Abstract
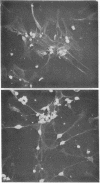
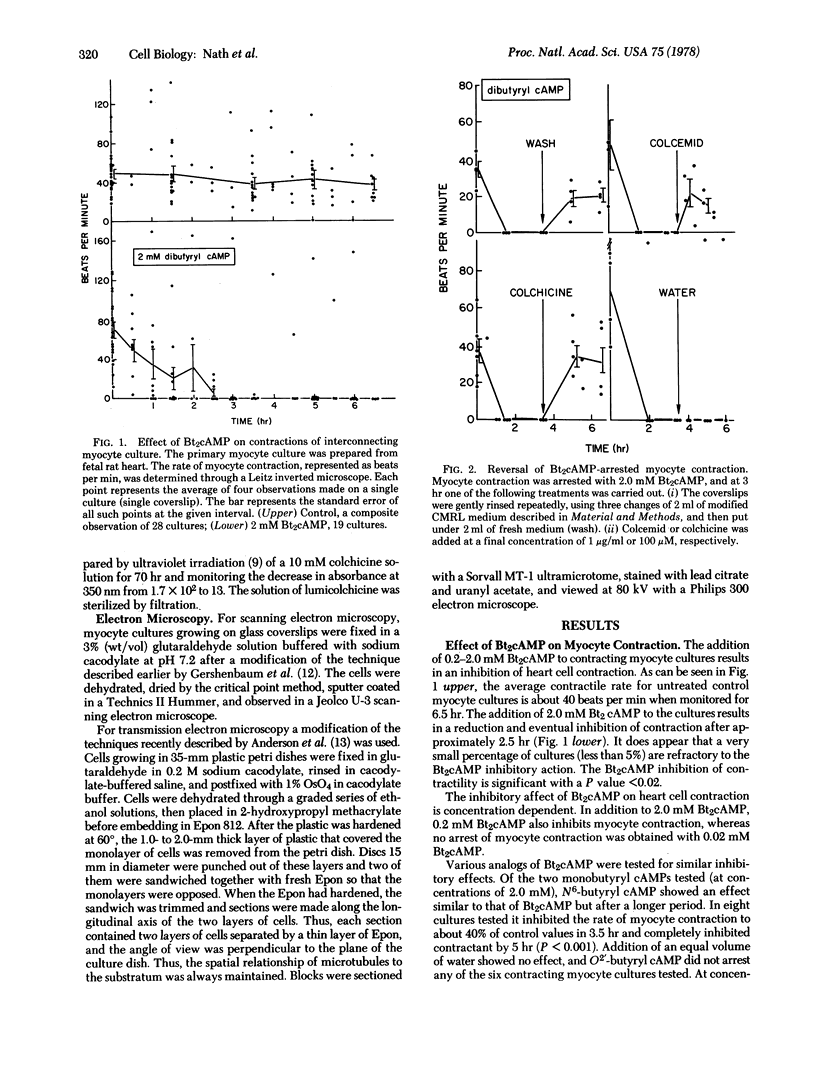
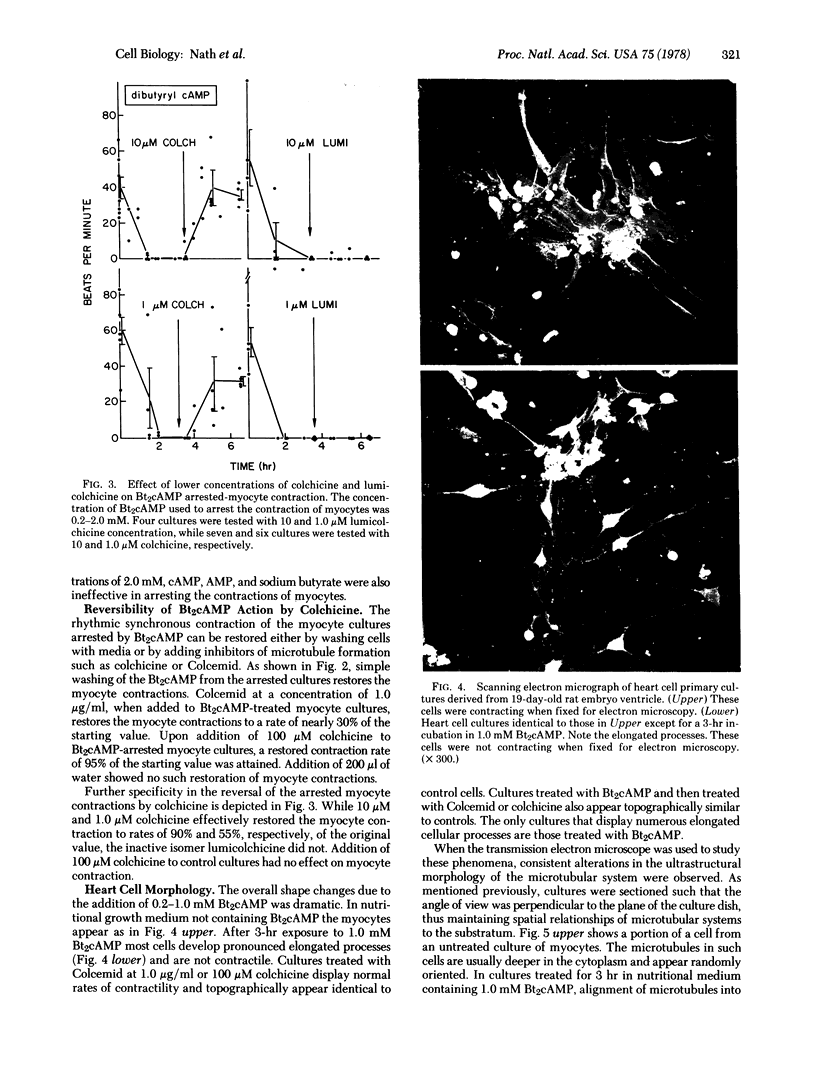
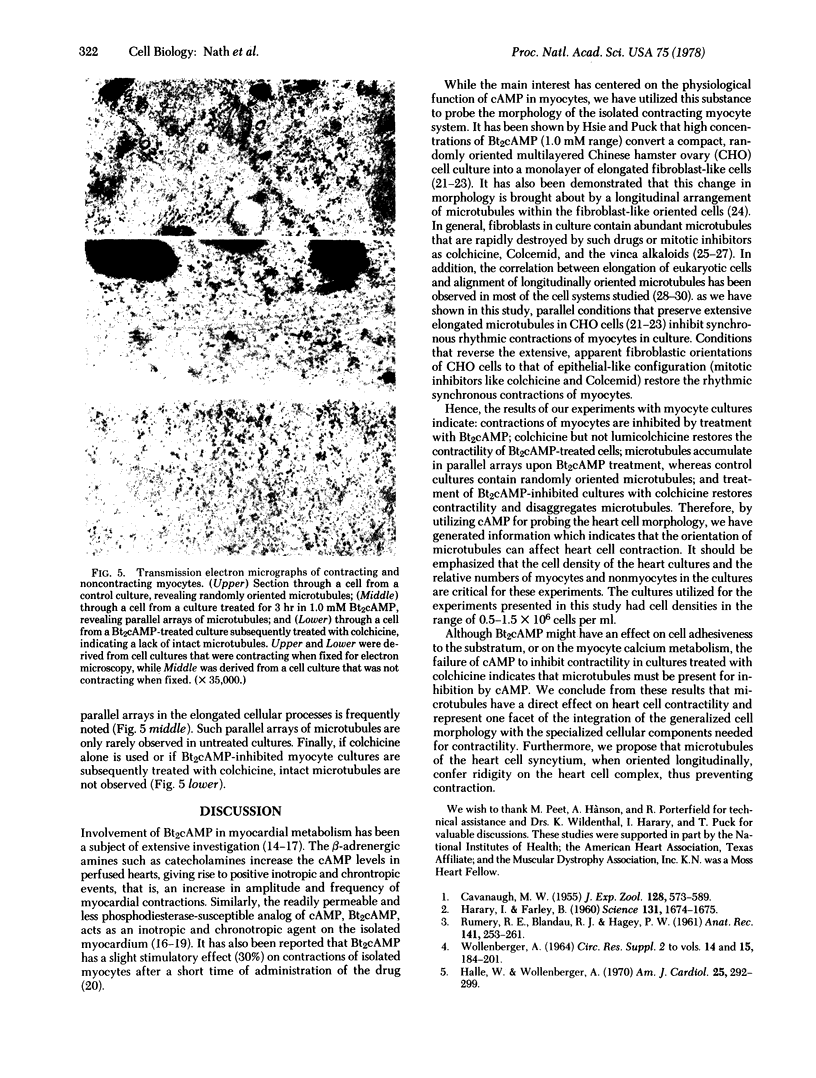
Rhythmic and synchronous contractions of interconnecting myocyte cultures prepared from fetal rat hearts are arrested upon the addition of 0.2--2.0 mM N6, O2'-dibuturyladenosine 3':5'-cyclic monophosphate (Bt2cAMP). The contractions arrested by Bt2cAMP are restored either by diluting the Bt2cAMP from the media or by adding colchicine. While colchicine restores Bt2cAMP-arrested myocyte contractions at concentrations as low as 1.0 micron, the inactive isomer lumicolchicine shows no effect. Morphologically, Bt2cAMP treatment of myocyte cultures results in the appearance of numerous elongated cellular processes not present in control cultures. Ultrastructural examination indicates that in Bt2cAMP-treated cells the intracellular distribution of microtubules is altered such that these organelles appear to accumulate in parallel arrays. In cells not treated with Bt2cAMP, the microtubules appear randomly oriented, while in cells treated with only colchicine, intact microtubules are not observed. The relationship between microtubules an heart cell contraction is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. G., Goldstein J. L., Brown M. S. Localization of low density lipoprotein receptors on plasma membrane of normal human fibroblasts and their absence in cells from a familial hypercholesterolemia homozygote. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2434–2438. doi: 10.1073/pnas.73.7.2434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borisy G. G., Taylor E. W. The mechanism of action of colchicine. Colchicine binding to sea urchin eggs and the mitotic apparatus. J Cell Biol. 1967 Aug;34(2):535–548. doi: 10.1083/jcb.34.2.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borman L. S., Dumont J. N., Hsie A. W. Relationship between cyclic AMP microtubule organization, and mammalian cell shape. Studies on Chinese hamster ovary cells and their variants. Exp Cell Res. 1975 Mar 15;91(2):422–428. doi: 10.1016/0014-4827(75)90123-8. [DOI] [PubMed] [Google Scholar]
- Drummond G. I., Hemmings S. J. Inotropic and chronotropic effects of dibutyryl cyclic AMP. Adv Cyclic Nucleotide Res. 1972;1:307–316. [PubMed] [Google Scholar]
- FANGE R., PERSSON H., THESLEFF S. Electrophysiologic and pharmacological observations on trypsin-disintegrated embryonic chick hearts cultured in vitro. Acta Physiol Scand. 1956 Dec 31;38(2):173–183. doi: 10.1111/j.1748-1716.1957.tb01381.x. [DOI] [PubMed] [Google Scholar]
- Goldman R. D. The role of three cytoplasmic fibers in BHK-21 cell motility. I. Microtubules and the effects of colchicine. J Cell Biol. 1971 Dec;51(3):752–762. doi: 10.1083/jcb.51.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies of single isolated beating heart cells. Science. 1960 Jun 3;131(3414):1674–1675. doi: 10.1126/science.131.3414.1674. [DOI] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies on single beating rat heart cells. I. Growth and organization. Exp Cell Res. 1963 Feb;29:451–465. doi: 10.1016/s0014-4827(63)80008-7. [DOI] [PubMed] [Google Scholar]
- HARARY I., FARLEY B. In vitro studies on single beating rat heart cells. II. Intercellular communication. Exp Cell Res. 1963 Feb;29:466–474. doi: 10.1016/s0014-4827(63)80009-9. [DOI] [PubMed] [Google Scholar]
- Halle W., Wollenberger A. Differentiation and behavior of isolated embryonic and neonatal heart cells in a chemically defined medium. Am J Cardiol. 1970 Mar;25(3):292–299. doi: 10.1016/s0002-9149(70)80006-6. [DOI] [PubMed] [Google Scholar]
- Handel M. A., Roth L. E. Cell shape and morphology of the neural tube: implications for microtubule function. Dev Biol. 1971 May;25(1):78–95. doi: 10.1016/0012-1606(71)90020-0. [DOI] [PubMed] [Google Scholar]
- Hardman J. G., Robison G. A., Sutherland E. W. Cyclic nucleotides. Annu Rev Physiol. 1971;33:311–336. doi: 10.1146/annurev.ph.33.030171.001523. [DOI] [PubMed] [Google Scholar]
- Henry P. D., Dobson J. G., Jr, Sobel B. E. Dissociations between changes in myocardial cyclic adenosine monophosphate and contractility. Circ Res. 1975 Mar;36(3):392–400. doi: 10.1161/01.res.36.3.392. [DOI] [PubMed] [Google Scholar]
- Hsie A. W., Puck T. T. Morphological transformation of Chinese hamster cells by dibutyryl adenosine cyclic 3':5'-monophosphate and testosterone. Proc Natl Acad Sci U S A. 1971 Feb;68(2):358–361. doi: 10.1073/pnas.68.2.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause E. G., Halle W., Wollenberger A. Effect of dibutyryl cyclic GMP on cultured beating rat heart cells. Adv Cyclic Nucleotide Res. 1972;1:301–305. [PubMed] [Google Scholar]
- Piatigorsky J., Webster H. D., Craig S. P. Protein synthesis and ultrastructure during the formation of embryonic chick lens fibers in vivo and in vitro. Dev Biol. 1972 Feb;27(2):176–189. doi: 10.1016/0012-1606(72)90096-6. [DOI] [PubMed] [Google Scholar]
- Porter K. R., Puck T. T., Hsie A. W., Kelley D. An electron microscopy study of the effects on dibutyryl cyclic AMP on Chinese hamster ovary cells. Cell. 1974 Jul;2(3):145–162. doi: 10.1016/0092-8674(74)90089-0. [DOI] [PubMed] [Google Scholar]
- Puck T. T., Waldren C. A., Hsie A. W. Membrane dynamics in the action of dibutyryl adenosine 3':5'-cyclic monophosphate and testosterone on mammalian cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1943–1947. doi: 10.1073/pnas.69.7.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUMERY R. E., BLANDAU R. J., HAGEY P. W. Observations on living myocardial cells from cultured 48-hour chick hearts. Anat Rec. 1961 Nov;141:253–261. doi: 10.1002/ar.1091410310. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Sobel B. E., Mayer S. E. Cyclic adenosine monophosphate and cardiac contractility. Circ Res. 1973 Apr;32(4):407–414. doi: 10.1161/01.res.32.4.407. [DOI] [PubMed] [Google Scholar]
- Stebbings H. Influence of vinblastine sulphate on the deployment of microtubules and ribosomes in telotrophic ovarioles. J Cell Sci. 1971 Jan;8(1):111–125. doi: 10.1242/jcs.8.1.111. [DOI] [PubMed] [Google Scholar]
- Warren R. H. Microtubular organization in elongating myogenic cells. J Cell Biol. 1974 Nov;63(2 Pt 1):550–566. doi: 10.1083/jcb.63.2.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]