways to cloak themselves from immune-surveillance – either by down-regulating their tumor-specific antigens, or by suppressing the anti-tumor immune cells – leading to immune evasion and escape1,2. Furthermore, tumor cells may manipulate the immune system in ways that support tumor growth and sustain a microenvironment favorable for cancer progression and hostile to different means of anti-cancer treatment modalities – be it chemotherapy, radiation, or targeted therapy 2. This type of tumor-associated inflammation has recently been appreciated as a novel “enabling characteristic” in the latest “Hallmarks of cancer” review, because it can facilitate the acquisition of multiple already defined hallmarks of cancer, thus pointing out its critical role in carcinogenesis 3.
Pancreatic ductal adenocarcinoma (PDAC) represents the quintessential example of an inflammation-driven cancer. This is supported by epidemiological data as well as evidence from preclinical models and clinical studies. In this review we discuss the recent advances pertaining to the link between inflammation, microbiome and pancreatic cancer, and present our perspective on this topic.
Inflammation and Pancreatic Cancer
In the case of pancreatic cancer, inflammation is relevant both as a risk factor for and as a consequence of the cancer. Patients with hereditary autoimmune pancreatitis have an estimated lifetime risk for PDAC development of 40% 4, while patients suffering from chronic pancreatitis carry a 13-fold higher risk of PDAC development 5. The duration of pancreatitis appears to correlate positively with the possibility of Kras mutations 6, which suggests a possible mutagenic role for repetitive bouts of inflammation. Interestingly, however, the majority of PDAC cases develop in the absence of clinically evident overt pancreatitis 7. Therefore we can conclude that either subclinical, low-grade inflammation is sufficient to promote carcinogenesis, or that inflammation is a consequence of the earliest events in the stepwise process of pancreatic carcinogenesis. This low-grade inflammation that is a result of cellular stress and malfunction has been termed “parainflammation” and is hypothesized to either contribute to cellular adaptation to the noxious environment or to promote senescence in order to prevent malignant transformation 8.
A considerable amount of evidence has accumulated in the past decade from work done on genetically-engineered mouse models (GEMMs) that recapitulate the sequence of events occurring in human PDAC 9. Kras is mutated in more than 90% of human PDAC cases and constitutive activation within the pancreas results in PDAC development in mice in a fashion very similar to its human counterpart 10,11. There appears to be a cooperative relationship between Kras activation and inflammation 12,13. Specifically, p48+/Cre;LsL-KrasG12D (“KC”) mice, which have mutant Kras expressed prenatally in all exocrine pancreatic lineages, exhibit pancreatitis as one of the earliest morphologic changes in their pancreas. In addition, treatment of these mice with only a few doses of caerulein – a cholecystokinin analog that hyperstimulates the pancreas and induces pancreatitis if injected repeatedly – dramatically accelerates the progression to advanced pancreatic intraepithelial neoplasia (PanIN) and invasive cancer within a few weeks (compared to months in untreated mice) 14.
A seminal discovery was made by Guerra et al, who used a Cre / Tet-off system to activate the Kras mutation in acinar cells of adult mice. Surprisingly, these mice only developed pancreatic cancer when chronic pancreatitis was induced with caerulein, suggesting that Kras mutation alone – in the absence of pancreatitis – is not sufficient to induce pancreatic cancer in adulthood 15. The same group has shown that chronic pancreatitis enables Kras-driven carcinogenesis by thwarting oncogene-induced senescence (OIS) – a homeostatic mechanism that diverts stressed cells prone to malignant transformation towards cell cycle arrest and quiescence 16. Concurrent treatment of the mice with a COX1/2 inhibitor not only prevented the progression of early PanIN to advanced PanIN and invasive cancer but also decreased the number of early PanIN lesions, pointing to additional mechanisms through which inflammation promotes pancreatic carcinogenesis 16.
Logsdon and colleagues have studied the role of inflammation and its influence on Kras-driven carcinogenesis from a different perspective 17,18. They showed that even when Kras is mutated and constitutively active, it cannot reach the expected theoretical levels of activity and remains at levels close to the basal state 17. However, inflammatory insults such as caerulein and lipopolysaccharide (LPS) can hyperstimulate Kras, bringing its activity above the hypothetical threshold necessary for the initiation of the sequence of carcinogenesis 17,18. Furthermore, constitutive activation of both Kras and either IKK2 (an activator of the NF-κB pathway) or COX2 (a downstream effector of the NF-κB pathway) in acinar cells dramatically accelerates carcinogenesis 18. On the other hand, ablation of IKK2 mitigated the caerulein-induced inflammation and fibrosis, decreased the levels of active Kras, and protected against PanIN formation 18. Similar results were observed with inhibition of COX2 18. Additional evidence was provided by a different study, which showed that mutant Kras induces the transcription of IL-1α through AP-1. IL-1α in turn activates the NF-κB pathway leading to production of more IL-1α as well as activation of the signaling adaptor p62 which prolongs the activity of this pathway 19. A very intriguing finding was that, in the context of chronic pancreatitis and tissue injury, Kras mutation can give rise to PDAC originating from insulin-positive endocrine cells 20. The significance of this lies in the fact that chronic inflammation can induce de-differentiation of committed epithelial cells and thence promote carcinogenesis. In summary, inflammation synergizes with Kras through the establishment of a positive feedback loop that is dependent on NF-κB and COX2 and leads to sustained Kras activity; on the other hand, Kras activity promotes an IL-1α- and p62-mediated feed-forward loop that sustains NF-κB pathway activity.
Several other mechanisms contributing to inflammation-driven carcinogenesis in PDAC have been identified to date. A notable example is STAT3, which appears to be a central player in pancreatic carcinogenesis. STAT3 is activated in mice challenged with caerulein. In wild-type (WT) mice, the activation status reverts to baseline after a few days consistent with recovery from acute pancreatitis. In contrast, in KC mice STAT3 remains persistently activated 21. This is a consequence of communication between the epithelial cells and the surrounding stromal cells. Specifically, the Kras-mutant epithelial cells recruit myeloid cells which secrete IL-6 and activate the STAT3 pathway in the epithelial cells via IL-6 trans-signaling, thus completing a positive feedback loop 22. Persistent STAT3 activation drives pancreatic cancer progression through upregulation of anti-apoptotic and pro-proliferative proteins such as Bcl-XL, Mcl-1, Survivin, c-Myc and Cyclin D1 21-23. In incipient carcinogenesis, this may be critical for the evasion of OIS, while later on it may be more important for proliferation under the adverse conditions of the hypoxic tumor microenvironment. Moreover, STAT3 activation in epithelial cells promotes the secretion of pro-inflammatory mediators which further recruit leukocytes; at the same time, epithelial STAT3 signaling induces the expression of matrix metallopeptidase 7 (MMP7) which supports tumor growth and metastasis 21. Genetic ablation of IL-6 or STAT3 , neutralization of IL-6 trans-signaling, as well as deletion of STAT3 exclusively in the epithelial cells dampens tumor-associated inflammation and protects from spontaneous and caerulein-induced PanIN formation and PDAC development 21,22.
The pancreatic tumor microenvironment is rife with factors that can attract inflammatory cells and entrain them to support the process of carcinogenesis and shield the cancer cells from the anti-tumorigenic arm of the immune system. Kras-mutant epithelial cells secrete granulocyte-macrophage colony-stimulating factor (GM-CSF) to recruit myeloid cells that suppress CD8+ cytotoxic T cells 24,25. The secretion of GM-CSF begins early on, and its neutralization depletes myeloid-derived suppressor cells (MDSCs) and enables efficient tumor killing by CD8+ T cells 24,25. In a similar fashion, pancreatic cancer cells produce the chemokine CCL2 that mobilizes inflammatory monocytes from the bone marrow and recruits them to the pancreas as well as to premetastatic niches such as the liver to promote tumor growth and metastasis, respectively 26. Cancer cells also secrete CCL5 and other ligands that recruit regulatory T cells (Treg) in a CCR5-dependent manner and thus contribute to an immunosuppressive tumor microenvironment 27.
Several studies have shown that cancer cells can also influence the immune response indirectly, by manipulating other stromal cells. Notably, cancer-associated fibroblasts (CAFs) – which in pancreatic cancer may originate from pancreatic stellate cells (PSCs) – are induced to express an NF-κB-dependent pro-inflammatory gene signature that enhances tumor growth, vascularization and macrophage recruitment 28. Secondly, inflammatory mediators such as IL-1β and TNF released by the cancer cells instruct CAFs to release thymic stromal lymphopoietin (TSLP), which in turn signals to myeloid dendritic cells (DCs) to skew CD4+ T cells towards TH2 polarization 29. TH2-deviated CD4+ T cells have a pro-carcinogenic role in the pancreas through the perpetuation of pancreatic fibro-inflammation and the recruitment of M2 macrophages 29,30. Thirdly, activated PSCs upregulate adhesion molecules and secrete chemokines – the most prominent being CXCL12 – that sequester CD8+ T cells around them and prevent them from attacking the cancer cells 31. Another study validated these results and further showed that fibroblast activation protein (FAP)-expressing CAFs secreting CXCL12 are the limiting factor for the efficacy of T cell checkpoint inhibitors 32. Inhibition of CXCL12 reverses the immunosuppressive effects of PSC/CAFs and synergizes with anti-PD-L1 immunotherapy leading tumor elimination 31,32.
Given the fact that inflammation is such an important contributor and is present very early in the stepwise process of pancreatic carcinogenesis, it is reasonable to assume that it would be an attractive target for chemoprevention. Indeed, multiple approaches have been adopted using either natural compounds with diverse effects or synthetic compounds that have specific targets 33. For example, inhibition of cyclooxygenases has been investigated in multiple studies using both non-steroidal anti-inflammatory drugs (aspirin, nimesulide, etodolac, sulindac etc) and COX2-specific inhibitors (e.g. celecoxib) 33,34. Several of those have been associated with decreased risk of pancreatic cancer 33,34. Curcumin – an agent with pleiotropic effects on the tumor stroma and the associated inflammation – also seems to have a protective effect in pancreatic carcinogenesis 33. Prospective studies in larger cohorts are required to address the efficacy of such compounds in preventing pancreatic cancer.
Microbiome and Pancreatic Cancer
Infectious agents are estimated to be responsible for 10%-20% of all cancers globally 35, yet none has been established as causative for pancreatic cancer so far. However, in the last few years several studies have presented tangential evidence that suggests a possible role of microbes in the pathogenesis of pancreatic cancer (Tables 1 and 2). Previous studies have attempted to associate pathogens such as Helicobacter pylori with pancreatic cancer using serologic and culture-based methods (Table 2). However, the fact that an overwhelming percentage of the commensal microflora are non-cultivable has precluded objective investigation of their influence in such diseases as pancreatitis 36. New techniques such as next generation sequencing and metagenomics now enable a representative evaluation of the microbiotic communities in health and disease, and their dynamic interactions with their human host 37. More importantly, the human microbiome has only recently been appreciated as an indispensable factor for the normal development of the immune system as well as a key modulator of disease when the homeostatic relations between host and microorganisms are deranged 38-40. In the latter state – called dysbiosis – certain members of the microbial community may decrease in numbers and their place may be taken by other, less prevalent bacteria that can become pathogenic if they reach high concentrations – hence termed “pathobionts”. The role of such global shifts in the microbiome composition rather than causative role of specific pathogens has not been evaluated in the context of pancreatic carcinogenesis.
Table 1.
Preclinical evidence supporting a role of microbial pathogens in pancreatic carcinogenesis
| Model | Microbe / Microbial component investigated | Mediator(s) / Mechanism(s) | Findings | Refs |
|---|---|---|---|---|
| Caerulein-induced pancreatitis | LPS | - | • LPS synergizes with caerulein to induce severe acute pancreatitis. | 52 |
| Caerulein-induced pancreatitis; L-arginine-induced pancreatitis | LPS; cultivable bacteria* | TLR4 CD14 |
•Genetic ablation of TLR4 or CD14 protects mitigates acute pancreatitis •Inoculation of blood agar plates with blood and pancreas samples from mice undergoing pancreatitis did not result in bacterial growth * •No LPS was detected in any tissue sample higher than 1 unit of LPS per ml (using the chromogenic Limulus amebocyte lysate assay) |
36 |
| Caerulein-induced pancreatitis | - | TLR9 NLRP3 inflammasome |
•TLR9 blockade mitigates pancreatitis •Genetic ablation of TLR9, or the inflammasome components ASC, NLRP3, and Caspase 1 decreased the severity of pancreatitis |
55 |
| Caerulein-induced pancreatitis | intestinal microbiota; ampicillin- and kanamycin-resistant E. coli | TLR4 NOD1 |
•TLR4−/− and NOD1−/− mice but not TLR2−/− or TLR9−/− are protected from acute pancreatitis •Bowel sterilization with broad-spectrum antibiotics protected from acute pancreatitis •Repeated administration of antibiotic-resistant E. coli to antibiotic-pretreated mice exacerbated acute pancreatitis |
56 |
| Combination of caerulein and glycodeoxycholic acid-induced pancreatitis; concurrent terminal loop ileostomy | Small bowel -vs- colon microbiota | Small bowel–mesenteric lymph nodes–pancreas as source of superinfection during pancreatitis | •Selective decontamination of the small bowel reduced bacterial overgrowth in the small bowel, bacterial translocation to mesenteric lymph nodes, and subsequent superinfection of pancreatic necrosis •Selective decontamination of the colon did not have significant effects |
59 |
| Human PDAC cell lines | H. pylori | NF-κB, AP-1, CagA | •Human PDAC cell lines exposed to H. pylori increased the activities of proliferation factors NF-kB, AP-1, and SRE, and secreted higher levels of IL-8 and VEGF •H. pylori secreted CagA into pancreatic cancer cells |
60 |
| p48+/Cre;LsL-KrasG12D/+ | LPS | TLR4 | •LPS accelerates pancreatic carcinogenesis •TLR4 and TRIF blockade attenuate carcinogenesis •MyD88 blockade exacerbates carcinogenesis through DC-mediated skewing of CD4+ T cells towards TH2 phenotype |
51 |
| Ela-CreERT;LsL-KrasG12D/+ | LPS | NF-κB | • LPS synergizes with Kras mutation in acinar cells to induce pancreatitis and accelerate pancreatic carcinogenesis | 18 |
| p48+/Cre;LsL-KrasG12D/+ | ssRNA | TLR7 | •ssRNA accelerates pancreatic carcinogenesis •Ablation of TLR7 in immune cells attenuate carcinogenesis |
50 |
AP-1, activator protein 1; CagA, cytotoxin-associated gene-A; DC, dendritic cell; E. coli, Escherichia coli; H. pylori, Helicobacter pylori; IL-8, interleukin-8; LPS, lipopolysaccharide; MyD88, myeloid differentiation primary response gene (88); NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3, NOD-like receptor family, pyrin domain containing 3; NOD1, nucleotide-binding oligomerization domain-containing protein 1; PDAC, pancreatic ductal adenocarcinoma; SRE, serum response elements; ssRNA, single-stranded RNA; TRIF, TIR-domain-containing adapter-inducing interferon-β; TLR, toll-like receptor; VEGF, vascular endothelial growth factor.
We question the reliability of these results due to significant limitations of the techniques employed37.
Table 2.
Human studies investigating a role of bacterial pathogens in pancreatic carcinogenesis
| Association tested | Study design | Patients & Samples | Implicated Microbe(s) | Conclusions | Refs |
|---|---|---|---|---|---|
| Composition of the oral microbiome | Case-control | Saliva samples Discovery phase: 10 resectable PDAC, 10 controls Validation phase: 28 resectable PDAC, 27 chronic pancreatitis, 28 controls |
N. elongata; S. mitis; Granulicatella adiacens | •Specific oral bacterial profiles are associated with pancreatitis and pancreatic cancer •The levels of N. elongata and S. mitis decreased in pancreatic cancer while G. adiacens levels increased •Combination of N. elongata and S. mitis yields 96.4% sensitivity and 82.1% specificity in distinguishing pancreatic cancer patients from controls |
48 |
| Periodontal disease | Prospective population-based | Questionnaire-based Male health professionals 40-75y (N=51,529) 216 PDAC cases over 16y follow-up |
- | •History of periodontal disease is associated with increased risk of pancreatic cancer overall and in never smokers •Recent tooth loss further increases the risk |
44 |
| Antibodies to specific oral bacteria | Prospective population-based | Blood samples (pre-diagnosis) Ages 35-70y (N=519,978) 405 PDAC cases, 416 controls |
P. gingivalis ATCC 53978; commensal oral bacteria | •High levels of antibodies against P. gingivalis ATCC 53978 confer higher risk of pancreatic cancer •High levels of antibodies against commensal oral bacteria decrease the risk of pancreatic cancer |
61 |
| H. pylori seropositivity | Case-control | Blood samples; tumor samples (n=20) 92 PDAC cases; 30 gastric cancer and 35 colorectal cancer cases and 27 healthy volunteers as controls |
H. pylori | •Pancreatic cancer cases had equal risk of H. pylori seropositivity as gastric cancer cases but higher than colorectal cancer cases or healthy controls. •H. pylori could not be identified in pancreatic tissue using conventional H&E stain. |
62 |
| H. pylori seropositivity, H. pylori CagA+ strains | Prospective case-control | Blood samples Male smokers 50-69y (ABTC study) 123 exocrine panc. cancer cases, 226 controls |
H. pylori | •Patients with exocrine pancreatic cancer had higher rates of seroprevalence for H. pylori, compared to controls •CagA+ seropositivity further elevates the risk |
63 |
| H. pylori seropositivity, specific H. pylori antigens | Prospective case-control | Blood samples, multiplex serology assay Male smokers 50-69y (ABTC study, longer follow-up) 353 exocrine panc. cancer cases, 353 controls |
H. pylori | • Neither specific H. pylori antigens in serum nor the overall seropositivity (positive for ≥4 antigens) was associated with development of pancreatic cancer. | 64 |
| H. pylori seropositivity, H. pylori CagA+ strains | Prospective case-control | Blood samples 373 newly-diagnosed PDAC cases, 690 controls |
H. pylori | • H. pylori colonization is associated with higher risk of pancreatic cancer, especially for individuals with non-O blood types. | 65 |
CagA, cytotoxin-associated gene-A; G. adiacens, Granulicatella adiacens; H&E, hematoxylin and eosin; H. pylori, Helicobacter pylori;
Environmental insults can alter the composition of the intestinal microflora and also increase the intestinal permeability, allowing pathobionts to gain access to the bloodstream and reach distant organs. For example, alcohol consumption – the most common cause for chronic pancreatitis – has been linked to dysfunction of the intestinal barrier function and overgrowth of Gram-negative bacteria in the intestine, leading to elevated systemic levels of LPS 41-43. In addition, bacteria administered orally to healthy WT mice can reach the pancreas and persist there for several hours (C.P.Z. and G.M., unpublished data). We believe that reflux of intestinal contents through the main pancreatic duct may be a second route through which bacteria can access the pancreas.
Another situation that may permit bacterial translocation is poor oral hygiene and associated diseases. It is well established that individuals with periodontitis and tooth loss – conditions caused by dysbiosis of oral bacteria – are at increased risk for pancreatic cancer 44-47. A recent epidemiologic study presented more convincing evidence for this by revealing associations between specific profiles of oral bacteria and increased risk of pancreatitis and pancreatic cancer 48 (Table 2). Interestingly, this latter study investigated the utility of the discovered bacterial profiles as biomarkers for pancreatitis and pancreatic cancer and has thus exemplified how perturbations in the microbiome of the gastrointestinal tract may be exploited as biomarkers for non-invasive screening of pancreatic disease 48.
The most plausible mechanism for the pro-carcinogenic effect of microbes seems to involve chronic low-grade activation of the immune system and perpetuation of tumor-associated inflammation, rather than direct mutagenic effects (Figure 1). Toll-like receptors (TLRs) represent the best described family of pattern-recognition receptors (PRRs). They are present on most types of immune cells and they bind a variety of microbe-associated molecular patterns (MAMPs, such as LPS) as well as byproducts of dying cells and sterile inflammation denoted DAMPs (damage-associated molecular patterns) 49. Upon ligand binding, they recruit either the MyD88 or the TRIF adaptor molecules (depending on the specific TLR) to transduce activation signals to the NF-κB and MAPK pathways. We found that TLR4 and TLR7 are upregulated within the tumor microenvironment of pancreatic cancer 50,51. Further, we and others have shown that TLR activation can fuel pancreatitis and can synergize with Kras to dramatically accelerate pancreatic carcinogenesis in mice 18,36,50-52. These pro-carcinogenic effects of TLRs can be prevented through inhibition of either NF-κB or MAPK pathway 51. Furthermore, mice deficient in several TLRs are protected from acute pancreatitis (Table 1). Direct inhibition of TLR4 as well as TLR7 protects KC mice from pancreatic carcinogenesis 50,51.
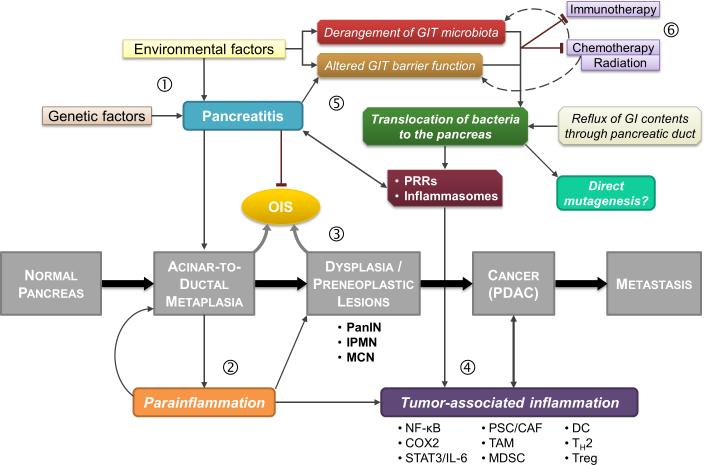
Figure 1. Summary of the interplay between tumor-associated inflammation and microbiome, and their key roles in pancreatic carcinogenesis.
Pancreatic cancer progresses through a series of defined stages that involve acinar-to-ductal metaplasia (ADM) in response to repetitive injury, development of pre-neoplastic lesions, and eventually invasive cancer. (1) Pancreatitis can be caused by genetic and environmental factors (e.g. alcohol), and can promote pancreatic carcinogenesis by inducing ADM while inhibiting OIS. (2) Noxious stimuli to the pancreas result in a low-grade maladaptive inflammatory response termed “parainflammation” that can synergize with mutant Kras in tumor development and evasion of OIS (3), and recruit immune cells that promote cancer-associated inflammation (4). Environmental factors, such as antibiotics and alcohol consumption, as well as pancreatitis can cause derangement of the gut microbiome and compromise the intestinal barrier function to promote translocation of bacteria to the pancreas (5). Translocated dysbiotic bacteria (pathobionts) can stimulate PRRs and inflammasomes and perpetuate tumor-associated inflammation. Treatment modalities such as chemotherapy and ratiotherapy can also influence the microbiome; alterations in the microbiome (dysbiosis) can have detrimental effects on the efficacy of chemotherapy and immunotherapy (6).
CAF, cancer-associated fibroblasts; DC, dendritic cells; GIT, gastrointestinal tract; IPMN, intraductal papillary mucinous neoplasm; MCN, mucinous cystic neoplasm; MDSC, myeloid-derived suppressor cells; OIS, oncogene-induced senescence; PanIN, pancreatic intraepithelial neoplasia; PRRs, pattern-recognition receptors; PSC, pancreatic stellate cells; TAM, tumor-associated macrophages.
Other PRRs and associated signaling molecules have been implicated in the pathogenesis of inflammation-driven cancer. Nucleotide-binding domain and leucine-rich repeat containing molecules or NOD-like receptors (NLRs) are cytoplasmic PRRs. When engaged by their ligands, they can activate the NF-κB pathway but they also associate with other molecules to form large oligomeric complexes called inflammasomes 53. Caspase-1 is a key component of activated inflammasomes and is responsible for the proteolytic cleavage and maturation of the pro-inflammatory cytokines IL-1β and IL-18 53. NLRs and their downstream effectors are necessary for keeping the intestinal microbiota under control. For example, mice deficient in several NLRs, Caspase-1, or IL-18 exhibit alterations in the gut microbiome and dysbiosis, and are highly susceptible to colorectal carcinogenesis 54. Consistent with their pro-inflammatory role, the inflammasomes have been found to contribute to the pathogenesis of pancreatitis 55,56. Specifically, administration of a NOD1 agonist synergized with caerulein in the induction of acute pancreatitis 56. This was a result of NOD1 activation in acinar cells, which promoted acinar NF-κB and STAT3 signaling, and CCL2-mediated recruitment of CCR2+ pro-inflammatory cells 56.Furthermore, genetic ablation of NOD1, Nlrp3, Caspase-1, or ASC (another component of the inflammasome) protects from caerulein-induced acute pancreatitis 55,56.
The implication of bacterial dysbiosis in pancreatic carcinogenesis has very interesting ramifications. Even though genetically-identical, the current genetically-engineered mouse models of pancreatic cancer exhibit variability in the rate and extent of tumor progression, such that even age-matched littermate mice can have significantly different tumors 10. This variability may well be explained by variations in the commensal microbiota of these mice. In the same manner, variations in the gastrointestinal tract microbiome may have prognostic significance in patients with newly-diagnosed pancreatic cancer, with certain bacterial profiles correlating with more aggressive disease. Conversely, alterations of the intestinal microbiome that occur before the appearance of clinically detectable disease may be exploited towards the development of new preclinical screening tests for the early diagnosis of pancreatic cancer. These hypotheses deserve further investigation.
Recent studies on the influence of the microbiome in carcinogenesis have highlighted crucial roles in other gastrointestinal malignancies such as colon and liver cancer – both of which have an important inflammatory component (studies summarized in 40). An invaluable tool for these studies has been the ability to generate and maintain germ-free mice. In most models, germ-free mice have been found to be less prone to carcinogenesis, likely because of less tumor-associated inflammation 40. These results have also been reproduced in using antibiotic-treated mice that decrease the microbial load of the gut 40. Similar experiments need to be conducted using mouse models of pancreatic cancer. Notably, bowel sterilization with broad-spectrum antibiotics has been shown to have a protective effect in acute pancreatitis 56. On the other hand, administration of antibiotic-resistant Escherichia coli to bowel-sterilized mice undergoing acute pancreatitis results in more severe disease 56.
Since the intestinal microbiome is modifiable, it may represent a potential new target for therapeutic intervention. This may be achieved by targeted antibiotic therapy to eradicate the microbial species that are associated with an increased pancreatic cancer risk as well as other methods such as probiotics to (re)introduce the species that are associated with a decreased cancer risk. Moreover, modification of the intestinal microbiome may serve as a prophylactic approach for preventing pancreatic cancer development in high-risk individuals such as patients with strong family histories or those harboring high grade PanIN lesions.
An unexpected role of dysbiosis has been revealed very recently through two seminal studies that investigated its impact on the efficacy of anticancer chemotherapy and immunotherapy 57,58. The rationale behind the studies was that patients treated with chemotherapy often develop mucositis, which impairs the intestinal barrier function, and neutropenia, which requires the administration of broad-spectrum antibiotics that can potentially cause perturbations in the gut microbiome. The first study showed that cyclophosphamide alters the composition of the gut microbiome in mice and promotes the translocation of certain Gram-positive bacteria to secondary lymphoid organs 57. The bacteria in turn prime the immune system to generate TH1 and TH17-deviated T cells that are necessary for the immune-mediated tumoricidal effects of chemotherapy 57. The second study had similar findings with oxaliplatin and further expanded to show that the absence of gut microbiota compromises the efficacy of CpG- and anti-IL-10-based immunotherapy due to ineffective priming of tumor-infiltrating myeloid cells and consequently lack of ROS-dependent apoptosis and TNF-dependent necrosis 58. These findings highlight how iatrogenic factors can lead to disequilibrium in the commensal flora and how this can affect not only disease progression but also response to treatment.
Conclusion
It is well established that inflammation is a critical factor in pancreatic carcinogenesis, beginning at a very early stage. The mechanisms involved are just beginning to be uncovered in detail. Furthermore, the microbiome seems to be intricately connected to cancer-associated inflammation. The elucidation of the interplay between these two factors will allow us to identify novel targets for intervention in order to both prevent and treat pancreatic cancer more efficiently. Finally, the microbiome holds promise as a biomarker for early detection of pancreatic cancer. Further studies are urgently needed to address the exciting hypothesis of a pro-carcinogenic role of perturbed gut microbiome in pancreatic cancer.
Acknowledgments
Research reported in this publication was supported by NCI 1CA168611 (G.M.), the Lustgarden Foundation (G.M. and D.S.), and the National Pancreas Foundation (C.Z.).
Grant Support: Research reported in this publication was supported by NCI 1CA168611 (G.M.), the Lustgarden Foundation (G.M. and D.S.), and the National Pancreas Foundation (C.Z.).
Footnotes
Conflict of Interest
None declared.
REFERENCES
- 1.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117(5):1137–46. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vesely MD, Kershaw MH, Schreiber RD, et al. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–71. doi: 10.1146/annurev-immunol-031210-101324. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Bartsch DK, Gress TM, Langer P. Familial pancreatic cancer--current knowledge. Nature reviews Gastroenterology & Hepatology. 2012;9(8):445–53. doi: 10.1038/nrgastro.2012.111. [DOI] [PubMed] [Google Scholar]
- 5.Yadav D, Lowenfels AB. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144(6):1252–61. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lohr M, Kloppel G, Maisonneuve P, et al. Frequency of K-ras mutations in pancreatic intraductal neoplasias associated with pancreatic ductal adenocarcinoma and chronic pancreatitis: a meta-analysis. Neoplasia. 2005;7(1):17–23. doi: 10.1593/neo.04445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yeo TP, Lowenfels AB. Demographics and epidemiology of pancreatic cancer. Cancer Journal. 2012;18(6):477–84. doi: 10.1097/PPO.0b013e3182756803. [DOI] [PubMed] [Google Scholar]
- 8.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–35. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 9.Mazur PK, Siveke JT. Genetically engineered mouse models of pancreatic cancer: unravelling tumour biology and progressing translational oncology. Gut. 2012;61(10):1488–500. doi: 10.1136/gutjnl-2011-300756. [DOI] [PubMed] [Google Scholar]
- 10.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–50. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 11.di Magliano MP, Logsdon CD. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144(6):1220–9. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ancrile BB, O'Hayer KM, Counter CM. Oncogenic ras-induced expression of cytokines: a new target of anti-cancer therapeutics. Molecular Interventions. 2008;8(1):22–7. doi: 10.1124/mi.8.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pylayeva-Gupta Y, Grabocka E, Bar-Sagi D. RAS oncogenes: weaving a tumorigenic web. Nat Rev Cancer. 2011;11(11):761–74. doi: 10.1038/nrc3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carriere C, Young AL, Gunn JR, et al. Acute pancreatitis markedly accelerates pancreatic cancer progression in mice expressing oncogenic Kras. Biochem Biophys Res Commun. 2009;382(3):561–5. doi: 10.1016/j.bbrc.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guerra C, Schuhmacher AJ, Canamero M, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11(3):291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Guerra C, Collado M, Navas C, et al. Pancreatitis-induced inflammation contributes to pancreatic cancer by inhibiting oncogene-induced senescence. Cancer Cell. 2011;19(6):728–39. doi: 10.1016/j.ccr.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang H, Daniluk J, Liu Y, et al. Oncogenic K-Ras requires activation for enhanced activity. Oncogene. 2014;33(4):532–5. doi: 10.1038/onc.2012.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daniluk J, Liu Y, Deng D, et al. An NF-kappaB pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122(4):1519–28. doi: 10.1172/JCI59743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ling J, Kang Y, Zhao R, et al. KrasG12D-induced IKK2/beta/NF-kappaB activation by IL-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21(1):105–20. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gidekel Friedlander SY, Chu GC, Snyder EL, et al. Context-dependent transformation of adult pancreatic cells by oncogenic K-Ras. Cancer Cell. 2009;16(5):379–89. doi: 10.1016/j.ccr.2009.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fukuda A, Wang SC, Morris JPt, et al. Stat3 and MMP7 contribute to pancreatic ductal adenocarcinoma initiation and progression. Cancer Cell. 2011;19(4):441–55. doi: 10.1016/j.ccr.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lesina M, Kurkowski MU, Ludes K, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19(4):456–69. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 23.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nature Reviews Cancer. 2009;9(11):798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pylayeva-Gupta Y, Lee KE, Hajdu CH, et al. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell. 2012;21(6):836–47. doi: 10.1016/j.ccr.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bayne LJ, Beatty GL, Jhala N, et al. Tumor-derived granulocyte-macrophage colony-stimulating factor regulates myeloid inflammation and T cell immunity in pancreatic cancer. Cancer Cell. 2012;21(6):822–35. doi: 10.1016/j.ccr.2012.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanford DE, Belt BA, Panni RZ, et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clinical Cancer Research : An Official Journal of the American Association for Cancer Research. 2013;19(13):3404–15. doi: 10.1158/1078-0432.CCR-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tan MC, Goedegebuure PS, Belt BA, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. Journal of Immunology. 2009;182(3):1746–55. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erez N, Truitt M, Olson P, et al. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell. 2010;17(2):135–47. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 29.De Monte L, Reni M, Tassi E, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med. 2011;208(3):469–78. doi: 10.1084/jem.20101876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Demols A, Le Moine O, Desalle F, et al. CD4(+ )T cells play an important role in acute experimental pancreatitis in mice. Gastroenterology. 2000;118(3):582–90. doi: 10.1016/s0016-5085(00)70265-4. [DOI] [PubMed] [Google Scholar]
- 31.Ene-Obong A, Clear AJ, Watt J, et al. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145(5):1121–32. doi: 10.1053/j.gastro.2013.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feig C, Jones JO, Kraman M, et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(50):20212–7. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stan SD, Singh SV, Brand RE. Chemoprevention strategies for pancreatic cancer. Nature reviews Gastroenterology & Hepatology. 2010;7(6):347–56. doi: 10.1038/nrgastro.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cui XJ, He Q, Zhang JM, et al. High-dose aspirin consumption contributes to decreased risk for pancreatic cancer in a systematic review and meta-analysis. Pancreas. 2014;43(1):135–40. doi: 10.1097/MPA.0b013e3182a8d41f. [DOI] [PubMed] [Google Scholar]
- 35.Chang AH, Parsonnet J. Role of bacteria in oncogenesis. Clin Microbiol Rev. 2010;23(4):837–57. doi: 10.1128/CMR.00012-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharif R, Dawra R, Wasiluk K, et al. Impact of toll-like receptor 4 on the severity of acute pancreatitis and pancreatitis-associated lung injury in mice. Gut. 2009;58(6):813–9. doi: 10.1136/gut.2008.170423. [DOI] [PubMed] [Google Scholar]
- 37.Fraher MH, O'Toole PW, Quigley EM. Techniques used to characterize the gut microbiota: a guide for the clinician. Nature reviews Gastroenterology & Hepatology. 2012;9(6):312–22. doi: 10.1038/nrgastro.2012.44. [DOI] [PubMed] [Google Scholar]
- 38.Kau AL, Ahern PP, Griffin NW, et al. Human nutrition, the gut microbiome and the immune system. Nature. 2011;474(7351):327–36. doi: 10.1038/nature10213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Human Microbiome Project C. Structure, function and diversity of the healthy human microbiome. Nature. 2012;486(7402):207–14. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13(11):800–12. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Purohit V, Bode JC, Bode C, et al. Alcohol, intestinal bacterial growth, intestinal permeability to endotoxin, and medical consequences: summary of a symposium. Alcohol. 2008;42(5):349–61. doi: 10.1016/j.alcohol.2008.03.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yan AW, Schnabl B. Bacterial translocation and changes in the intestinal microbiome associated with alcoholic liver disease. World Journal of Hepatology. 2012;4(4):110–8. doi: 10.4254/wjh.v4.i4.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yan AW, Fouts DE, Brandl J, et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011;53(1):96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Michaud DS, Joshipura K, Giovannucci E, et al. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J Natl Cancer Inst. 2007;99(2):171–5. doi: 10.1093/jnci/djk021. [DOI] [PubMed] [Google Scholar]
- 45.Stolzenberg-Solomon RZ, Dodd KW, Blaser MJ, et al. Tooth loss, pancreatic cancer, and Helicobacter pylori. Am J Clin Nutr. 2003;78(1):176–81. doi: 10.1093/ajcn/78.1.176. [DOI] [PubMed] [Google Scholar]
- 46.Hiraki A, Matsuo K, Suzuki T, et al. Teeth loss and risk of cancer at 14 common sites in Japanese. Cancer Epidemiol Biomarkers Prev. 2008;17(5):1222–7. doi: 10.1158/1055-9965.EPI-07-2761. [DOI] [PubMed] [Google Scholar]
- 47.Hujoel PP, Drangsholt M, Spiekerman C, et al. An exploration of the periodontitis-cancer association. Ann Epidemiol. 2003;13(5):312–6. doi: 10.1016/s1047-2797(02)00425-8. [DOI] [PubMed] [Google Scholar]
- 48.Farrell JJ, Zhang L, Zhou H, et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut. 2012;61(4):582–8. doi: 10.1136/gutjnl-2011-300784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Takeuchi O, Akira S. Pattern recognition receptors and inflammation. Cell. 2010;140(6):805–20. doi: 10.1016/j.cell.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 50.Ochi A, Graffeo CS, Zambirinis CP, et al. Toll-like receptor 7 regulates pancreatic carcinogenesis in mice and humans. J Clin Invest. 2012;122(11):4118–29. doi: 10.1172/JCI63606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ochi A, Nguyen AH, Bedrosian AS, et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J Exp Med. 2012;209(9):1671–87. doi: 10.1084/jem.20111706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding SP, Li JC, Jin C. A mouse model of severe acute pancreatitis induced with caerulein and lipopolysaccharide. World J Gastroenterol. 2003;9(3):584–9. doi: 10.3748/wjg.v9.i3.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Franchi L, Munoz-Planillo R, Nunez G. Sensing and reacting to microbes through the inflammasomes. Nature Immunology. 2012;13(4):325–32. doi: 10.1038/ni.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen GY, Nunez G. Inflammasomes in intestinal inflammation and cancer. Gastroenterology. 2011;141(6):1986–99. doi: 10.1053/j.gastro.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoque R, Sohail M, Malik A, et al. TLR9 and the NLRP3 inflammasome link acinar cell death with inflammation in acute pancreatitis. Gastroenterology. 2011;141(1):358–69. doi: 10.1053/j.gastro.2011.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsuji Y, Watanabe T, Kudo M, et al. Sensing of commensal organisms by the intracellular sensor NOD1 mediates experimental pancreatitis. Immunity. 2012;37(2):326–38. doi: 10.1016/j.immuni.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342(6161):971–6. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342(6161):967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fritz S, Hackert T, Hartwig W, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg. 2010;200(1):111–7. doi: 10.1016/j.amjsurg.2009.08.019. [DOI] [PubMed] [Google Scholar]
- 60.Takayama S, Takahashi H, Matsuo Y, et al. Effects of Helicobacter pylori infection on human pancreatic cancer cell line. Hepato-gastroenterology. 2007;54(80):2387–91. [PubMed] [Google Scholar]
- 61.Michaud DS, Izard J, Wilhelm-Benartzi CS, et al. Plasma antibodies to oral bacteria and risk of pancreatic cancer in a large European prospective cohort study. Gut. 2013;62(12):1764–70. doi: 10.1136/gutjnl-2012-303006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Raderer M, Wrba F, Kornek G, et al. Association between Helicobacter pylori infection and pancreatic cancer. Oncology. 1998;55(1):16–9. doi: 10.1159/000011830. [DOI] [PubMed] [Google Scholar]
- 63.Stolzenberg-Solomon RZ, Blaser MJ, Limburg PJ, et al. Helicobacter pylori seropositivity as a risk factor for pancreatic cancer. J Natl Cancer Inst. 2001;93(12):937–41. doi: 10.1093/jnci/93.12.937. [DOI] [PubMed] [Google Scholar]
- 64.Yu G, Murphy G, Michel A, et al. Seropositivity to Helicobacter pylori and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2013;22(12):2416–9. doi: 10.1158/1055-9965.EPI-13-0680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Risch HA, Yu H, Lu L, et al. ABO blood group, Helicobacter pylori seropositivity, and risk of pancreatic cancer: a case-control study. J Natl Cancer Inst. 2010;102(7):502–5. doi: 10.1093/jnci/djq007. [DOI] [PMC free article] [PubMed] [Google Scholar]