Abstract
Skeletal muscle tissue engineering holds promise for the replacement of muscle due to an injury and for the treatment of muscle diseases. Although RGD substrates have been widely explored in tissue engineering, there is no study aimed at investigating the combined effects of RGD nanoscale presentation and matrix stiffness on myogenesis. In the present work, we use polyelectrolyte multilayer films made of poly(L-lysine) (PLL) and poly(L-glutamic) acid (PGA) as substrates of tunable stiffness that can be functionalized by a RGD adhesive peptide to investigate important events in myogenesis, including adhesion, migration, proliferation and differentiation. C2C12 myoblasts were used as cellular models. RGD presentation on soft films and increased film stiffness could both induce cell adhesion, but integrins involved in adhesion were different in case of soft and stiff films. Moreover, soft films with RGD peptide appeared to be the most appropriate substrate for myogenic differentiation while the stiff PLL/PGA films significantly induced cell migration, proliferation and inhibited myogenic differentiation. The ROCK kinase was found to be involved in myoblast response to the different films. Indeed, its inhibition was sufficient to rescue the differentiation on stiff films, but no significant changes were observed on stiff films with the RGD peptide. These results suggest that different signaling pathways may be activated depending on mechanical and biochemical properties of the multilayer films. This study emphasizes the superior advantage of the soft PLL/PGA films presenting the RGD peptide in terms of myogenic differentiation. This soft RGD-presenting film may be further used as coating of various polymeric scaffolds for muscle tissue engineering.
Keywords: Extracellular matrix, RGD, substrate stiffness, polyelectrolyte multilayer films, myogenesis
1. Introduction
Regenerative medicine and tissue engineering make use of injected cells or of biomaterials to support cell attachment and to provide them with the appropriate cues to guide their differentiation. Skeletal muscle tissue engineering holds promise for the replacement of muscle due to an injury following a surgery or due to a trauma and for the treatment of muscle diseases, such as muscle dystrophies or paralysis. Adult skeletal muscle progenitor cells are considered as a powerful source for the generation of several tissues, especially skeletal muscles [1] but also smooth muscle [2], bone [3] [4] or fat tissue [5] [6]. The process of muscle formation requires that muscle precursor cells become activated, proliferate, differentiate, and fuse together to form multinucleated myotubes. Proliferation and differentiation of skeletal myoblasts are mutually exclusive events, which are governed by the upregulation of transcriptional activators [7]. A major limitation to the clinical application of muscle progenitors is a rapid loss of their muscle stem cell properties once they are removed from their in vivo environment [8].
The development of skeletal muscles is known to depend on the interaction of muscle cells with their surrounding extra-cellular matrix (ECM) [9]. Transmembrane receptors like the dystrophin-glycoprotein complex are known to be important [10] [11]. However, other transmembrane receptors of the integrin family [12] have been shown to be crucial for skeletal muscle development and function [13] [14].
Tissue engineering requires a combination of engineering methods, cell biology and materials. In this context, a goal of biomaterials scientists is to design biocompatible scaffolds in which cells can adhere, proliferate, differentiate and synthesize their own matrix to regenerate tissues. Adhesive properties can be provided either by grafting or by physically adsorbing cell adhesion molecules.
The tripeptide sequence RGD is present in many ECM proteins, including fibronectin, vitronectin, fibrinogen, von Willebrand factor, thrombospondin, laminin, osteopontin, bone sialo protein, and some collagen isoforms [15]. It binds to a wide range of integrin receptors in a non selective manner, i.e. not specifically to a given integrin receptor. To achieve better selectivity and/or target only one type of integrin receptor, several strategies can be applied (for review, see [16]). In vitro, ligands containing the RGD peptide have already been used in the field of biomaterials to increase early adhesion of anchorage-dependent cells [17]. This was especially targeted to osteoblasts on peptide-grafted poly(ethylene glycol) hydrogels [18], to fibroblasts on ethylene-acrylic copolymer film with immobilized peptides [19] and to endothelial cells on polyurethane [20]. Also, self-assembled monolayers have emerged as a method that allows creating discrete regions of controlled peptide identity and density within a bioinert background [21] [22] [23]. However, so far, studies of muscle cell differentiation in vitro rather used full-length ECM proteins such as fibronectin [24], laminin [25], or collagen [26], which are difficult to couple to synthetic or natural biomaterials. In addition, the functionality of these ECM proteins may be altered by adsorption or chemical coupling onto materials. Mooney and coworkers [27] showed that RGD coupling improved the initial adhesion and enabled the differentiation of myoblast cultured on 2D alginate gels or inside 3D alginate gels.
Besides biochemical cues, matrix stiffness has also been shown to be an important parameter in regulating function of various tissue and cell types in vivo and in vitro [28] [29]. Indeed, a number of pathologies, including muscle pathologies, involve changes in matrix properties. In dystrophic muscles, a more fibrotic tissue and an increased rigidity of the diaphragm have been observed as compared to a normal diaphragm [30]. In vitro, it is now acknowledged that mechanical properties of the substrate can affect muscle cell adhesion, spreading, proliferation and differentiation. This has been shown using different types of synthetic and natural materials, such as model synthetic polyacrylamide (PA) gels coated with collagen [26], poly(ethylene glycol) (PEG) hydrogels [31], alginate gels of varying stiffness [27] and polyelectrolyte multilayer films made of biopolymers [32]. Recently, Post and coworkers [33] showed, using PA gels of varying rigidity and protein coating that proliferation was influenced only by rigidity, whereas differentiation was influenced both by rigidity and by protein coating.
However, to date, there is no study aimed to investigate the combined effects of RGD nanoscale presentation and matrix stiffness in myoblast adhesion, proliferation and differentiation. Polyelectrolyte multilayer films [34] are currently emerging as a new kind of biomaterials coating that can be used to guide cell fate [35] [36]. Advantageously, the architecture of the films, their biodegradability and bioactivity can be controlled [37]. The films can also be micropatterned to have (X-Y) architecture by combining with microfabrication techniques such as photolithography, microcontact printing or microfluidics [38] [39] [40]. Furthermore, they can be deposited on various types of supporting materials, including metals, polymers and ceramics, which are already approved as implantable materials [37].
In this study, we investigated the influence of substrate rigidity and RGD nanoscale presentation alone or in combination on C2C12 myoblast adhesion, proliferation and differentiation. To this end, we cultured the myoblasts on polyelectrolyte multilayer films made of poly(L-lysine) (PLL) and poly(L-glutamic) acid (PGA) whose rigidity can be tuned by chemical cross-linking [41]. Moreover, such films are of particular interest since they are made of biodegradable polymers and appear to be biocompatible [42].
In addition, presentation of a RGD-containing peptide was achieved by chemically grafting the peptide to PGA [43] and adsorbing it as final layer of the film. Such covalent grafting provides a good control of surface composition, a stable link and limits release of the functional group into the culture medium.
We studied the combined effects of RGD nanoscale presentation and matrix stiffness on early adhesion of the myoblasts to their late differentiation in myotubes after 9 days in culture, until the formation of sarcomeres.
2. Materials and methods
2.1. Covalent grafting of RGD adhesion peptide to PGA (poly(L-glutamic) acid)
The type I collagen-derived peptide was chosen according to a published sequence that was shown to induce adhesion of human primary osteoblasts in vitro [43]. The 15-amino-acid peptide containing a central RGD (Arg-Gly-Asp) sequence (Cys-Gly-Pro-Lys-Gly-Asp-Arg-Gly-Asp-Ala-Gly-Pro-Lys-Gly-Ala, CGPKGDRGDAGPKGA) was purchased from GeneCust (Dudelange, Luxembourg). The peptide was grafted as described previously [43]. Briefly, the first step consisted in grafting maleimide groups onto PGA (P-4886, Sigma) chains. To accomplish this grafting, 60 mg of PGA were dissolved in 3 mL of a solution containing 10 mM HEPES buffer (pH 6.5), 20 mg of EDC, and 3 mg of sulfo-NHS in an inert atmosphere (nitrogen gas) under magnetic stirring. Then, 24 mg of N-(2-aminoethyl) maleimide trifluoroacetate was added. The reaction was allowed to proceed at room temperature (RT) for 24 h. After removal of the byproducts via dialysis against water, the PGA–maleimide was freeze-dried. The average number of maleimide groups bound to PGA was equal to 16% ((i.e. in average 16 maleimide groups every one hundred repeating PGA units), as determined via 1H NMR analysis. In the second step, the PGA–maleimide was reacted with the peptide to form the PGA-RGD: 5 mg of PGA-maleimide were mixed with 5 mg of peptide in 1.5 mL of 10 mM HEPES buffer (pH 7.4) and maintained for 24 h under magnetic stirring at RT. An excess of mercaptopropionic acid was used to neutralize the unreacted maleimide groups. The solution was dialyzed against water and freeze-dried. The quantitative grafting ratio of the peptide was determined by 1H NMR, and the effective degree of grafting was found to be 10% analysis.
2.2. Polyelectrolyte solutions and PEM film buildup
Poly(L-lysine) (PLL, P2636, Sigma) and poly(L-glutamic) acid (PGA, P-4886, Sigma) were dissolved at 0.5 mg/mL in a HEPES-NaCl buffer (20 mM HEPES, 150 mM NaCl, at pH 7.4). For all experiments, films were manually constructed in 96-well plates starting with a first layer of poly(ethyleneimine) (PEI) at 5 mg/ml. To deposit the subsequent polyelectrolyte layers, 50 μL of the polyelectrolyte solution was deposited in each well and let for 8 min before being rinsed twice for 30 sec and 5 min, respectively, with 100 μL of 150 mM NaCl (pH 6.5). This sequence was repeated until the buildup of a (PGA/PLL)6 film was achieved. Then, the last layer of PGA (0.5 mg/mL) or of the PGA-RGD (0.5 mg/mL) was added, giving (PGA/PLL)6-PGA and (PGA/PLL)6-PGA-RGD films.
In order to increase the stiffness, (PGA/PLL)6-PGA films were chemically cross-linked to give [(PGA/PLL)6-PGA]CL films. To obtain the cross-linked films functionalized with PGA-RGD, cross-linking was done after (PGA/PLL)6, and PGA-RGD was added after the cross-linking, giving [(PGA/PLL)6]CL-PGA-RGD films. For the cross-linking, 100 μL of EDC/sulfo-NHS solution in 150 mM NaCl pH 5.5 (mixed v/v with final EDC concentrations of 15 mg/mL and 5.5 mg.mL) were deposited in the wells and incubated at 4° C overnight. Finally, the films were thoroughly washed with the HEPES-NaCl buffer. The nomenclature of the film is given in Table 1.
Table 1. Summary of the conditions used for the buildup of the films.
| Film design | Film architecture | Film nomencature | ||
|---|---|---|---|---|
| PGA | Cross-linking | PGA-RGD | ||
| x | (PAG/PLL)6-PAG | NCL | ||
| x | (PAG/PLL)6-PAG-RGD | NCL-RGD | ||
| x | x | [(PAG/PLL)6-PAG]CL | CL | |
| x | x | [(PAG/PLL)6]CL-PAG-RGD | CL-RGD | |
2.3. Quartz Crystal Microbalance with dissipation monitoring (QCM-D)
Film buildup was followed by in situ quartz crystal microbalance (QCM D300, QSense, Sweden) using a previously published procedure [44]. PLL, PGA and PGA with grafted RGD peptide prepared at 0.5 mg/mL in the HEPES-NaCl buffer were successively injected in the cell. They were let to adsorb for 8 min and rinsed for 5 min with the HEPES-NaCl buffer.
When a mass Δm is adsorbed at the crystal and the measurements are conducted in air, the resulting decrease Δf typically obeys the Sauerbrey equation:
where C is the mass sensitivity constant (17.7 ng/cm2/Hz at 5 MHz), and n is the overtone number.
2.4. C2C12 culture
C2C12 cells (from ATCC, used at passages 5-15) were maintained in polystyrene dishes in an incubator at 37° C and 5% CO2 and cultured in growth medium (GM) composed of Dulbecco’s modified Eagle’s medium (DMEM)/F12 medium (1:1; Gibco, Invitrogen, Cergy-Pontoise, France) supplemented with 10% fetal bovine serum (PAA Laboratories, Les Mureaux, France) containing 10 U/mL of penicillin G and 10 μg/mL of streptomycin (Gibco, Invitrogen, Cergy-Pontoise, France). Cells were subcultured prior to reaching 60–70% confluence (approximately every 2 days). For all experiments, C2C12 cells were first allowed to adhere in a serum-free medium (SFM) composed of DMEM/F12 1:1 and supplemented with antibiotics. After 1 h of adhesion, the cells were fixed or the SMF was replaced by the GM, depending on the type of experiment (see below). Cell were differentiated in a differentiation medium (DM) composed of DMEM/F12 (1:1) supplemented with 2% horse serum (PAA Laboratories, Les Mureaux, France) and antibiotics.
2.5. Cell adhesion, proliferation, migration and differentiation assays
For cell adhesion tests, C2C12 cells were seeded at 15 000 cells/cm2 in 96-well plates and allowed to adhere in SFM for 1 h. For the short-term adhesion tests (1 h), cells were then fixed in 3.7% formaldehyde. For adhesion tests at 4 h, the medium was changed to GM after 1 h and the cells were fixed at 4 h.
Cell proliferation was quantified by a BrdU (5-bromo-2′-deoxyuridine) assay (Cell Proliferation Kit, RPN20, GE Healthcare) following the manufacturer instructions. Three time points were chosen: 4, 24, and 48 h. The cells were incubated for 1 h at 37°C. Nuclei were counter-stained with Hoechst 3342 (Invitrogen). The images of BrdU-positive nuclei taken by phase contrast microscopy and the images of Hoechst-labeled nuclei taken using an inverted fluorescence microscope were merged to determine the ratio of BrdU positive nuclei.
To follow cell migration, C2C12 cells were mixed 1:1 with C2C12 HB1 GFP cells (kindly provided by E.Gomes, Institut de Myology, Paris) and seeded at 15 000 cells/cm2 in 96-well plates. Images of the fluorescent nuclei were taken every 30 min during 5 h. For analysis, at least 20 cells were tracked using ImageJ (v1.45d, NIH, Bethesda).
For differentiation assays, cells were seeded at 30 000 cells/cm2 and allowed to adhere for 1 h in SFM. Cells were then grown for 1 day in GM and then switched to DM. The medium was changed twice a week. For the proliferation and differentiation tests in the presence of the ROCK kinase inhibitor (Y-27632, Calbiochem), 5 μM of inhibitor was added at day 0 in DM. Fresh drug was then added every 24 h.
For the quantitative analysis of adhesion and differentiation, at least 50 cells of at least ten different fields (430 μm × 320 μm) were analyzed per condition. To characterize cell adhesion, cell number per field, cell area and circularity were quantified. Cell circularity is a parameter defined by the formula: Circularity = 4π(A/P2), (A being the cell area and P its perimeter) that allows to characterize cell morphology: a circularity value of 1.0 indicates a perfect circle and a decrease toward 0 indicates an increasingly elongated polygon.
The differentiation was characterized by the fusion index, which is a ratio of the nuclei contained in myotubes reported to the total number of nuclei [45], and by the percentage of striated myotubes.
2.6. Transfection by siRNA
Cells were transfected with siRNA against β1 and β3 integrins (ON-TARGET plus SMARTpool, respectively Mouse ITGB1 and Mouse ITGB3, Thermo Scientific Dharmacon) individually or at the same time, a scrambled siRNA (All Stars negative Control siRNA, Qiagen) being taken as control. For this, the cells were seeded at 30 000 cells/cm2 in a 6-well plate and cultured in GM (2 mL per well) for 15 h. The transfection mix was prepared as following: for one well, 6 μL of lipofectamine RNAiMAX Reagent (Invitrogen) were added to 305 μL of Opti-MEM medium (Gibco) and 0.72 μL of 1 mM siRNA were added to another 305 μL of Opti-MEM medium. Lipofectamine-containing mix was added to siRNA-containing mix and incubated for 20 min at room temperature. Previously to transfection, the GM of the wells was replaced by the GM without antibiotics. Then, 610 μL of the final mix were added to each well. After 24 h of incubation at 37°C, the cells were transfected for the second time following the protocol described above and incubated for another 24h. Then the cells were detached by trypsin-EDTA, seeded in GM at 20 000 cells/cm2 on the films built in 96-well plate and allowed to adhere for 4 h. Then, the cells were fixed and their area was quantified.
2.7. Immuno-staining
Cells were first rinsed in PBS and fixed in 3.7% formaldehyde for 30 min at RT before being permeabilized in 0.5% Triton X-100 for 4 min. After rinsing with PBS, samples were incubated for 1 h in 0.1 % BSA in TRIS-buffered saline (TBS, 50 mM TRIS, 150 mM NaCl, 0.1% NaN3, pH 7.4). Actin was labeled with phalloidin-TRITC (1:800, Sigma) for 30 min. Cell nuclei were stained with Hoechst 33342 (Invitrogen) at 5 μg/ml for 10 min. After the incubations with the primary antibodies (diluted in 0.2% TBS-gelatin) for 30 min at RT, cells were washed 3 times in TBS and incubated for 30 min with the secondary antibodies: rabbit anti-FAK pY397 antibody, (1:200, Invitrogen), myogenin (rabbit anti-myogenin antibody (1:30, Tebu-Bio) and myosin heavy chain (mouse anti-myosin heavy chain (1:500, Sigma). Alexa-Fluor 388 or 558 conjugated antibodies (Invitrogen) were used at 1:1000. Images were taken by means of Zeiss Axiovert 200 inverted or Zeiss LSM 700 confocal microscope.
2.8. Statistics
The results represent three independent experiments. Data are reported as means ± standard deviation. Statistical comparisons were performed using SigmaPlot Version 11.0 software and based on an analysis of variance (ANOVA) followed by an appropriate pairwise comparison or comparison versus control group procedure (P < 0.05 was considered significant). Statistically different values are reported on the figures.
3. Results
3.1. Density of RGD peptide on the film surface
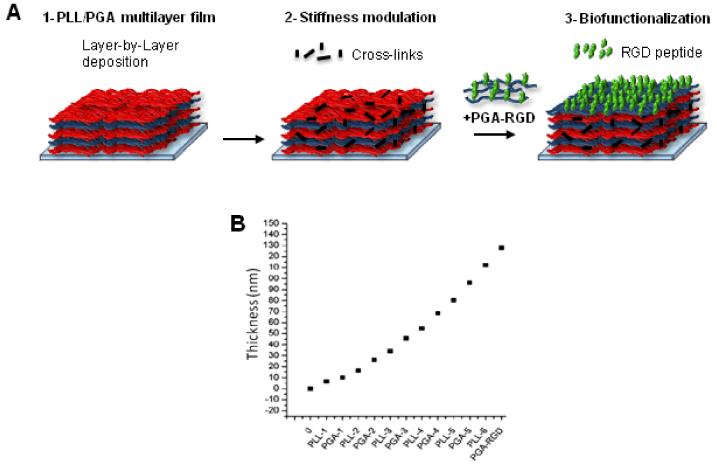
The principle of film buildup with or without subsequent cross-linking (CL) and/or functionalization with the RGD peptide is depicted in Figure 1A. The four different film architecture studied are given in Table 1: NCL, NCL-RGD, CL and CL-RGD. The film buildup was followed in situ by Quartz Crystal Microbalance with dissipation monitoring (QCM-D) (Fig. 1B), which allowed us to measure film thickness using the Voigt model [46]. Film thickness was 120 nm for a film made of 6 layer pairs.
FIGURE 1. Design of biomimetic thin film combining physical and biochemical cues.
(A) 1- a polyelectrolyte multilayer film (PEM) is built onto a substrate by alternating deposits of PLL and of PGA. 2- The PEM film can be covalently cross-linked using a water-soluble carbodiimide to modulate its stiffness. 3- Biochemical functionality is provided by adding a final layer of PGA grafted with a RGD-containing peptide. (B) Exponential growth of the film followed by QCM-D.
Knowing the grafting density of the RGD peptide to PGA (10%) the amount of RGD peptide present at the film surface was quantified. The adsorbed mass of PGA-RGD was 400 ng/cm2, which corresponded to a RGD surface density of 0.78 molecules of peptide per nm2 (or 300 pmol/cm2). This is a relatively high surface coverage. The Young’s modulus of these films deposited on a thick polyelectrolyte cushion has previously been measured to be 51 ± 17 kPa for NCL films and 230 ± 70 kPa for CL ones [41].
3.2. Effect of RGD-functionalization and film cross-linking on C2C12 myoblast adhesion, spreading and morphology
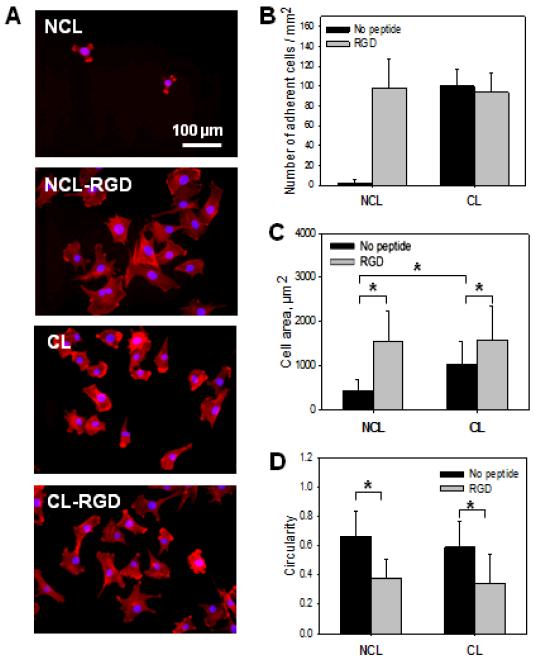
Adhesion is the very first and important step of cell-substrate interactions, which is especially important for anchorage-dependent cells. To evaluate the effect of film stiffness and RGD-functionalization on C2C12 myoblast adhesion, cells were cultured on the four different types of films NCL, NCL-RGD, CL and CL-RGD. The cells were allowed to adhere for 1 h in serum free medium (SFM) to eliminate any effect of serum on early adhesion. The number of adherent cells as well as their spreading area and morphology (circularity) were evaluated. Actin and nuclei staining of C2C12 cells (Fig. 2A) revealed the presence of adherent cells on NCL-RGD, CL and CL-RGD films but of very few cells on NCL ones. In addition, these cells were poorly spread. Quantitative measurements of the number of adherent cells confirmed the microscopy observations with no statistical differences between NCL-RGD, CL and CL-RGD (Fig. 2B). However, after 1 h, cell area was significantly higher on films presenting PGA-RGD (Fig. 2C) as compared to those ending with PGA only. Cells spread also about two times more on CL films as compared to NCL ones. Thus, both substrate stiffness and RGD-functionalization had an effect on cell spreading. The circularity index was significantly lower for films containing the RGD peptide as compared to films without peptide (NCL and CL) (Fig. 2D). This indicated that the presence of RGD but not film stiffness influenced the cell circularity.
FIGURE 2. Adhesion and spreading of C2C12 myoblasts at early time.
Initial C2C12 cell adhesion and spreading were observed 1 h after plating the cells on NCL, CL, NCL-RGD and CL-RGD films. (A) Actin (red) and nuclei (blue) staining of C2C12 cells to visualize adhesion and spreading on the four types of films (B) Number of adherent cells. (C) Spreading area. (D) Cell circularity quantification. Error bars correspond to SD, *: p < 0.05.
As only few cells adhered on NCL films, these films were discarded for the subsequent experiments on cell differentiation. It is interesting to note that they constitute the “blank slate” [35] for the specific effect of RGD grafting on PGA.
3.3. Characterization of adhesion via integrin receptors and cell migration
The cells are mechano-sensors that actively sense their environment via specific cell surface receptors, especially integrins [47]. Several integrins can interact with RGD ligand, especially α5β1 and αVβ3. Cell sensing and integrin activation lead to the formation of focal adhesions (FA), sites where the cells contact with the matrix to exert cell traction [48] [49]. FA, which are enriched in integrins and in several other proteins, are linked to actin stress fibers.
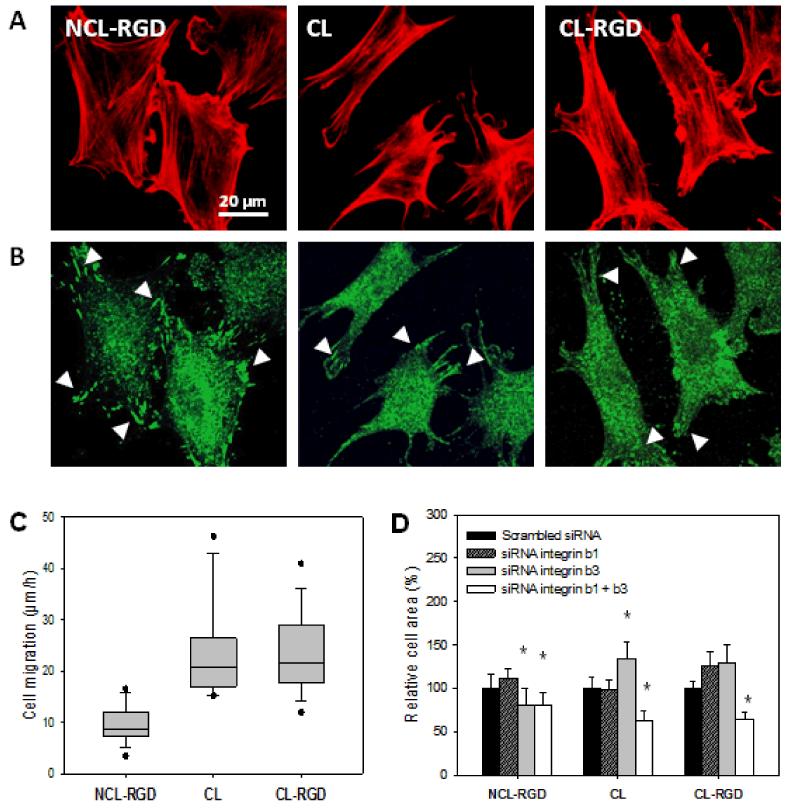
To characterize cell interaction with the substrate via integrin receptors, the formation of stress fibers and the presence of focal adhesions were first analyzed in C2C12 cells cultured on the different films after 4 h (Fig. 3A and B). Focal adhesions were visualized by labeling phosphorylated focal adhesion kinase (pFAK) an important component of mature FA (Fig 3A). Robust focal adhesions or even fibrillar adhesions (small dashes at the cell periphery) were formed only on NCL-RGD films, while only small and thin focal adhesions or focal complexes (small dots) were visible on CL and CL-RGD films (Fig. 3B). These results showed that both film stiffness and RGD-functionalization played a role in the organization of the actin cytoskeleton and in the formation of focal adhesions as well. The presence of the adhesive ligand on soft films led to the formation of numerous focal and fibrillar adhesions, while only focal complexes formed on the stiffest films, even in the presence of RGD.
FIGURE 3. Effect of film stiffness and RGD fonctionalization on cytoskeletal organization, focal adhesions and migration.
(A) Staining of actin cytoskeleton (red) after 4 h of culture. (B) Staining of phosphorylated focal adhesion kinase (pFAK Y397, green) after 4 h of culture. (C) Myoblast migration measured over 5 h after seeding. (D) Effect of blocking β1 and/or β3 integrins using siRNA: quantification of the cell area after 4 h of adhesion (* p < 0.05 compared to scrambled siRNA). Focal adhesions/complexes are indicated by white arrowheads.
As stress fibers and focal complexes/adhesions play a key role in cell migration, we investigated whether cell migration is influenced by the substrates. To this end, cell migration on the different films over 5 h after cell seeding was followed by tracking individual cells. The results for the migration speed on the different films are shown in Fig. 3C. Cell migrated at ~20 μm/h on stiff films (CL and CL-RGD), which was about two times faster than cells on NCL-RGD films (10 μm/h). Thus, the decrease of focal adhesions on stiff films (CL and CL-RGD) correlated with an enhanced migration on these films.
In order to investigate the possible role of beta chain integrins in cell adhesion, the knockdown of β1 or β3 integrin or both using siRNA approach was studied. These integrins are known to be involved in cell mechano-sensing and β1 and β3 integrins are present in C2C12 myoblasts [50] [51]. The cell area of the transfected cells was quantified after 4 h of adhesion (Fig. 3D). On NCL-RGD films, only β3 blocking but not of β1 lead to a slight decrease in cell spreading. Double tranfection of β1 and β3 siRNA gave similar results. These data suggested that myoblast interaction with the RGD-containing peptide involved partly β3 integrins. On CL and CL-RGD films, siRNA against β1 or β3 alone did not decrease cell spreading, and even significantly increased it in the case of siRNA β3 treatment on CL film. However, when the cells were transfected with both β1 and β3 siRNA, cell area decreased significantly on CL and CL-RGD films, suggesting that both β1 and β3 integrins can be used by the cells in a commutable way to interact with these films. Thus, it appeared that integrins involved in cell spreading on the different films are different.
3.4. Effect of film cross-linking and RGD-functionalization on myogenic differentiation
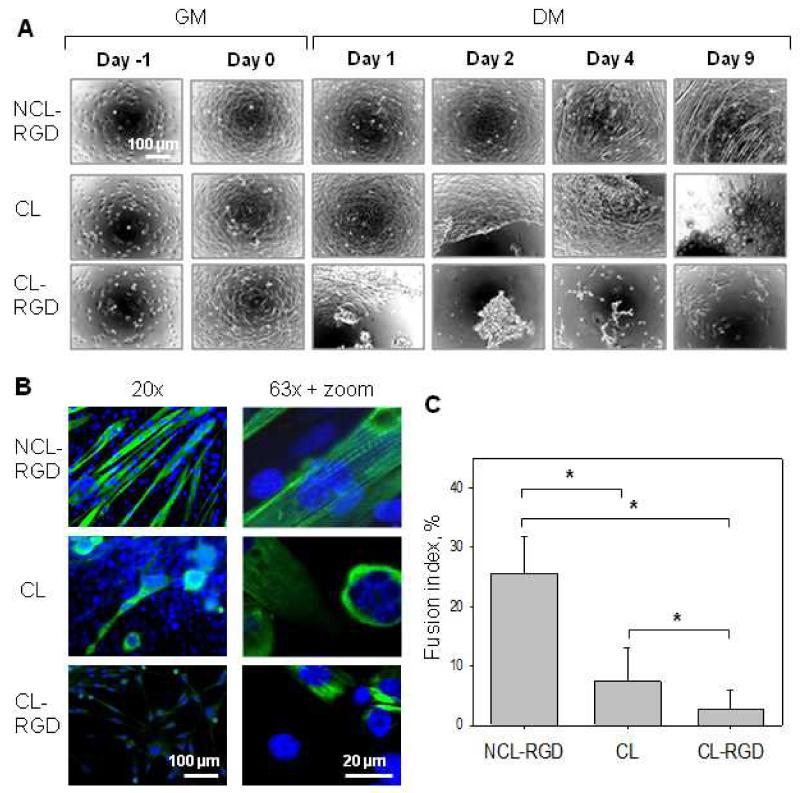
C2C12 myoblasts are a well-known model for the in vitro study of myogenic differentiation due to their ability to reproduce processes that take place during in vivo differentiation of skeletal muscle progenitors [52]. The effect of film stiffness and presentation of the RGD ligand on myoblast differentiation in myotubes were studied over 9 days. Phase contrast microscopy images of myogenic differentiation are shown in Fig. 4A. The formation of myotubes was observed on NCL-RGD films, while cell aggregation followed by detachment occurred on both CL and CL-RGD films, after 1-2 days on CL-RGD films and after 2-3 on CL ones. Some detached cells were able to form aggregates that remained adherent until day 9 of differentiation.
FIGURE 4. Myogenic differentiation of C2C12 myoblasts is effective on NCL-RGD films.
(A) Phase contrast microscopy observations of C2C12 cell differentiation on the NCL-RGD, CL, and CL-RGD films. After 24 h of proliferation in GM (ie Day −1), the cells were put in DM (ie Day 0) and were let to differentiate until Day 9. Cell detachment was observed on CL and CL-RGD films after few days in DM. (B) Myosin heavy chain (green) and nuclei (blue) labeling (20 × and 63 × magnification). (C) Quantification of the fusion index. Error bars correspond to SD, *: p < 0.05.
Staining of myosin heavy chain (MHC), a late marker of myogenic differentiation [7] was used to characterize myogenic differentiation and the formation of myotubes. MHC was well-expressed only in myoblast cultured on NCL-RGD films, while its expression on CL and CL-RGD films was very weak (Fig. 4B). The fusion index was of 25% of NCL-RGD films and only of 8% for CL and 3% for CL-RGD films (Fig. 4C). Thus, only a small fraction of the remaining cell adhered on CL and CL-RGD films was able to fuse. Moreover, the multinucleated cells on these films neither had the typical elongated morphology of myotubes nor were striated. It was solely for cells grown on NCL-RGD films that striation reached 67% of the myotubes, indicating that their maturation was very good (Fig. 4B, upper image of right column).
These results showed that the soft RGD-functionalized film used in our study was the only film architecture enabling myoblast fusion and differentiation in an efficient manner. Conversely, CL and CL-RGD films were not appropriate for long term differentiation in myotubes.
3.5. C2C12 myoblasts poor differentiation on stiff films is associated with a decreased myogenin expression and enhanced proliferation
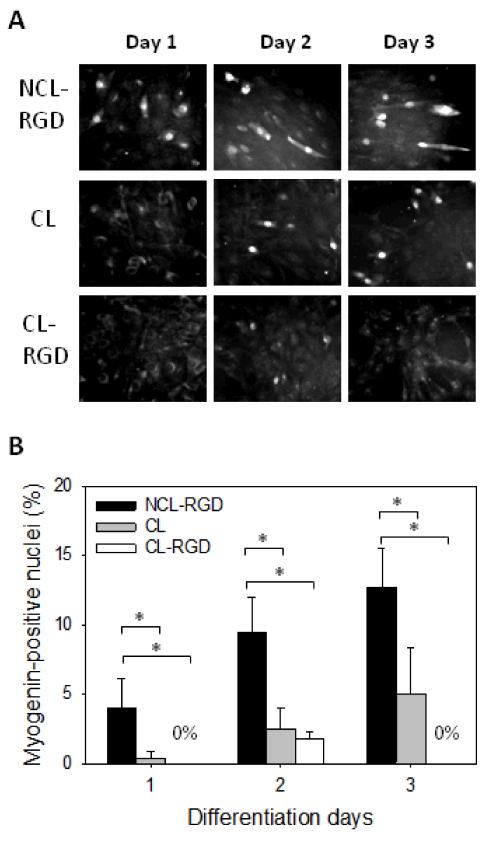
For skeletal myoblasts, cell cycle arrest is necessary to undergo differentiation. During myogenic differentiation, a highly ordered process of temporally separable events that begins with the expression of myogenic transcription factors and is followed by cell cycle arrest takes place [7]. In order to further understand the origin of the inappropriate differentiation on stiff films, we quantified the expression of the transcription factor myogenin at early times of the differentiation process (Day 1 to 3) (Fig. 5). On NCL-RGD films, myogenin was already expressed at day 1 (4% of nuclei) and steadily increased until day 3 (13% of nuclei). On CL films, only 1% of nuclei were myogenin-positive at Day 1 and 5% at Day 3. On CL-RGD films, no positive nuclei was detected at Day 1 or 3, but about 2.5% of myogenin-positive cells were observed at Day 2. These results showed that myogenin expression is decreased on stiff films, especially on CL-RGD one where no positive nuclei were found at Day 3.
FIGURE 5. Myogenin expression is decreased on stiff films.
After 24 h of proliferation in GM, the medium was changed to DM and cells were let to differentiate for 2 days. (A) Myogenin labeling at day 1, 2 and 3 of differentiation. (B) Quantification of the percentage of myogenin expressing cells. Error bars correspond to SD, *: p < 0.05.
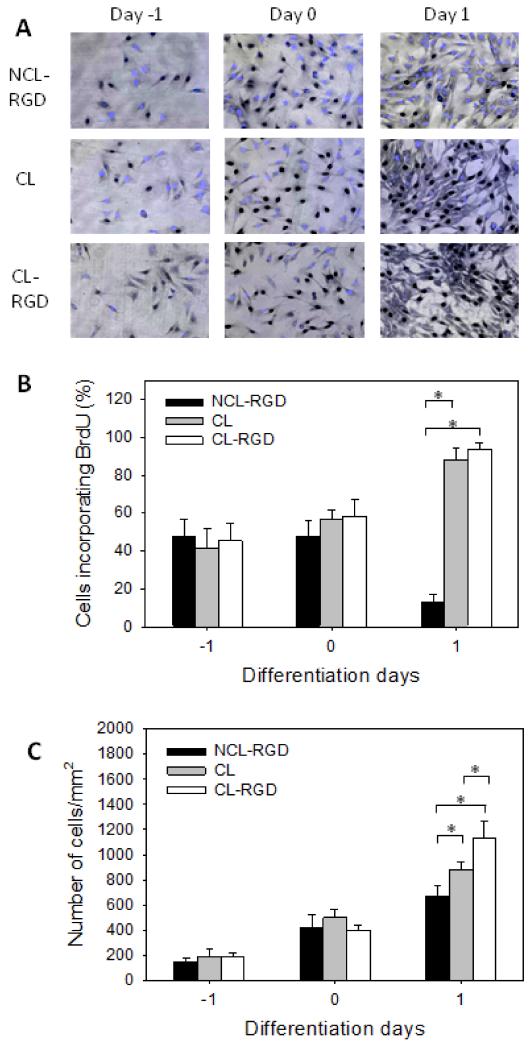
As down-regulation of proliferation is needed for myogenesis to occur [7], we also quantified cell proliferation on the different types of films before and after addition of the DM (Day −1 to 1) by a BrdU incorporation assay (Fig. 6). Day −1 and Day 0 represent the initial growth phase in GM at 4 h and 24 h after cell seeding, respectively, before switching to DM. No difference in the percentage of proliferating cells between the three types of films was observed in GM (Fig. 6B). However, significant differences were observed after 24 h of culture in DM, noted here Day 1. As anticipated, the rate of proliferating cells decreased drastically on NCL-RGD films from 50 % to 10 %, which corresponded to the cell cycle arrest. Conversely, on CL and CL-RGD films, these rates not only failed to decrease but still increased to reach ~90%. As a consequence, at Day −1 and Day 0, the total cell number on the three types of films was similar (Fig. 6C) but at Day 1, the number of cells on stiff films was higher than on NCL-RGD. The highest cell number was found on CL-RGD film.
FIGURE 6. Proliferation is enhanced on CL films resulting in an increased cell number.
After 24 h of proliferation in GM (Day −1), the medium was changed to DM (Day 0) and cells were let to differentiate for one day. (A) Staining of BrdU in the nuclei (in black) associated to a fluorescent labeling of total nuclei (blue). (B) Percentage of BrdU-positive cells. (C) Quantification of the total number of adherent cells. Error bars correspond to SD, *: p < 0.05.
These results showed that two key events in myogenic differentiation, i.e. myogenin expression and cell cycle arrest, were altered on stiff films. Cells on stiff films bypass the cell cycle exit induced by growth factor deprivation. Cell detachment observed on stiff films may thus be due to enhanced proliferation leading to excessive cell confluence, and/or to enhanced cell migration.
3.6. Effect of inhibition of ROCK kinase on myogenin expression, proliferation and differentiation on stiff films
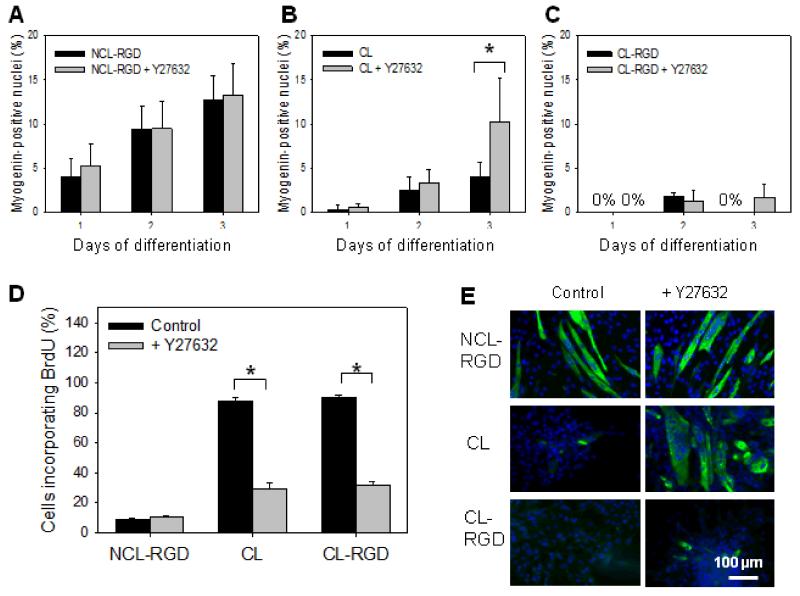
Previous studies have shown that myoblast differentiation is regulated through Rho/ROCK pathways that must be downregulated to allow myogenesis [53]. These authors have shown that constitutive activation of ROCK resulted in decreased myogenin expression and inhibition of myogenic differentiation in C2C12, while inhibition of ROCK led to an accelerated exit from the cell cycle and induced myogenin expression. These data suggested that ROCK is involved in keeping the myoblasts cycling and prevents commitment to differentiation [54]. We thus hypothesized that decreased myogenin expression and excessive proliferation on CL films, resulting in poor differentiation, may be due to an enhanced ROCK activity. To investigate if ROCK pathway was involved in inappropriate cell differentiation on stiff films, the ROCK inhibitor Y27632 was added at Day 0 of differentiation. Its effect on myogenin expression was evaluated at Days 1, 2 and 3, and on proliferation at Day 1. In addition, MHC was labeled at Day 6.
First, we studied the effect of ROCK inhibitor on myogenin expression (Fig. 7A-C). On NCL-RGD films, Y27632 treatment did not alter myogenin expression at any time (Fig. 7A). On CL films, a 2-fold increase in myogenin-positive nuclei was observed after 3 days of treatment, reaching 10% (Fig. 7B). On CL-RGD films, myogenin expression remained very weak at less than 3% in any conditions. However, it was still present at Day 3 when treated with ROCK inhibitor, while it was absent in a control (non-treated) condition (Fig. 7C). The effect of Y27632 on C2C12 myoblast proliferation at Day 1 was also tested (Fig 7D). There was no difference for NCL-RGD films, but we observed a 3-fold decrease in BrdU-incorporating cells on CL and CL-RGD films. This indicated that ROCK was involved in excessive C2C12 proliferation on these films.
FIGURE 7. ROCK kinase inhibition decreases myoblast proliferation and rescues differentiation on CL films.
After 24 h of proliferation in GM, cells were transferred to DM and let to differentiate for 6 days. (A, B, C) Myogenin labeling at day 1, 2 and 3 in DM. (D) Percentage of BrdU-positive cells at Day 1 of differentiation. (E) Myosin heavy chain labeling at Day 6 of differentiation. Error bars correspond to SD, *: p <0.05.
MHC expression on NCL-RGD films was not modified by Y27632 treatment (Fig. 7E). In contrast, on CL films, Y27632 treatment was sufficient to prevent cell detachment and to allow myogenic differentiation. Of note, the myotubes formed were not as elongated as on NCL-RGD films and nuclei were more clustered. Finally, no differentiation was observed on CL-RGD films upon Y27632 treatment, suggesting that ROCK inhibition alone was insufficient to restore the differentiation program, and that a more complex molecular mechanism was involved in the cell response to these films.
4. Discussion
It is becoming increasingly clear that mechanical and biochemical properties of the substrate both play an important role not only in cell adhesion, but in many other processes such as proliferation and differentiation [29]. However, the contribution of each type of signal, i.e substrate stiffness and adhesive ligand, is not always easy to decouple. Mechano-sensitivity studies often use synthetic hydrogels such as PA with ECM proteins or PEG grafted with ECM fragments [55]. It is already acknowledged that mechanical properties of the substrate can affect muscle cell adhesion and differentiation. This has been observed on PA surfaces [26], PEG hydrogels [31] and on PEM films made of PLL and hyaluronan [32] [56]. However, little work has been done on the role of RGD-containing peptides in myogenic differentiation. Rowley and Mooney [57] showed that RGD-peptide was necessary to promote myoblast attachment to alginate hydrogels and that myoblast differentiated only on alginate gels with specific combination of monomeric ratio and RGD grafting density [57]. In addition, RGD-peptides were found to significantly improve myoblast cell adhesion onto grooved polystyrene substrates [58]. Here, we used layer-by-layer films made of polypeptides as modular substrates, which can be stiffened by chemical cross-linking and can be specifically functionalized by grafting a RGD-containing peptide onto PGA [43]. In a previous study, the 15-amino-acid collagen type I-derived peptide containing an RGD adhesive sequence has been tested for both short-term adhesion properties and long-term proliferation of primary osteoblasts [43]. In the present work, four different types of films with or without cross-linking and with or without the RGD-peptide allowed investigation of the effect of mechanical and biochemical signals and their combinations as well, on important events of myogenesis. We especially focused on the sequence of events involved in C2C12 cell differentiation including early adhesion, migration, proliferation, differentiation and fusion of myoblast into myotubes.
The results obtained by QCM-D regarding (PLL/PGA) film growth (Fig. 1) are consistent with those obtained previously by optical waveguide lightmode spectroscopy on the same films [43], with however a higher thickness measured by QCM-D due to the water incorporated in the films.
While the cells spread more on the RGD-functionalized films, a more detailed analysis of cell interaction with the substrates showed that the stiffness was also very important: only cells on NCL-RGD film exhibited formation of robust focal adhesions and migrated at low speed. The presence of only small focal complexes on stiff films (CL and CL-RGD) (Fig. 3) correlated with an enhanced migration on these films (Fig. 6). Our results also suggested that soft films with RGD and stiff films recruit different combinations of integrin receptors: while β3 knockdown alone had an effect on myoblast spreading on NCL-RGD films, the knockdown of both β1 and β3 is required to affect myoblast spreading on CL and CL-RGD films (Fig 3D). However, the inhibition of cell spreading on the different films was never complete by blocking β1, β3 or both integrins at the same time (Fig 3D), suggesting that other integrin or non-integrin receptors may be involved. It has been shown using epithelial cells that β1 and β3 integrins promote different migration modes: adhesion by β3 resulted in static cell-matrix adhesions and persistent migration, while adhesion by β1 promoted highly dynamic cell-matrix interactions and random migration [59]. These results make a link between cell-surface interactions via specific integrin receptors, focal adhesion dynamics and cell migration. Our results on myoblasts show that there is a correlation between integrins that are recruited, size of focal adhesions/complexes and cell migration speed: involvement of β3 correlates with robust adhesion and low migration speed, while recruitment of both β1 and β3 are related to smaller adhesion complexes and enhanced cell migration (Fig. 3). Our results thus suggest that control of motile strategy by integrins may be a common feature of different cell types.
Interestingly, β3 integrin was found to be crucial for myogenic differentiation of C2C12 myoblasts, and to mediate satellite cell differentiation [51], while β1-integrin, which is constitutively expressed in skeletal muscle, has earlier been shown to be dispensable to myogenesis [60]. This is in agreement with our data showing that on PEM films differentiation was only possible on soft films with RGD peptide. The stiffest films offered unfavorable conditions for the differentiation as myogenin expression was decreased on stiff films (Fig. 7) but proliferation was enhanced (Fig. 6). Moreover, the cells detached from stiff films after few days in DM. We hypothesize that cell detachment may be due to enhanced proliferation leading to excessive cell confluence and/or to enhanced cell migration. The ROCK kinase, which is known to be involved in myogenic differentiation but also in cell blebbing may be responsible for cell detachment (for review, see [61]). Interestingly, myoblast differentiation could be partially rescued on CL films by treatment with ROCK inhibitor, which decreased proliferation level and increased myogenin expression (Fig 7B). However, on CL-RGD films, even if the myoblast treated with ROCK inhibitor showed a decreased proliferation level, they were still unable to express myogenin and to differentiate (Fig. 7C). Enhanced ROCK activity on stiff films may be the consequence of the engagement of β1 integrin on these films since it was reported that β1 induces a higher RhoA activity than β3 [59]. Both RhoA and its effector ROCK play a crucial role in myogenic differentiation as both activities must be downregulated to allow myogenesis to occur [53] [54] [62] [63].
In our previous work [32] we evaluated the adhesion and differentiation of C2C12 myoblasts using poly(L-lysine) and hyaluronan (PLL/HA) multilayer films of varying stiffnesses. On these films, formation of focal adhesions was increased on stiff films as compared to soft ones. Evaluation of the adhesive behaviour during the initial steps of spreading showed that blocking β3, but not β1 integrins inhibited cell adhesion on stiff (PLL/HA) films [56]. This is different from cross-linked (PLL/PGA) films, which are stiffer than (PLL/HA) films) and for which blocking of both β1 and β3 integrins was necessary to inhibit cell spreading. Thus, molecular mechanisms of cell/film interactions involved different integrins depending on film type, film stiffness and presence of a specific ligand (RGD peptide).
Cell adhesion to ECM influences cell proliferation by transducing signals through cell surface integrin receptors, and proliferation is generally low in soft matrix and high in stiff matrix [64] [65]. Proliferation of C2C12 myoblasts was also increased on stiff substrates as compared to soft ones [27] [32]. Here, we showed that the proliferation of C2C12 was significantly increased on stiff films via the activation of ROCK (Fig. 7). Rho/ROCK pathway is also known to be implicated in the remodeling of focal adhesions and migration of tumor cells [66]. Similarly, tumor malignancy and invasion is associated to matrix stiffening [67]. In this context, C2C12 cells on stiff films seemed to acquire some features of cancer cells: they bypassed the cell cycle exit induced by growth factor deprivation, showed an absence of mature focal adhesions and enhanced migration. Thus, CL and/or CL-RGD films may be used as a model basement membrane for studies of cancer cell behavior in response to matrix stiffening. Decreased myogenin expression and absence of exit from the cell cycle observed on cross-linked films could also be a potential tool for in vitro amplification of satellite cell while preserving their multiple differentiation potential. Indeed, these primary cells rapidly lose their stem properties and switch to differentiation upon removal from their niche and cultured in vitro [8].
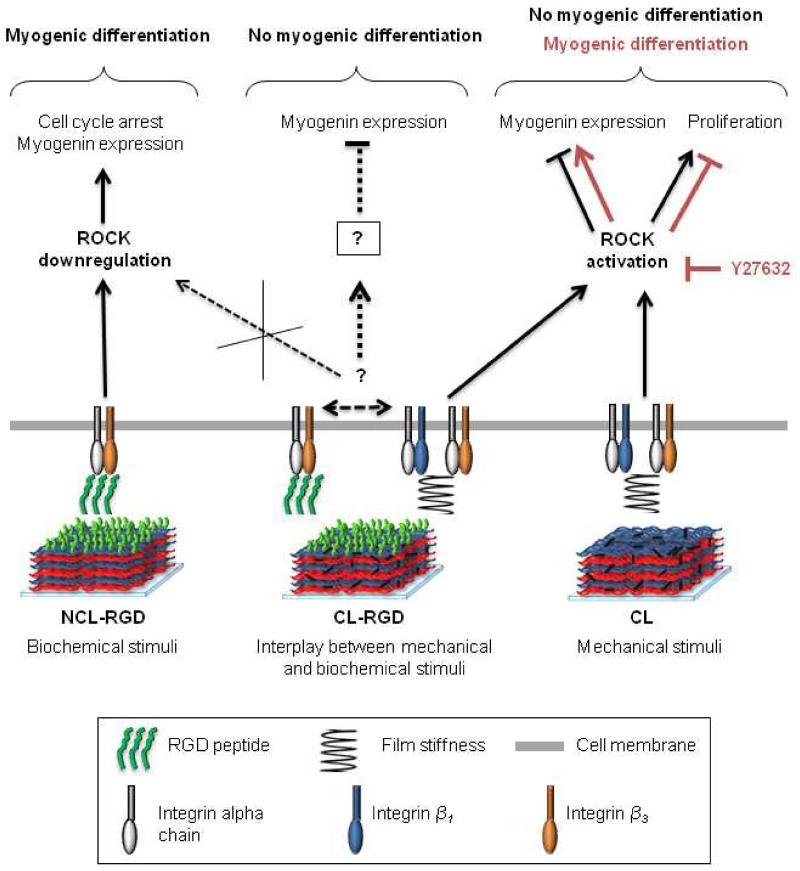
Based on our experimental data, we propose a model for the interplay between mechanical and biochemical stimuli during induction of C2C12 myogenic differentiation (Fig. 8). Adhesion on NCL-RGD films involved β3 integrins and provided favorable conditions for myogenic differentiation of C2C12 cells. Adhesion on stiff films (CL and CL-RGD) involved β1 integrins in addition to β3 integrins and promoted ROCK activation. This leads to a high proliferative state without myogenic differentiation. On CL films, ROCK inhibition allowed myogenic differentiation. However, on CL films ending by RGD peptide, ROCK inhibition was not sufficient to induce myogenin expression and to allow cells differentiation. We suggest that mechanical signals (stiffness) on CL-RGD films may affect cell interaction with biochemical signals (RGD peptide), resulting in the inhibition of β3 integrins by RGD peptide or by β1 integrins. Thus, our findings underline the importance of engineering substrates with well-controlled properties, as mechanical signals provided by the substrate can modify cell responses to biochemical cues. In this context, NCL-RGD represents a tool for the study of cell responses to RGD independently of the pathways activated by mechanical signal. Besides, in view of their excellent capacity to support myogenic differentiation, the soft films ending by RGD may be used as coating of various types of scaffolds used in muscle tissue engineering.
FIGURE 8. Model of the interplay between mechanical and biochemical stimuli during induction of C2C12 myogenic differentiation on soft/stiff films functionalized or not with the RGD peptide.
Adhesion involving β3 integrins on NCL-RGD films (soft films with covalently attached RGD peptide) provides favorable conditions for myogenic differentiation of C2C12 cells: when the medium is changed to DM, the rate of proliferating cells decreases and that of myogenin increases. Adhesion on CL and CL-RGD films (stiff films) involving β1 and β3 integrins promotes ROCK activation leading to a high proliferative state even in DM and to low myogenin expression. When the cells on stiff films are treated with ROCK inhibitor during differentiation, the rate of proliferating cells decreases significantly on both CL and CL-RGD films. Additionally, on CL films, myogenin expression increases, allowing the cells to undergo myogenic differentiation. However, on CL-RGD films, ROCK inhibition was not sufficient to induce myogenin expression and to allow cell to differentiate. We suggest that mechanical signals (stiffness) on CL-RGD films may affect cell interaction with biochemical signals (RGD peptide), resulting in the inhibition of β3 integrins by RGD peptide or by β1 integrins.
5. Conclusions
In the present work, four different types of PEM films, with or without cross-linking and with or without the RGD-peptide, allowed investigation of the effect of mechanical and biochemical signals and their combinations on important events of myogenesis. Soft films with RGD peptide appeared as the most appropriate for myogenic differentiation of C2C12 myoblasts, while stiff films (CL and CL-RGD) induced enhanced migration and proliferation and inhibited myogenic differentiation. ROCK inhibition was sufficient to rescue C2C12 differentiation on CL films but no significant changes were observed on CL-RGD films, showing that different signaling pathways were activated on each type of film depending on their mechanical and biochemical properties. Our model allowed highlighting how important events in myogenesis such as adhesion, migration proliferation, myogenin expression and fusion are regulated by substrate elasticity and presence of an adhesive ligand.
These results suggest that thin films with tunable mechanical and biochemical properties may be a useful tool for biophysical studies of muscle progenitors on controlled 2D microenvironments as well as for their expansion and differentiation in vitro. In addition, these films could be very easily be used to coat a wide range of 2D structured materials and 3D scaffolds.
Acknowledgements
This research was supported by Region Rhônes-Alpes via a PhD fellowship to VG. CP and RAV are members of the Institut Universitaire de France (IUF). CP wishes to thank the European Commission for support in the framework of FP7 via an ERC Starting grant 2010 (GA 259370, BIOMIM).
REFERENCES
- [1].Bischoff R. Regeneration of single skeletal muscle fibers in vitro. Anat Rec. 1975;182:215–35. doi: 10.1002/ar.1091820207. [DOI] [PubMed] [Google Scholar]
- [2].Le Ricousse-Roussanne S, Larghero J, Zini JM, Barateau V, Foubert P, Uzan G, et al. Ex vivo generation of mature and functional human smooth muscle cells differentiated from skeletal myoblasts. Exp Cell Res. 2007;313:1337–46. doi: 10.1016/j.yexcr.2007.01.022. [DOI] [PubMed] [Google Scholar]
- [3].Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, et al. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–66. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Schindeler A, Liu R, Little DG. The contribution of different cell lineages to bone repair: exploring a role for muscle stem cells. Differentiation. 2009;77:12–8. doi: 10.1016/j.diff.2008.09.007. [DOI] [PubMed] [Google Scholar]
- [5].Teboul L, Gaillard D, Staccini L, Inadera H, Amri EZ, Grimaldi PA. Thiazolidinediones and fatty acids convert myogenic cells into adipose-like cells. J Biol Chem. 1995;270:28183–7. doi: 10.1074/jbc.270.47.28183. [DOI] [PubMed] [Google Scholar]
- [6].Wada MR, Inagawa-Ogashiwa M, Shimizu S, Yasumoto S, Hashimoto N. Generation of different fates from multipotent muscle stem cells. Development. 2002;129:2987–95. doi: 10.1242/dev.129.12.2987. [DOI] [PubMed] [Google Scholar]
- [7].Andres V, Walsh K. Myogenin expression, cell cycle withdrawal, and phenotypic differentiation are temporally separable events that precede cell fusion upon myogenesis. J Cell Biol. 1996;132:657–66. doi: 10.1083/jcb.132.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Cosgrove BD, Sacco A, Gilbert PM, Blau HM. A home away from home: challenges and opportunities in engineering in vitro muscle satellite cell niches. Differentiation. 2009;78:185–94. doi: 10.1016/j.diff.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Thorsteinsdottir S, Deries M, Cachaco AS, Bajanca F. The extracellular matrix dimension of skeletal muscle development. Dev Biol. 2011;354:191–207. doi: 10.1016/j.ydbio.2011.03.015. [DOI] [PubMed] [Google Scholar]
- [10].Cohn RD, Henry MD, Michele DE, Barresi R, Saito F, Moore SA, et al. Disruption of DAG1 in differentiated skeletal muscle reveals a role for dystroglycan in muscle regeneration. Cell. 2002;110:639–48. doi: 10.1016/s0092-8674(02)00907-8. [DOI] [PubMed] [Google Scholar]
- [11].Han R, Kanagawa M, Yoshida-Moriguchi T, Rader EP, Ng RA, Michele DE, et al. Basal lamina strengthens cell membrane integrity via the laminin G domain-binding motif of alpha-dystroglycan. Proc Natl Acad Sci U S A. 2009;106:12573–9. doi: 10.1073/pnas.0906545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- [13].Mayer U. Integrins: redundant or important players in skeletal muscle? J Biol Chem. 2003;278:14587–90. doi: 10.1074/jbc.R200022200. [DOI] [PubMed] [Google Scholar]
- [14].Perkins AD, Ellis SJ, Asghari P, Shamsian A, Moore ED, Tanentzapf G. Integrin-mediated adhesion maintains sarcomeric integrity. Dev Biol. 2010;338:15–27. doi: 10.1016/j.ydbio.2009.10.034. [DOI] [PubMed] [Google Scholar]
- [15].Ruoslahti E. RGD and other recognition sequences for integrins. Annu Rev Cell Dev Biol. 1996;12:697–715. doi: 10.1146/annurev.cellbio.12.1.697. [DOI] [PubMed] [Google Scholar]
- [16].Gribova V, Crouzier T, Picart C. A material’s point of view on recent developments of polymeric biomaterials: control of mechanical and biochemical properties. J Mater Chem. 2011;21:14354–66. doi: 10.1039/C1JM11372K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hersel U, Dahmen C, Kessler H. RGD modified polymers: biomaterials for stimulated cell adhesion and beyond. Biomaterials. 2003;24:4385–415. doi: 10.1016/s0142-9612(03)00343-0. [DOI] [PubMed] [Google Scholar]
- [18].Benoit DS, Anseth KS. The effect on osteoblast function of colocalized RGD and PHSRN epitopes on PEG surfaces. Biomaterials. 2005;26:5209–20. doi: 10.1016/j.biomaterials.2005.01.045. [DOI] [PubMed] [Google Scholar]
- [19].Hirano Y, Kando Y, Hayashi T, Goto K, Nakajima A. Synthesis and cell attachment activity of bioactive oligopeptides: RGD, RGDS, RGDV, and RGDT. J Biomed Mater Res. 1991;25:1523–34. doi: 10.1002/jbm.820251209. [DOI] [PubMed] [Google Scholar]
- [20].Lin HB, Garcia-Echeverria C, Asakura S, Sun W, Mosher DF, Cooper SL. Endothelial cell adhesion on polyurethanes containing covalently attached RGD-peptides. Biomaterials. 1992;13:905–14. doi: 10.1016/0142-9612(92)90113-3. [DOI] [PubMed] [Google Scholar]
- [21].Houseman BT, Mrksich M. The microenvironment of immobilized Arg-Gly-Asp peptides is an important determinant of cell adhesion. Biomaterials. 2001;22:943–55. doi: 10.1016/s0142-9612(00)00259-3. [DOI] [PubMed] [Google Scholar]
- [22].Koepsel JT, Murphy WL. Patterning discrete stem cell culture environments via localized self-assembled monolayer replacement. Langmuir. 2009;25:12825–34. doi: 10.1021/la901938e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Koepsel JT, Loveland SG, Schwartz MP, Zorn S, Belair DG, Le NN, et al. A chemically-defined screening platform reveals behavioral similarities between primary human mesenchymal stem cells and endothelial cells. Integr Biol. 2012;4:1508–21. doi: 10.1039/c2ib20029e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bajaj P, Reddy B, Jr., Millet L, Wei C, Zorlutuna P, Bao G, et al. Patterning the differentiation of C2C12 skeletal myoblasts. Integr Biol. 2011;3:897–909. doi: 10.1039/c1ib00058f. [DOI] [PubMed] [Google Scholar]
- [25].Serena E, Zatti S, Reghelin E, Pasut A, Cimetta E, Elvassore N. Soft substrates drive optimal differentiation of human healthy and dystrophic myotubes. Integr Biol. 2010;2:193–201. doi: 10.1039/b921401a. [DOI] [PubMed] [Google Scholar]
- [26].Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J Cell Biol. 2004;166:877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Boontheekul T, Hill EE, Kong HJ, Mooney DJ. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007;13:1431–42. doi: 10.1089/ten.2006.0356. [DOI] [PubMed] [Google Scholar]
- [28].Chen WL, Simmons CA. Lessons from (patho)physiological tissue stiffness and their implications for drug screening, drug delivery and regenerative medicine. Adv Drug Deliv Rev. 2011;63:269–76. doi: 10.1016/j.addr.2011.01.004. [DOI] [PubMed] [Google Scholar]
- [29].Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–43. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- [30].Stedman HH, Sweeney HL, Shrager JB, Maguire HC, Panettieri RA, Petrof B, et al. The mdx mouse diaphragm reproduces the degenerative changes of Duchenne muscular dystrophy. Nature. 1991;352:536–9. doi: 10.1038/352536a0. [DOI] [PubMed] [Google Scholar]
- [31].Gilbert PM, Havenstrite KL, Magnusson KE, Sacco A, Leonardi NA, Kraft P, et al. Substrate elasticity regulates skeletal muscle stem cell self-renewal in culture. Science. 2010;329:1078–81. doi: 10.1126/science.1191035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ren K, Crouzier T, Roy C, Picart C. Polyelectrolyte multilayer films of controlled stiffness modulate myoblast cells differentiation. Adv Funct Mater. 2008;18:1378–89. doi: 10.1002/adfm.200701297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Boonen KJ, Rosaria-Chak KY, Baaijens FP, van der Schaft DW, Post MJ. Essential environmental cues from the satellite cell niche: optimizing proliferation and differentiation. Am J Physiol Cell Physiol. 2009;296:C1338–45. doi: 10.1152/ajpcell.00015.2009. [DOI] [PubMed] [Google Scholar]
- [34].Decher G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science. 1997;277:1232–7. [Google Scholar]
- [35].Tan G-K, Dinnes DLM, Butler LN, Cooper-White JJ. Interactions between meniscal cells and a self assembled biomimetic surface composed of hyaluronic acid, chitosan and meniscal extracellular matrix molecules. Biomaterials. 2010;31:6104–18. doi: 10.1016/j.biomaterials.2010.04.018. [DOI] [PubMed] [Google Scholar]
- [36].Samuel RE, Shukla A, Paik DH, Wang MX, Fang JC, Schmidt DJ, et al. Osteoconductive protamine-based polyelectrolyte multilayer functionalized surfaces. Biomaterials. 2011;32:7491–502. doi: 10.1016/j.biomaterials.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Boudou T, Crouzier T, Ren K, Blin G, Picart C. Multiple Functionalities of Polyelectrolyte Multilayer Films: New Biomedical Applications. Adv Mater. 2010;22:441–67. doi: 10.1002/adma.200901327. [DOI] [PubMed] [Google Scholar]
- [38].Berg MC, Yang SY, Hammond PT, Rubner MF. Controlling mammalian cell interactions on patterned polyelectrolyte multilayer surfaces. Langmuir. 2004;20:1362–8. doi: 10.1021/la0355489. [DOI] [PubMed] [Google Scholar]
- [39].Chien HW, Chang TY, Tsai WB. Spatial control of cellular adhesion using photo-crosslinked micropatterned polyelectrolyte multilayer films. Biomaterials. 2009;30:2209–18. doi: 10.1016/j.biomaterials.2008.12.060. [DOI] [PubMed] [Google Scholar]
- [40].Monge C, Ren K, Berton K, Guillot R, Peyrade D, Picart C. Engineering Muscle Tissues on Microstructured Polyelectrolyte Multilayer Films. Tissue Eng. 2012;18:1664–76. doi: 10.1089/ten.tea.2012.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schneider A, Bolcato-Bellemin AL, Francius G, Jedrzejwska J, Schaaf P, Voegel JC, et al. Glycated polyelectrolyte multilayer films: differential adhesion of primary versus tumor cells. Biomacromolecules. 2006;7:2882–9. doi: 10.1021/bm0605208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tryoen-Toth P, Vautier D, Haikel Y, Voegel JC, Schaaf P, Chluba J, et al. Viability, adhesion, and bone phenotype of osteoblast-like cells on polyelectrolyte multilayer films. J Biomed Mater Res. 2002;60:657–67. doi: 10.1002/jbm.10110. [DOI] [PubMed] [Google Scholar]
- [43].Picart C, Elkaim R, Richert L, Audoin F, Arntz Y, Da Silva Cardoso M, et al. Primary Cell Adhesion on RGD-Functionalized and Covalently Crosslinked Thin Polyelectrolyte Multilayer Films. Adv Funct Mater. 2005;15:83–94. [Google Scholar]
- [44].Crouzier T, Picart C. Ion pairing and hydration in polyelectrolyte multilayer films containing polysaccharides. Biomacromolecules. 2009;10:433–42. doi: 10.1021/bm8012378. [DOI] [PubMed] [Google Scholar]
- [45].Charrasse S, Comunale F, Fortier M, Portales-Casamar E, Debant A, Gauthier-Rouviere C. M-cadherin activates Rac1 GTPase through the Rho-GEF trio during myoblast fusion. Mol Biol Cell. 2007;18:1734–43. doi: 10.1091/mbc.E06-08-0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Voinova MV, Rodahl M, Jonson M, Kasemo B. Viscoelastic Acoustic Response of Layered Polymer Films at Fluid-Solid Interfaces: Continuum Mechanics Approach. Phys Scr. 1999;59:391. [Google Scholar]
- [47].Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nature Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- [48].Garcia AJ, Vega MD, Boettiger D. Modulation of cell proliferation and differentiation through substrate-dependent changes in fibronectin conformation. Mol Biol Cell. 1999;10:785–98. doi: 10.1091/mbc.10.3.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zamir E, Geiger B. Molecular complexity and dynamics of cell-matrix adhesions. J Cell Sci. 2001;114:3583–90. doi: 10.1242/jcs.114.20.3583. [DOI] [PubMed] [Google Scholar]
- [50].Ozeki N, Jethanandani P, Nakamura H, Ziober BL, Kramer RH. Modulation of satellite cell adhesion and motility following BMP2-induced differentiation to osteoblast lineage. Biochem Biophys Res Commun. 2007;353:54–9. doi: 10.1016/j.bbrc.2006.11.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Liu H, Niu A, Chen SE, Li YP. Beta3-integrin mediates satellite cell differentiation in regenerating mouse muscle. FASEB J. 2011;25:1914–21. doi: 10.1096/fj.10-170449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bach AD, Beier JP, Stern-Staeter J, Horch RE. Skeletal muscle tissue engineering. J Cell Mol Med. 2004;8:413–22. doi: 10.1111/j.1582-4934.2004.tb00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Nishiyama T, Kii I, Kudo A. Inactivation of Rho/ROCK signaling is crucial for the nuclear accumulation of FKHR and myoblast fusion. J Biol Chem. 2004;279:47311–9. doi: 10.1074/jbc.M403546200. [DOI] [PubMed] [Google Scholar]
- [54].Castellani L, Salvati E, Alema S, Falcone G. Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J Biol Chem. 2006;281:15249–57. doi: 10.1074/jbc.M601390200. [DOI] [PubMed] [Google Scholar]
- [55].Nemir S, West JL. Synthetic materials in the study of cell response to substrate rigidity. Ann Biomed Eng. 2010;38:2–20. doi: 10.1007/s10439-009-9811-1. [DOI] [PubMed] [Google Scholar]
- [56].Ren K, Fourel L, Rouviere CG, Albiges-Rizo C, Picart C. Manipulation of the adhesive behaviour of skeletal muscle cells on soft and stiff polyelectrolyte multilayers. Acta Biomater. 2010;6:4238–48. doi: 10.1016/j.actbio.2010.06.014. [DOI] [PubMed] [Google Scholar]
- [57].Rowley JA, Mooney DJ. Alginate type and RGD density control myoblast phenotype. J Biomed Mater Res. 2002;60:217–23. doi: 10.1002/jbm.1287. [DOI] [PubMed] [Google Scholar]
- [58].Wang PY, Thissen H, Tsai WB. The roles of RGD and grooved topography in the adhesion, morphology, and differentiation of C2C12 skeletal myoblasts. Biotechnol Bioeng. 2012;109:2104–15. doi: 10.1002/bit.24452. [DOI] [PubMed] [Google Scholar]
- [59].Danen EH, van Rheenen J, Franken W, Huveneers S, Sonneveld P, Jalink K, et al. Integrins control motile strategy through a Rho-cofilin pathway. J Cell Biol. 2005;169:515–26. doi: 10.1083/jcb.200412081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Hirsch E, Lohikangas L, Gullberg D, Johansson S, Fassler R. Mouse myoblasts can fuse and form a normal sarcomere in the absence of beta1 integrin expression. J Cell Sci. 1998;111(Pt 16):2397–409. doi: 10.1242/jcs.111.16.2397. [DOI] [PubMed] [Google Scholar]
- [61].Fackler OT, Grosse R. Cell motility through plasma membrane blebbing. J Cell Biol. 2008;181:879–84. doi: 10.1083/jcb.200802081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Charrasse S, Comunale F, Grumbach Y, Poulat F, Blangy A, Gauthier-Rouviere C. RhoA GTPase regulates M-cadherin activity and myoblast fusion. Mol Biol Cell. 2006;17:749–59. doi: 10.1091/mbc.E05-04-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Fortier M, Comunale F, Kucharczak J, Blangy A, Charrasse S, Gauthier-Rouviere C. RhoE controls myoblast alignment prior fusion through RhoA and ROCK. Cell Death Differ. 2008;15:1221–31. doi: 10.1038/cdd.2008.34. [DOI] [PubMed] [Google Scholar]
- [64].Mammoto A, Ingber DE. Cytoskeletal control of growth and cell fate switching. Curr Opin Cell Biol. 2009;21:864–70. doi: 10.1016/j.ceb.2009.08.001. [DOI] [PubMed] [Google Scholar]
- [65].Schaller MD. Cellular functions of FAK kinases: insight into molecular mechanisms and novel functions. J Cell Sci. 2010;123:1007–13. doi: 10.1242/jcs.045112. [DOI] [PubMed] [Google Scholar]
- [66].Narumiya S, Tanji M, Ishizaki T. Rho signaling, ROCK and mDia1, in transformation, metastasis and invasion. Cancer Metastasis Rev. 2009;28:65–76. doi: 10.1007/s10555-008-9170-7. [DOI] [PubMed] [Google Scholar]
- [67].Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, et al. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]