Abstract
Standard imaging in acute stroke is undertaken with the aim of diagnosing the underlying cause and excluding stroke mimics. In the presence of ischaemic stroke, imaging is also needed to assess patient suitability for treatment with intravenous thrombolysis. Non-contrast CT is predominantly used, but MRI can also exclude any contraindications to thrombolysis treatment. Advanced stroke imaging such as CT and MR angiography and perfusion imaging are increasingly used in an acute setting. In this review, we discuss the evidence for the application of these advanced techniques in the imaging of acute stroke.
STROKE PREVALENCE AND AETIOLOGY
Stroke is defined conventionally as a focal neurological deficit that persists for more than 24 h owing to interruption of the blood supply to the brain. This definition is used to differentiate stroke from a transient ischaemic attack (TIA), which does not persist beyond 24 h but can be otherwise clinically identical. Stroke is a major health concern worldwide, but it is a particular problem in less developed countries where there are increased risk factors and less dedicated care. But even in developed nations, stroke remains a significant concern among their increasingly aged populations. Recent figures from the World Health Organization show that in the UK, cerebrovascular disease causes approximately 10.6% of all deaths and has a death rate of 45.6 per 100,000 of population. This places the UK 34th in world rankings (19th within Europe), which range from 25.0 to 249.4 per 100,000; the worldwide median death rate from stroke is 101.8 per 100,000 of population.1
Advances in care over the past 20 years have greatly improved the outcome for stroke patients with a reduction in deaths and more patients ultimately achieving independence. Amongst several possible changes, three factors are largely responsible for this improvement. First was the emergence of dedicated stroke units that offer both acute and rehabilitative care for the various and variable needs of stroke patients; a systematic review of randomized trial data has shown that specialized stroke unit care improves the long-term outcome for stroke patients by reducing both death and dependency.2 Second was the introduction of intravenous thrombolysis, which represented the first effective treatment for acute ischaemic stroke (aspirin has a modest effect as described below).3,4 Third is a better understanding among the general public at large that stroke represents a potentially treatable medical emergency. The FAST (Face, Arm, Speech, Time) campaign has been shown to improve public awareness of stroke.5 Despite these advances, however, many stroke survivors remain disabled; in general, around one-third of stroke patients die, one-third remain disabled long term and one-third regain or retain their independence. Stroke-related disability has enormous implications and costs for both the patient and for society as a whole. It is estimated that stroke costs the UK economy around £9 billion per year. Up to 50% of this is billed directly to the National Health Services, 30% is for the cost of informal care, while loss of productivity is estimated to account for the remaining 20%.6,7
The majority of strokes are ischaemic (approximately 80%) and most of these relate to problems with arterial blood flow to the brain; ischaemic stroke secondary to venous insufficiency is much less common. Haemorrhagic stroke accounts for the remaining 20%. The aetiologies of ischaemic and haemorrhagic stroke are usually quite different, but ischaemic strokes are often complicated by secondary haemorrhagic transformation. Stroke aetiology also varies with patient age. In middle aged to elderly adults, large artery ischaemic stroke (affecting both the cortex and white matter of a specific arterial territory) is generally secondary to intra-arterial thrombus or thrombo- or cardioembolism. Occlusive atherosclerotic vascular disease represents the major underlying pathology. This can be further divided into cardioembolic (for example, cardiac emboli arising secondary to atrial fibrillation or valvular heart disease) and atherothromboembolic causes (thrombus or embolus developing from atheromatous plaques of the extra-and/or intracranial arteries). Hypertension, hypercholesterolaemia, diabetes and smoking act as major risk factors for the development of atherosclerotic disease. Lacunar ischaemic stroke is a subset of ischaemic stroke thought to be owing to intrinsic disease of a single perforating cerebral arteriole (“small vessel disease”) that affects only a small volume of subcortical tissue and causes distinct clinical syndromes. The cause of the perforating arteriolar disease is unknown but it is commonly referred to as lipohyalinosis or fibrinoid necrosis; cardioembolism and carotid stenosis are infrequent causes of lacunar stroke, but hypertension and diabetes are common risk factors for both large- and small-vessel disease. Haemorrhagic stroke can occur secondary to an underlying vascular abnormality such as aneurysm, amyloid angiopathy, small-vessel disease or venous sinus thrombosis or in the presence of medicinal anticoagulation (e.g. warfarin). Hypertension is also a major risk factor for haemorrhagic stroke, present in approximately 50% of cases.8 In children or young adults, stroke is much less common but is most likely to result from arterial dissection within the neck (secondary to trauma or in the presence of a connective tissue disorder such as Marfan's syndrome) or in the presence of a clotting disorder, infection or illicit drug use. Less commonly, both ischaemic and haemorrhagic stroke can occur in all age groups secondary to a variety of neurological diseases, including vasculitis, CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), Moyamoya syndrome, Sneddon's syndrome and MELAS (mitochondrial encephalomyopathy lactic acidosis and stroke-like episodes).
TREATMENT OF STROKE
Intravenous thrombolysis is an effective treatment for acute ischaemic stroke and acts by dissolving occlusive thrombus or embolus and thereby restoring arterial blood flow to the brain.9–21 A recently updated systematic review and meta-analysis incorporated data from the 12 major randomized controlled trials performed to date that tested intravenous recombinant tissue plasminogen activator (rt-PA). In 7012 patients randomized over a 20-year period, this meta-analysis showed that those treated with rt-PA within 6 h of stroke were significantly more likely to be alive and independent at follow-up (46.3% vs 42.1%, respectively; p = 0.001). This is despite evidence that there is an increase in early death secondary to intracerebral haemorrhage for those treated with rt-PA (8.9% vs 6.4%, respectively; p = 0.0003). The effectiveness of thrombolytic therapy declines with time and the greatest benefit is achieved if treatment begins within the first 3 h from ischaemic stroke onset (40.7% alive and independent <3 h vs 31.7% >3 h; p < 0.0001).3 Importantly, approximately 10 years of observational data from the safe implementation of thrombolysis in stroke (SITS) register, which monitors rt-PA use in routine clinical practice across Europe has shown, in nearly 30,000 patients that intravenous rt-PA can be used safely in this context. Consistent with the data from randomized controlled trials, SITS data show comparable rates of haemorrhage, death and good outcome among stroke patients treated with intravenous rt-PA within the first 3 h (n = 25,279) and those treated between 3.0 and 4.5 h (n = 4056); even those treated “off-licence”, that is, between 4.5 and 6.0 h from stroke onset (n = 283) did not show a significant increase in the rates of poor outcome, although the authors acknowledge the limitations of this latter comparison.22–25 Presently, there is no evidence to support the routine use of intravenous thrombolysis therapy beyond 6 h of ischaemic stroke onset and the license in most countries only extends to 4.5 h after stroke (3 h in the USA). Trials are ongoing to find out if the time window can be extended and in which patients. Small amounts of data on patients with lacunar stroke in randomized controlled trials show that these patients achieved similar outcomes after intravenous rt-PA when compared with patients with other stroke subtypes.12,21 In the UK, recent audit data (excluding Scotland) show that approximately 75% of patients deemed eligible to receive thrombolysis currently do so (equivalent to 11% of all stroke patients), this compares with a national average of 7% of eligible patients in 2008.26,27
Patients with ischaemic stroke also benefit from early aspirin treatment.28 Two very large randomized controlled trials found in a total of nearly 40,000 acute ischaemic stroke patients that those randomized to receive daily aspirin suffered fewer (10 per 1000) recurrent strokes and fewer deaths in the first 6 months after stroke.4,29 Recent data show that 63% of all stroke patients in the UK are regularly taking an antiplatelet (e.g. aspirin or clopidogrel) 6 months from stroke onset.27 On a population level therefore, aspirin is likely to have a larger clinical impact on acute stroke treatment than does thrombolysis.
Endovascular retrieval of intra-arterial clot (mechanical embolectomy) or direct intra-arterial delivery of thrombolysis might also restore blood flow to the brain during acute ischaemic stroke when there is a proven arterial occlusion on angiography. However, as discussed below, catheter angiography carries an inherent level of risk and likely takes longer to perform than the time needed for administration of intravenous thrombolysis. An important consideration therefore is whether the perceived benefit in physically and actively removing clot or targeting thrombolysis delivery directly to the affected arteries, confers better outcomes on patients over those who receive intravenous thrombolysis alone. Several recent trials have failed to show benefit for these interventional procedures, therefore the balance of risk to benefit currently favours intravenous thrombolysis alone.30–32 The Interventional Management of Stroke III trial (IMS III) randomized patients to receive intravenous thrombolysis alone or intravenous thrombolysis in addition to some form of endovascular therapy (clot retrieval or intra-arterial thrombolysis—decided by the operator) within 3 h of acute stroke onset. IMS III was, however, stopped early due to futility since the interventional approach failed to show benefit (improved functional outcome) over intravenous delivery of thrombolysis alone in 656 patients.30 Similarly, the mechanical retrieval and recanalization of stroke clots using embolectomy (MR-RESCUE) trial randomized 118 patients with persistent large vessel occlusion, following intravenous thrombolysis, either to undergo clot retrieval or to continue with standard therapy but found no difference in outcome between the two groups.31 Finally, the SYNTHESIS trial randomized 362 patients to receive either endovascular therapy or intravenous thrombolysis but also found no significant differences in outcome between groups.32 Hence, it remains unclear where and when these more invasive procedures provide the most effective ratio of risk to benefit for acute stroke patients—consequently, at present, there is no place for routine intra-arterial thrombolysis or use of mechanical devices in patients with acute ischaemic stroke but trials are ongoing [e.g. pragmatic ischaemic stroke thrombectomy evaluation (PISTE)33] to test this therapy further.
Patients with TIA are at high risk of subsequent ischaemic stroke. Thus, if the symptoms of stroke are transient, the patient should be investigated rapidly for underlying stroke risk factors and started on secondary prevention therapy as fast as possible to avoid recurrent stroke—antiplatelet drugs, a statin and blood pressure reduction are guideline based;34 treatment of diabetes if present and general lifestyle advice such as smoking cessation and exercise are also important. Specific underlying risk factors should be identified and treated rapidly, especially symptomatic carotid stenosis (>50%) and atrial fibrillation.
The treatment for haemorrhagic stroke is currently primarily supportive unless there is a specific underlying cause such as aneurysm.
A large number of trials of other agents that might accelerate medical thrombolysis or offer neuroprotection in both ischaemic and haemorrhagic stroke have been and continue to be tested. As yet, no new agents have been convincingly identified—several alternative thrombolytic drugs are being tested in randomized controlled trials. Similarly, trials of surgical intervention [e.g. hemicraniectomy for patients at risk of malignant middle cerebral artery (MCA) syndrome] have shown mixed results. An in-depth discussion of these trials is beyond the scope of this article; readers interested in learning more are encouraged to visit The Internet Stroke Center (www.strokecenter.org), which provides an independent repository of stroke trial data, past and present.
EVIDENCE FOR CURRENT IMAGING PRACTICE IN STROKE
To allow for the prompt delivery of appropriate treatment, it is important to make a rapid and accurate diagnosis of the stroke type as soon as possible following symptom onset—imaging is necessary to differentiate infarct from haemorrhage and exclude stroke mimics. Until recently, imaging in early stroke has primarily been used to exclude haemorrhage and non-vascular causes of stroke (i.e. structural stroke mimics such as encephalitis, acute demyelination, meningitis, abscess, subdural haematoma or other space-occupying lesions) rather than confirm infarct. Consider that haemorrhage (or a structural lesion) is a contraindication to intravenous thrombolysis but the absence of an infarct is not. However, with advances in understanding of the differences in stroke pathology between large artery atheroma and small-vessel lacunar stroke, it is becoming increasingly necessary to identify positively the causative ischaemic lesion. Non-contrast CT scanning has become the primary imaging modality in the initial assessment of acute stroke for several reasons. Firstly, CT is widely available whereas MRI is not, especially out of hours.35 Secondly, on modern equipment, a non-contrast brain CT can be performed in seconds, whereas even a few basic MRI sequences can take several minutes. Thirdly, owing to the inherent differences in machine structure and function, CT makes it easier to manage an unstable patient during scanning and is better tolerated by those who suffer claustrophobia. Finally, as a means to answer the basic imaging question, of whether an acute stroke is ischaemic, haemorrhagic or due to a non-vascular cause, CT is very accurate for identifying haemorrhage and non-vascular causes36,37 and can frequently identify early ischaemic changes in patients with moderate-to-severe stroke although small ischaemic lesions in patients with mild stroke are difficult to identify on CT.
In addition to differentiating ischaemic from haemorrhagic stroke, non-contrast CT also provides some information on the presence of arterial thrombus (the hyperdense artery sign) and on the extent of ischaemia (loss of grey–white matter differentiation, hypoattenuation of brain tissue and evidence of swelling) (Figure 1). The hyperdense artery sign can be subjectively defined as any artery that appears more dense than do adjacent or equivalent contralateral arteries,38,39 but more objective definitions (>43 Hounsfield units or >1.2 times the density of a normal contralateral vessel) have been proposed.40 The hyperdense artery sign is a highly specific and moderately sensitive marker of true arterial thrombus as demonstrated using angiography and compared with the other non-contrast CT features of stroke is consistently identified even among non-specialist observers.41,42 Furthermore, identification of a hyperdense artery makes it more likely that an observer will also correctly identify concurrent ischaemic changes.43 A high specificity means that if a hyperdense artery is correctly identified, one can be reasonably confident that it correlates with intra-arterial thrombus; some difficulty can arise in the presence of small volume arterial calcification where it may not be possible to accurately assess density or location in the vessel wall rather than lumen. With only moderate sensitivity, the absence of a hyperdense artery sign does not mean that angiography will definitely be normal. It is worth noting that sensitivity is improved when thin-slice (<1.25 mm) volumetric CT is used.42 Non-contrast CT provides a reliable means of identifying medium-to-large areas of ischaemic change. There is, however, no evidence that stratifying patients by extent of ischaemia as assessed on non-contrast CT reliably predicts outcome following treatment with thrombolysis. Data from two large randomized trials failed to show an association between Alberta stroke program early CT score (ASPECTS), which measures the extent of MCA territory involvement in stroke, and outcome following treatment with rt-PA.44,45 Small volume infarct in the early stages of stroke can be significantly more difficult to detect, particularly for those with less experience of doing so and especially if insufficient time is allowed for assessment.41,43 Non-contrast CT also provides valuable information on the rest of the imaged brain. For example, the identification of background leukoaraiosis has been shown in meta-analysis of case series and observational studies (there is limited randomized controlled trial data), to increase the risk of haemorrhage following treatment with rt-PA,46 while an unexpected subacute infarct, that is, a silent infarct that occurred several days prior to the presenting clinical deterioration,47 would represent contraindication to the use of intravenous thrombolysis.
Figure 1.
Acute stroke changes on non-contrast CT. Left image shows an area of hypodensity and loss of grey–white matter differentiation affecting the right basal ganglia and insular cortex (within box) consistent with an acute infarct. Compare with the normal left side. Right image shows a hyperdense mainstem of left middle cerebral artery in a different patient (arrow).
MRI is used for assessing acute stroke in many centres. As a minimum, MRI in stroke requires at least four sequences, namely T2 weighted, diffusion-weighted imaging (DWI), T2* and fluid-attenuated inversion recovery (FLAIR),48,49 in order to identify haemorrhage, ischaemia, underlying structural changes (mimics) and factors such as leukoaraiosis. In this context, MRI is usually most appropriate in patients with mild stroke since these minimum four sequences will require approximately 15 min of in-scanner time for the patient using standard modern equipment, and this will increase if other sequences, that is, T1 weighted, time-of-flight angiography, are applied. In some series, up to 45% of patients with moderate or severe stroke were unable to complete MRI examinations.50 Depending on availability, MRI may be used instead of non-contrast CT but only if blood-sensitive sequences like T2* (or susceptibility-weighted imaging) are used—MRI without these is not able to differentiate haemorrhage reliably, especially if imaging is performed hyperacutely; both modalities are excellent at differentiating acute ischaemia from haemorrhage in the first 5 days after stroke if suitable MRI sequences are used. In addition, blood-sensitive MRI sequences enable detection of subclinical microhaemorrhages, which are not routinely identifiable on CT. It has been postulated that these might predict an increased risk of symptomatic haemorrhage following thrombolysis, but with the limited data available, such a link remains unproven,51,52 although microhaemorrhages are associated with increased risk of spontaneous haemorrhage.53 MRI also has other advantages over non-contrast CT. The main advantage of MRI in acute stroke relates specifically to the use of DWI, which can provide very clear evidence of the extent of parenchymal ischaemia and/or infarction within minutes of stroke onset and can do so more sensitively than non-contrast CT within the first 6 h from the onset; 40–73% for CT vs 58–97% for MRI.54–56 Early parenchymal changes on MRI are best appreciated as restriction in the normal diffusivity of intracellular water (i.e. restricted diffusion—high signal on DWI sequences and correspondingly low on apparent diffusion coefficient maps) (Figure 2). It should be noted that DWI provides most benefit in identifying patients with hyperacute, small volume (e.g. focal cortical or lacunar infarct as in the example provided) or atypical infarcts since these are more likely to be overlooked using non-contrast CT. International consensus standards for the reporting of small subcortical infarcts and lacunes have recently been proposed [STandards for ReportIng Vascular changes on nEuroimaging (STRIVE)].57 However, even with very sensitive diffusion imaging,58 DWI may not be positive in up to one-third of those with minor stroke and in two-thirds of those with TIA.59,60 Large volume infarcts are usually readily apparent on both CT and MRI, especially when they are imaged subacutely. Changes on the other standard MRI sequences (i.e. increased T2 signal within affected tissues, best seen on FLAIR) occur later with the development of parenchymal oedema and are therefore seen in a similar time course (and with no greater sensitivity) than the changes described above for non-contrast CT. This time difference between DWI and other MRI sequences has been used to estimate the time of stroke onset by assessing for a DWI–FLAIR mismatch. It has been shown that although DWI changes can be identified within minutes of stroke onset in some patients, in a large proportion of patients, FLAIR changes may not be apparent until several hours from the onset. Patients with large areas of mismatch are therefore deemed to have had a more recent onset time of stroke and could potentially be eligible for intravenous thrombolysis even if an absolute stroke onset time is not available.61,62 This concept is being tested in randomized trials and until the results of those trials are known, there is no current basis on which to use DWI–FLAIR mismatch for deciding whether or not to use intravenous thrombolysis in routine practice.
Figure 2.
Acute stroke changes on MRI. Acute lacunar infarct within the left thalamus as demonstrated on MRI. Infarct is high signal on T2 weighted (left) and diffusion-weighted imaging (middle) but correspondingly low signal on apparent diffusion coefficient imaging (right).
ADVANCED STROKE IMAGING USING ANGIOGRAPHY AND PERFUSION
To identify features of acute stroke not routinely accessible through non-contrast CT or standard stroke MRI such as arterial patency and blood flow dynamics within brain, many centres now perform advanced forms of imaging (CT and MRI angiography and/or perfusion imaging) during the very early stages of stroke as part of their routine acute stroke assessment protocol. In fact, a recent multicentre review of the imaging received by >12,000 stroke patients in the USA prior to administration of intravenous thrombolysis, showed that over a 4-year period, the number of patients being imaged with non-contrast CT alone dropped from 64% to 55%.63 Similar figures for the UK are not available but would be interesting to compare given that the USA uses a fee-for-service model of funding radiological services.
Angiography in acute stroke aims to provide the location and extent of any potentially treatable thrombus or embolus causing acute arterial occlusion whether this affects intra- or extracranial vessels. We know, for example, from large trial data that stroke patients with a hyperdense artery sign (a surrogate for arterial occlusion) on initial imaging achieve better long-term outcomes if the hyperdensity (and by extrapolation, the occlusion) does not persist.64,65 A large systematic review, including >1000 patients, similarly concluded that recanalization (identified using a range of methods) increases the odds of good outcome at 3 months, especially if achieved early.66 The expectation is that angiography could improve intravenous thrombolysis treatment decision-making by focusing treatment on patients with a blocked artery, thus avoiding exposing patients without a blocked artery to the risk of rt-PA.
Perfusion imaging aims to provide an accurate and understandable depiction of the non-salvageable infarct extent (core of dead tissue) and to contrast this with the volume of potentially salvageable tissue that is ischaemic and at risk but has not yet infarcted (penumbra). This is an important distinction to make prior to the restoration of arterial blood flow to an underperfused area of tissue. The concern is that infarcted tissue is more likely than ischaemic tissue to undergo reperfusion injury and subsequent haemorrhage following restoration of blood flow.67 It is hoped therefore that perfusion imaging can isolate patients with more penumbra and less core. Theoretically, such patients could gain the most benefit from arterial reperfusion therapy at the lowest risk of adverse outcome irrespective of the time elapsed from stroke onset, or even when an accurate onset time cannot be determined. This has not yet been proven clinically, but trials are in progress.68,69 It is hoped that in future, an accurate assessment of tissue perfusion following stroke could allow extension of current time limits for reperfusion therapy and may even lead to individualized treatment decisions for each patient.
Any additional “advanced” imaging adds to the time needed for patient assessment. The thrombolysis trials have demonstrated very clearly that “time is brain”, so the negative effects of any additional time taken to assess the patient has to be traded against the potential benefits of the information provided by those additional tests and whether or not it translates into more effective treatment. If not, then the additional information adds little value. So far, there is generally limited evidence that the inclusion of more expensive and time-consuming imaging adds to patient care or improves outcomes. In the realm of acute stroke care where time is paramount, there remain several unanswered questions regarding the correct use and interpretation of advanced imaging. In this review, we discuss angiography and perfusion imaging techniques and explore the evidence for using them in an acute clinical setting.
ANGIOGRAPHY
Catheter angiography remains the gold standard for assessing arterial (and venous) flow in real time. It provides excellent information on vascular anatomy, vessel patency and the extent of collateral supply (including the length of time that it takes and the direction travelled by the contrast as it bypasses an occlusion). Catheter angiography is, however, an invasive procedure that comes with its own risks. Neurologic complications of any type occur in up to 3% of stroke cases, while permanent disability or death is reported in <1%.70–72
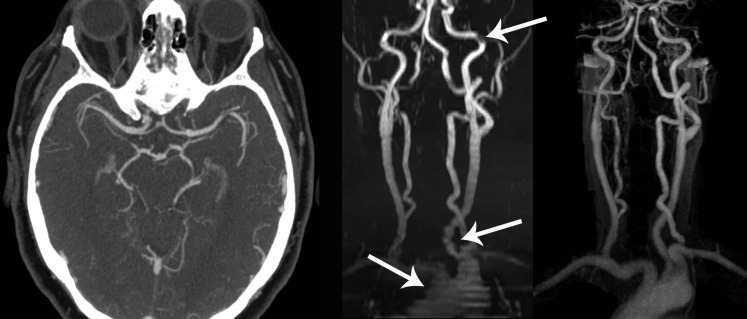
CT and MRI offer a means of obtaining minimally and non-invasive angiography. Minimally invasive indicates that the patient will require an intravenous injection of contrast (CT or MRI). Non-invasive implies that no contrast is given (MRI only). Angiography performed with contrast generally provides more robust imaging than does angiography without contrast since the former is less likely to be affected by imaging artefacts. In an acute setting, however, it can be difficult to obtain the baseline renal status or allergy profile of a stroke patient. In addition, many stroke patients will be dehydrated at presentation, which can further exacerbate the problem. In these scenarios, intravenous contrast may be contraindicated. Non-contrast time-of-flight MRI (saturates signal from non-moving tissue to isolate the signal returned from flowing blood) provides an alternative option, but as alluded to above, this approach is potentially limited by imaging artefact. Figure 3 displays contrast-enhanced CT angiography (CTA) and compares MR time-of-flight angiography with contrast-enhanced MR angiography. Notwithstanding, angiography performed with either CT or MRI (CTA and MRA, respectively) is known to provide very accurate information of arterial flow when compared with the gold standard, catheter angiography.73–78 The major limitation is that a standard CTA or MRA obtains imaging over a short time period (in the case of CT, this is only a few seconds) and therefore provides no information on the speed or directionality of blood flow (information that can be especially important if collateral supply is being assessed). To incorporate this, four-dimensional CTA and MRA have been developed. These techniques reimage the field of interest at several time points following the injection of contrast or initiation of the stimulatory radiofrequency pulse. The resultant dynamic imaging provides flow information very similar to that obtained by catheter angiography.79–81
Figure 3.
CT and MR angiography. Left image shows normal CT angiography through the circle of Willis. Time-of-flight MR angiography (middle image) and contrast-enhanced MR angiography (right image) of the neck arteries are compared in the coronal plane. Note significant artefacts on time-of-flight images (arrows).
CT and MR angiography in stroke are commonly assessed using either the thrombolysis in cerebral infarction (TICI) score or the Mori scale.82,83 These scales were, however, originally developed for the assessment of catheter angiography. As such, they assess distal tissue perfusion in addition to focal arterial luminal narrowing and were developed to be used both in the initial assessment of an arterial occlusion and also to measure the extent of recanalization following treatment, that is, several different components of tissue blood supply are being conflated into one scoring system. Comparatively, standard CTA and MRA only allow interpretation of luminal narrowing or focal occlusion (unless they are performed in four-dimensions as described above) and therefore the standard catheter angiography scores are not wholly appropriate in this context.84 Many of the studies that have used these scales for assessing CTA or MRA have therefore adopted modified (and non-standardized) versions.85 Consequently, it is difficult to assess the wider application of angiography rating scales from the available literature; as recommended in a recent consensus group statement, greater standardization is required.86 The acute stroke imaging research roadmap II87 aims to do exactly that and has suggested use of their modified TICI scale for studies assessing CT and MR angiography.
A possible association has been identified between the expansion of haematoma on the follow-up imaging of haemorrhagic stroke patients (a poor prognostic indicator) and the presence of the so-called “spot sign” on CTA (contrast extravasation within the haematoma) performed acutely. A recent systematic review has however failed to clarify this association owing to the heterogeneity of data.88
A major benefit of performing angiography with CT or MRI rather than via an intra-arterial catheter is the additional brain parenchymal imaging that can simultaneously be obtained. For a few extra minutes in the scanner, CTA may be preceded by a non-contrast brain CT, whereas MRA may be enhanced with a few basic stroke sequences (e.g. FLAIR, DWI and T2*). In fact, even if angiography sequences were acquired in isolation, additional parenchymal information will still be available. For example, when the window settings of CTA images are reset to those of a standard non-contrast brain, a simple “perfusion” study is achieved [a crude snapshot assessment of cerebral blood flow (CBF)—see below for definition]. Such use of the CTA source images has been shown to enhance the interpretation of non-contrast CT and CTA, providing results that are equivalent to MRI in stroke by improving the sensitivity of CT for detecting infarct and predicting outcome, particularly among less experienced observers89–92 (Figure 4).
Figure 4.
CT angiography (CTA) source images. CTA (left image) demonstrates an occlusion within the proximal left middle cerebral artery (arrow). When window settings are adjusted to view brain (right image), these “CTA source images” reveal that the left middle cerebral artery territory (within dotted line) is underperfused; affected area is hypodense relative to contralateral normal brain.
PERFUSION IMAGING
Standard perfusion imaging uses dynamic contrast-enhanced images to calculate several flow-related variables within each voxel of interest, that is, the same slice or block of tissue is scanned continuously or repeatedly as a contrast bolus passes through. The change in density (CT perfusion) or T1 signal intensity (MR perfusion) from baseline through the period of contrast inflow, at peak and during contrast outflow is used to estimate blood flow and blood volume dynamics within the tissue of interest. Most commonly, the output variables include CBF (the total volume of blood flowing through a voxel of interest in a given unit of time), cerebral blood volume (CBV—the total blood volume within the voxel of interest), mean transit time (MTT—mean time taken for a contrast to pass through the voxel of interest and is equivalent to CBV/CBF) and time to peak (time taken to reach maximal contrast enhancement). The results for each variable are then presented as colour-coded perfusion maps (Figure 5). In cases where intravenous contrast is contraindicated, an MR technique called arterial spin labelling (ASL) may offer an alternative means to obtain perfusion mapping for acute stroke. ASL works by stimulating flowing blood before it reaches the tissue of interest. Stimulated blood then attenuates signal from the surrounding tissue. Subtraction of a labelled image from a pre-stimulation control image gives a measure of stimulated blood inflow to the tissue of interest, i.e. CBF.93 Early clinical testing has shown this non-invasive technique is comparable to contrast-enhanced perfusion studies in acute stroke, however, it is very sensitive to artefact from movement, which may limit its use in acute stroke; work is ongoing.94,95
Figure 5.
CT perfusion maps showing commonly provided perfusion parameters of cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT) and time to peak (TTP). Anterior left middle cerebral artery territory perfusion defect showing decreased CBF and increased MTT and TTP. CBV is marginally increased. The difference between CBV and the other parameters suggests ischaemic but not yet infarcted tissue (i.e. penumbra, at risk). This patient proceeded to treatment with intravenous thrombolysis. P, posterior; R, right.
While cerebral perfusion maps can indicate areas of altered blood flow dynamics, the technique is not fully developed such that it can be used routinely and consistently in non-specialist clinical practice. The problem with current CT or MR perfusion imaging is that it is difficult to standardize.96 One of the major difficulties faced by researchers in this area is the plethora of perfusion parameters and thresholds that have been suggested as differentiating core from penumbra and the large variability in the current definitions of core and penumbra.97 A recent systematic review found that in 69 articles discussing perfusion imaging (49 MRI and 20 CT), there were 38 different parameter definitions for tissue state, within the range from normal tissue, to tissue at risk, to non-viable tissue and that the threshold values used for each varied widely; as an example, the CBF threshold used to differentiate non-viable tissue from tissue at risk (core vs penumbra) was in the range 9–20 ml per 100 g min−1 in only three articles (Table 1 compares definitions for tissue at risk assessed using initial perfusion imaging only).97 Secondly, owing to differences in acquisition, CT and MR perfusion are likely to give quite different results, even for the same patient.98,99 Thirdly, there is significant variability of image processing methods. Different scanner manufacturers may use different image analysis algorithms to calculate the same perfusion parameters (i.e. a CBF from one manufacturer is not the same as a CBF from another manufacturer100), and may potentially do so from images acquired using different techniques (e.g. spin echo vs gradient echo).101 It is worth noting that these differences are compounded on the variability that we would expect even in normal imaging practice where patients, imaging equipment and applied protocols are in constant flux.
Table 1.
Comparison of different CT and MRI perfusion definitions used to assess tissue at risk (i.e. penumbra) on initial perfusion imaging in acute ischaemic stroke as identified in a recent systematic review
| CT perfusion | MR perfusion |
|---|---|
| Volume of hypoperfusion assessed using MTT only | Entire volume of hypoperfused tissue in the acute MRP lesion |
| Volume of hypoperfusion assessed using CBF only | Volume of tissue present in the acute MRP lesion but not in the acute diffusion weighted imaging lesion (diffusion mismatch) |
| Volume of hypoperfusion assessed using MTT and CBV | |
| Volume of hypoperfusion assessed using CBF, CBV and TTP | |
| Volume of hypoperfusion assessed using TTP and CBV | |
| Volume of hypoperfusion on CTP as a percentage of the affected hemisphere | |
| Volume of tissue identified using pre-determined CBF thresholds (>12 but ≤24 ml per 100 g−1 min−1) |
CBF, cerebral blood flow; CBV, cerebral blood volume; CTP, CT perfusion; MRP, MR perfusion; MTT, mean transit time; TTP, time to peak.
Reproduced from Dani et al97 with permission from John Wiley and Sons.
Several other definitions (not listed here) also used follow-up imaging to make assessment.
PRACTICALITIES OF ADVANCED IMAGING
It is estimated that during an acute stroke, approximately 2 million neurones, 14 billion synapses and 12 km of myelinated fibres are destroyed per minute, that is, time is brain.102 Therefore, every intervention or investigation that occurs between the acute presentation of a stroke patient and the initiation of definitive reperfusion therapy needs to be justified and proven to provide benefit by improving outcome, while not causing undue harm. Recent national UK data show that only 40% of acute stroke patients in the UK (excluding Scotland) are scanned within 1 h of arrival in hospital (median delay, 83 min).27 Inevitably, performing more scanning takes more time, increases radiation dose or the risk of aspiration while supine and reduces oxygen saturation.103 Prior to the widespread utilization of an advanced imaging modality in acute stroke, therefore, several factors need to be considered.
The first consideration is whether the information from imaging can be consistently and repeatedly extracted. As alluded to above, there is some variability in the interpretation of non-contrast CT for ischaemic stroke, especially in the identification of subtle parenchymal changes. A systematic review of interobserver agreement for the detection of stroke on non-contrast CT demonstrated κ statistics in the range 0.14–0.78 for the identification of any relevant CT finding, but the experience level of observers or their number was not clearly stated in all cases.104 Two more recent observer reliability studies, including large numbers of observers (>200) with varying levels of experience and expertise, showed that while expert neuroradiologists were more likely than non-neuroradiologists to identify subtle stroke signs, years of experience was less important.41,43 Notwithstanding, such reader variability must be considered as the baseline on which more advanced imaging techniques should be build, that is, advanced techniques should not significantly increase reader variability, as this might diminish the value of performing those additional techniques in the first place.
By contrast, there is little information on the observer reliability of perfusion or angiographic imaging in stroke. Using observational data, some authors have compared standard non-contrast CT or MRI with CT perfusion and MR perfusion, respectively, for the identification of acute stroke, including assessment using visual scales such as ASPECTS; in all cases, perfusion imaging was found to provide higher levels of interobserver agreement in the range 0.67–0.92 among a limited number of observers (up to six).105–108 There is even less work evaluating the reliability of intracranial CT or MR angiography in stroke. One small study of 17 patients and 4 observers reported interobserver agreement for the identification of arterial occlusion in acute stroke using MRA as κ = 0.78.109 Another small study assessed the degree of chronic arterial stenosis in 25 patients using 2 observers and found better agreement using MRA (κ = 0.78) than CTA (κ = 0.51);110 more work is needed here.
Other issues to consider relate to the technical aspects in implementing an advanced imaging technique. These include, a reasonable estimate of the average time that each additional imaging study is likely to take, a measure of how consistently complex imaging is acquired, the expected increase in radiation exposure for CT-based modalities and whether additional hardware or software are required for either the creation or the interpretation of imaging (i.e. departmental extra costs).
Very little data exist to accurately quantify the extra time that is required for additional angiographic or perfusion imaging following non-contrast CT or MRI in acute stroke. In general, advocates for these techniques suggest and imply that these extra sequences add only a few minutes to the basic imaging requirement but rarely publish actual results. In reality, staff working in busy imaging centres are likely to have variable levels of experience, and the efficiency of performing complex imaging is also likely to drop when performed out of hours. Additionally, both angiography and perfusion imaging require more time for image processing (especially perfusion imaging) and for interpretation. It has been reported that CTA and CT perfusion require approximately 15–20 min each for acquisition and interpretation, whereas a full-stroke MR protocol can take more than 20 min.89,111–114
Compared with non-contrast scanning, the acquisition of more complex imaging requires greater planning, and therefore more opportunity to introduce human error. For example, good contrast-enhanced angiography requires images to be obtained during the peak of the arterial contrast bolus. Similarly, perfusion imaging requires the arterial inflow and venous outflow be identified. Finally, many perfusion imaging techniques require that a few representative image slices are chosen for analysis (unless whole-brain volumetric perfusion is performed). Sections incorporating the middle cerebral artery territory are often chosen, this may miss infarcts occurring elsewhere.
Although radiation dose is rarely a concern when assessing acutely unwell patients with life threatening pathology, not all of those suffering from an acute stroke can be described as such. The radiosensitive corneas are especially at risk from too much radiation, as these often lie directly within the X-ray beam when head CT is performed and can develop radiation-induced cataracts in the medium term. Moreover, many stroke patients are of an age where the long-term effects of too much radiation, that is, development of a radiation-induced malignancy remains a concern. It should therefore be noted that a non-contrast CT brain requires a median total dose (including planning sequences) of approximately 2 mSv (interquartile range, 2–3 mSv). In comparison, a full-stroke protocol CT requires a median dose of 14 mSv (interquartile range, 9–20 mSv), that is, equivalent to an additional 5 years of normal background radiation over non-contrast CT alone. At these doses, it is estimated that approximately 1 in every 2000 patients aged 60 years undergoing a stroke protocol CT will develop a radiation-induced cancer; the estimated frequency is 2–3 times higher for younger patients.115
IMPACT OF ADVANCED IMAGING ON ACUTE STROKE TREATMENT AND OUTCOME
Compared with standard non-contrast imaging, advanced stroke imaging techniques such as angiography and perfusion clearly provide extra information on the arterial supply to and flow dynamics within the brain, but it is less clear whether this information is helpful when making accurate treatment decisions in acute stroke. The final, and perhaps most important, consideration for the adoption of an advanced imaging modality in any clinical sphere is how the results of that test might alter treatment decisions and ultimately affect subsequent outcomes.
Only a few retrospective observational analyses have tested whether occlusion identified on CTA or MRA during acute ischaemic stroke is associated with the response to intravenous thrombolysis. In 1 analysis of 188 patients with occlusion on CTA and severe stroke (National Institutes of Health Stroke Scale >10), those given intravenous tissue plasminogen activator (t-PA) were more likely to have a good outcome (Rankin score ≤2) at 6 months (35% vs 17% if not given t-PA; p = 0.031).116 Another analysis comparing those with occlusion with those without occlusion on CTA (n = 168 and n = 119, respectively) found no difference in 3-month outcome whether intravenous rt-PA was given or not (p = 0.94).117 Angiography data from the Third International Stroke Trial (IST-3), one of the major randomized controlled trials that tested intravenous rt-PA in acute ischaemic stroke, is currently being analysed and may help to clarify this issue.84
At the time of writing, there is also insufficient data to assess how the results of perfusion imaging might be used to guide treatment in acute ischaemic stroke. A meta-analysis exploring the importance of perfusion/diffusion mismatch on MRI found that while patients with mismatch (vs no mismatch) had a non-significant increased risk of infarct growth on radiological follow-up (n = 79), these results did not change with thrombolysis.118 A more recent systematic review assessing only patients with mismatch treated after 3 h (n = 502) similarly found no increase in favourable outcome for those given intravenous thrombolysis, despite the fact that patients achieving reperfusion did have more favourable outcomes.119 Some other individual studies have shown further promise in this area. For example, perfusion imaging has been used to predict which patients are more likely to suffer from haemorrhagic transformation of infarct following thrombolysis.120,121 However, these results need to be replicated in larger trials. Perfusion CT might help to determine when patients with an uncertain time of stroke onset remain safe to receive intravenous thrombolysis,122 but this application also needs to be proven in randomized trials before being used in this way in clinical practice. As discussed above, few perfusion parameters or thresholds have been validated independently. For now, those interested in perfusion imaging should consider participating in an appropriate imaging trial and should be aware of the lack of standardization when using perfusion imaging in clinical practice. At present, there is no good evidence to support the routine use of perfusion imaging in acute stroke assessment.
CONCLUSIONS
Despite recent advances in treatment and assessment, stroke remains a major health concern in the UK and globally, with high levels of death, disability and economic cost. Intravenous thrombolysis remains the only treatment that is proven to work in randomized controlled trials and is licenced for use in stroke.
In order for acute stroke patients to be appropriately considered as potential candidates for intravenous thrombolysis, rapid imaging is required to exclude any therapeutic contraindication, especially haemorrhage. This imaging can be performed using either CT or MRI, but CT is more commonly utilized thanks to its widespread availability, suitability for the unwell and medically unstable patient and accuracy in excluding contraindications to intravenous thrombolysis. In addition, when assessed by capable individuals, non-contrast CT provides complimentary information on the presence of early infarct and, in some cases, provides a specific measure of arterial occlusion when a hyperdense artery is identified. The major benefit of performing MRI hyperacutely in stroke, particularly in mild stroke, is that small-volume infarct that may be subtle on non-contrast CT is likely to be more readily apparent on DWI. T2* or an equivalent blood-sensitive sequence should always be used in stroke MRI.
Advanced stroke imaging, in the form of CT and MRI angiography and perfusion, is being used more commonly in general clinical practice. Although CT and MR angiography do provide an accurate assessment of intra- and extracranial arterial flow, it is not clear what benefit this extra information conveys when making treatment decisions for acute stroke. Similarly, perfusion imaging tempts us with the possibility of accurately determining whether a stroke patient has more salvageable than dead brain tissue, irrespective of the presumed onset time for their stroke, but, as yet, the ideal perfusion imaging parameters to achieve this (and their accuracy) remain elusive.
Unfortunately, both of these advanced imaging techniques are limited by a lack of standardization. Without standardization, it will be impossible to assess the impact of these technologies on stroke treatment. Specifically, we need to know whether angiographic occlusion or its absence should alter the decision to treat with intravenous thrombolysis and at what ratio of core to penumbra should we withhold treatment. This vital information is not currently available. Consequently, it is hard to argue that delaying a stroke patient perhaps 20 min from a proven therapy or increasing their radiation burden five-fold is a reasonable trade off, given the extremely time-sensitive nature of acute stroke. Until more evidence is available, advanced stroke imaging should remain the preserve of research departments. Initiatives like the Acute Stroke Imaging Research Roadmap should help to achieve these aims, and use of their recommendations is to be encouraged.87
REFERENCES
- 1.Mathers CD, Bernand C, Iburg KM, Inoue M, Ma Fat D, Shibuya K, et al. Global burden of disease: data sources, methods and results. World Health Organization; 2004. [Cited 30 June 2014.] Available from: http://www.who.int/healthinfo/global_burden_disease/en/ [Google Scholar]
- 2.Collaborative systematic review of the randomised trials of organised inpatient (stroke unit) care after stroke. Stroke Unit Trialists' Collaboration. BMJ 1997; 314: 1151–9. [PMC free article] [PubMed] [Google Scholar]
- 3.Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012; 379: 2364–72. doi: 10.1016/S0140-6736(12)60738-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The International Stroke Trial (IST): a randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997; 349: 1569–81. [PubMed] [Google Scholar]
- 5.Dombrowski SU, Mackintosh JE, Sniehotta FF, Araujo-Soares V, Rodgers H, Thomson RG, et al. The impact of the UK “Act FAST” stroke awareness campaign: content analysis of patients, witness and primary care clinicians' perceptions. BMC Public Health 2013; 13: 915. doi: 10.1186/1471-2458-13-915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing 2009; 38: 27–32. doi: 10.1093/ageing/afn281 [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence (NICE). Stroke quality standard (QS2). 2010. [Cited 30 June 2014.] Available from: http://www.nice.org.uk/guidance/QS2
- 8.Lavy S, Melamed E, Cahane E, Carmon A. Hypertension and diabetes as risk factors in stroke patients. Stroke 1973; 4: 751–9. [DOI] [PubMed] [Google Scholar]
- 9.Mori E, Yoneda Y, Tabuchi M, Yoshida T, Ohkawa S, Ohsumi Y, et al. Intravenous recombinant tissue plasminogen activator in acute carotid artery territory stroke. Neurology 1992; 42: 976–82. [DOI] [PubMed] [Google Scholar]
- 10.Yamaguchi T, Hayakawa T, Kiuchi H; Japanese Thrombolysis Study Group. Intravenous tissue plasminogen activator ameliorates the outcome of hyperacute embolic stroke. Cerebrovasc Dis 1993; 3: 269–72. [Google Scholar]
- 11.Haley EC, Jr, Brott TG, Sheppard GL, Barsan W, Broderick J, Marler JR, et al. Pilot randomized trial of tissue plasminogen activator in acute ischemic stroke. The TPA Bridging Study Group. Stroke 1993; 24: 1000–4. [DOI] [PubMed] [Google Scholar]
- 12.Tissue plasminogen activator for acute ischaemic stroke. The National Institute of Neurological Disorders and Stroke (NINDS) rt-PA Stroke Study Group. N Engl J Med 1995; 333: 1581–7. [DOI] [PubMed] [Google Scholar]
- 13.Hacke W, Kaste M, Fieschi C, Toni D, Lesaffre E, von Kummer R, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA 1995; 274: 1017–25. [PubMed] [Google Scholar]
- 14.Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet 1998; 352: 1245–51. [DOI] [PubMed] [Google Scholar]
- 15.Clark WM, Wissman S, Albers GW, Jhamandas JH, Madden KP, Hamilton S. Recombinant tissue-type plasminogen activator (Alteplase) for ischemic stroke 3 to 5 hours after symptom onset. The ATLANTIS Study: a randomized controlled trial. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. JAMA 1999; 282: 2019–26. [DOI] [PubMed] [Google Scholar]
- 16.Clark WM, Albers GW, Madden KP, Hamilton S. The rtPA (alteplase) 0- to 6-hour acute stroke trial, part A (A0276g): results of a double-blind, placebo-controlled, multicenter study. Thromblytic therapy in acute ischemic stroke study investigators. Stroke 2000; 31: 811–16. [DOI] [PubMed] [Google Scholar]
- 17.Albers GW, Clark WM, Madden KP, Hamilton SA. ATLANTIS trial: results for patients treated within 3 hours of stroke onset. Alteplase Thrombolysis for Acute Noninterventional Therapy in Ischemic Stroke. Stroke 2002; 33: 493–5. [DOI] [PubMed] [Google Scholar]
- 18.Wang SY, Wang XL, Zeng H, Zuo Y, Hu N, Li XY, et al. Early intravenous thrombolysis with recombinant tissue plasminogen activator for acute cerebral infarction. [In Chinese.] Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2003; 15: 542–5. [PubMed] [Google Scholar]
- 19.Davis SM, Donnan GA, Parsons MW, Levi C, Butcher KS, Peeters A, et al. Effects of alteplase beyond 3 h after stroke in the Echoplanar Imaging Thrombolytic Evaluation Trial (EPITHET): a placebo-controlled radomised trial. Lancet Neurol 2008; 7: 299–309. [DOI] [PubMed] [Google Scholar]
- 20.Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med 2008; 359: 1317–29. doi: 10.1056/NEJMoa0804656 [DOI] [PubMed] [Google Scholar]
- 21.The IST-3 Collaborative Group. The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 2012; 379: 2352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet 2007; 369: 275–82. doi: 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 23.Wahlgren N, Ahmed N, Dávalos A, Hacke W, Millan M, Muir K, et al. Thrombolysis with alteplase 3-4.5 h after acute ischaemic stroke (SITS-ISTR): an observational study. Lancet 2008; 372: 1303–9. doi: 10.1016/S0140-6736(08)61339-2 [DOI] [PubMed] [Google Scholar]
- 24.Ahmed N, Wahlgren N, Grond M, Hennerici M, Lees KR, Mikulik R, et al. Implementation and outcome of thrombolysis with alteplase 3-4.5 h after an acute stroke: an updated analysis from SITS-ISTR. Lancet Neurol 2010; 9: 866–74. doi: 10.1016/S1474-4422(10)70165-4 [DOI] [PubMed] [Google Scholar]
- 25.Ahmed N, Kellert L, Lees KR, Mikulik R, Tatlisumak T, Toni D; SITS Investigators. Results of intravenous thrombolysis within 4.5 to 6 hours and updated results within 3 to 4.5 hours of onset of acute ischemic stroke recorded in the Safe Implementation of Treatment in Stroke International Stroke Thrombolysis Register (SITS-ISTR): an observational study. JAMA Neurol 2013; 70: 837–44. [DOI] [PubMed] [Google Scholar]
- 26.Royal College of Physicians on behalf of the Intercollegiate Stroke Working Party. National Sentinel Stroke Audit Phase II (clinical audit) 2008. 2009. [Cited 30 June 2014.] Available from: https://www.rcplondon.ac.uk/sites/default/files/stroke-audit-report-2008.pdf
- 27.Royal College of Physicians on behalf of the Intercollegiate Stroke Working Party. Sentinel Stroke National Audit Programme (SSNAP) Clinical Audit Oct–Dec 2013 Public Report. 2014. [Cited 30 June 2014.] Available from: http://www.rcplondon.ac.uk/sites/default/files/ssnap_public_report_oct-dec_2013.pdf
- 28.Swain S, Turner C, Tyrrell P, Rudd A; Guideline Development Group. Diagnosis and initial management of acute stroke and transient ischaemic attack: summary of NICE guidance. BMJ 2008; 337: 291–3. [DOI] [PubMed] [Google Scholar]
- 29.CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet 1997; 349: 1641–9. [PubMed] [Google Scholar]
- 30.Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013; 368: 893–903. doi: 10.1056/NEJMoa1214300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013; 368: 914–23. doi: 10.1056/NEJMoa1212793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013; 368: 904–13. doi: 10.1056/NEJMoa1213701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muir K, White P. PISTE Trial Steering Committee. Protocol 12PRT/8832: Pragmatic Ischaemic Stroke Thrombectomy Evaluation (PISTE): a randomised controlled trial (NCT01745692). 2013. [Cited 30 June 2014.] Available from: http://www.thelancet.com/protocol-reviews/12PRT-8832
- 34.National Institute for Health and Care Excellence (NICE). Diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). Clinical Guideline CG68. 2008. [Cited 30 June 2014.] Available from: http://guidance.nice.org.uk/CG68
- 35.Kane I, Whiteley WN, Sandercock PA, Wardlaw JM. Availability of CT and MR for assessing patients with acute stroke. Cerebrovasc Dis 2008; 25: 375–7. doi: 10.1159/000120688 [DOI] [PubMed] [Google Scholar]
- 36.Wardlaw JM, Keir SL, Seymour J, Lewis S, Sandercock PA, Dennis MS, et al. What is the best imaging strategy for acute stroke? Health Technol Assess 2004; 8: 1–180. [DOI] [PubMed] [Google Scholar]
- 37.Kidwell CS, Chalela JA, Saver JL, Starkman S, Hill MD, Demchuk AM, et al. Comparison of MRI and CT for detection of acute intracerebral hemorrhage. JAMA 2004; 292: 1823–30. doi: 10.1001/jama.292.15.1823 [DOI] [PubMed] [Google Scholar]
- 38.Mullins ME. The hyperdense cerebral artery sign on head CT scan. Semin Ultrasound CT MR 2005; 26: 394–403. [DOI] [PubMed] [Google Scholar]
- 39.Jha B, Kothari M. Pearls & oy-sters: hyperdense or pseudohyperdense MCA sign: a Damocles sword? Neurology 2009; 72: e116–17. [DOI] [PubMed] [Google Scholar]
- 40.Koo CK, Teasdale E, Muir KW. What constitutes a true hyperdense middle cerebral artery sign? Cerebrovasc Dis 2000; 10: 419–23. [DOI] [PubMed] [Google Scholar]
- 41.Wardlaw JM, Farrall AJ, Perry D, von Kummer R, Mielke O, Moulin T, et al. Factors influencing the detection of early CT signs of cerebral ischemia: an internet-based, international multiobserver study. Stroke 2007; 38: 1250–6. [DOI] [PubMed] [Google Scholar]
- 42.Kim EY, Lee SK, Kim DJ, Suh SH, Kim J, Heo JH, et al. Detection of thrombus in acute ischemic stroke: value of thin-section noncontrast-computed tomography. Stroke 2005; 36: 2754–7. [DOI] [PubMed] [Google Scholar]
- 43.Wardlaw JM, von Kummer R, Farrall AJ, Chappell FM, Hill M, Perry D. A large web-based observer reliability study of early ischaemic signs on computed tomography. The Acute Cerebral CT Evaluation of Stroke Study (ACCESS). PLoS One 2010; 5: e15757. doi: 10.1371/journal.pone.0015757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Demchuk AM, Hill MD, Barber PA, Silver B, Patel SC, Levine SR. Importance of early ischemic computed tomography changes using ASPECTS in NINDS rtPA Stroke Study. Stroke 2005; 36: 2110–15. doi: 10.1161/01.STR.0000181116.15426.58 [DOI] [PubMed] [Google Scholar]
- 45.Dzialowski I, Hill MD, Coutts SB, Demchuk AM, Kent DM, Wunderlich O, et al. Extent of early ischemic changes on computed tomography (CT) before thrombolysis: prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke 2006; 37: 973–8. [DOI] [PubMed] [Google Scholar]
- 46.Whiteley WN, Bruins Slot K, Fernandes P, Sandercock P, Wardlaw J. Risk factors for intracranial hemorrhage in acute ischemic stroke patients treated with recombinant tissue plasminogen activator: a systematic review and meta-analysis of 55 studies. Stroke 2012; 43: 2904–9. [DOI] [PubMed] [Google Scholar]
- 47.Slot KB, Berge E, Wardlaw J. Haemorrhagic transformation of a recent silent cerebral infarct during thrombolytic stroke treatment. BMJ Case Rep 2008; 2008: bcr0620080266. doi: 10.1136/bcr.06.2008.0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albers GW, Lansberg MG, Norbash AM, Tong DC, O'Brien MW, Woolfenden AR, et al. Yield of diffusion-weighted MRI for detection of potentially relevant findings in stroke patients. Neurology 2000; 54: 1562–7. [DOI] [PubMed] [Google Scholar]
- 49.Tsushima Y, Aoki J, Endo K. Brain microhemorrhages detected on T2*-weighted gradient-echo MR images. AJNR Am J Neuroradiol 2003; 24: 88–96. [PMC free article] [PubMed] [Google Scholar]
- 50.von Kummer R. Clinical efficacy of MRI in stroke. In: von Kummer R, Back T, eds. Magnetic resonance imaging in ischemic stroke. 1st edn. Berlin, Heidelberg: Springer; 2006. pp. 17–21. [Google Scholar]
- 51.Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke 2013; 8: 348–56. doi: 10.1111/j.1747-4949.2012.00869.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and the risk of intracerebral haemorrhage after thrombolysis for acute ischaemic stroke: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2013; 84: 277–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Charidimou A, Kakar P, Fox Z, Werring DJ. Cerebral microbleeds and recurrent stroke risk: systematic review and meta-analysis of prospective ischemic stroke and transient ischemic attack cohorts. Stroke 2013; 44: 995–1001. doi: 10.1161/STROKEAHA.111.000038 [DOI] [PubMed] [Google Scholar]
- 54.Fiebach JB, Schellinger PD, Jansen O, Meyer M, Wilde P, Bender J, et al. CT and diffusion-weighted MR imaging in randomized order: diffusion-weighted imaging results in higher accuracy and lower interrater variability in the diagnosis of hyperacute ischemic stroke. Stroke 2002; 33: 2206–10. [DOI] [PubMed] [Google Scholar]
- 55.Mullins ME, Schaefer PW, Sorensen AG, Halpern EF, Ay H, He J, et al. CT and conventional and diffusion-weighted MR imaging in acute stroke: study in 691 patients at presentation to the emergency department. Radiology 2002; 224: 353–60. doi: 10.1148/radiol.2242010873 [DOI] [PubMed] [Google Scholar]
- 56.Saur D, Kucinski T, Grzyska U, Eckert B, Eggers C, Niesen W, et al. Sensitivity and interrater agreement of CT and diffusion-weighted MR imaging in hyperacute stroke. AJNR Am J Neuroradiol 2003; 24: 878–85. [PMC free article] [PubMed] [Google Scholar]
- 57.Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wardlaw JM. Neuroimaging in acute ischaemic stroke: insights into unanswered questions of pathophysiology. J Intern Med 2010; 267: 172–90. doi: 10.1111/j.1365-2796.2009.02200.x [DOI] [PubMed] [Google Scholar]
- 59.Doubal FN, Dennis MS, Wardlaw JM. Characteristics of patients with minor ischaemic strokes and negative MRI: a cross sectional study. J Neurol Neurosurg Psychiatry 2011; 82: 540–2. [DOI] [PubMed] [Google Scholar]
- 60.Brazzelli M, Chappell FM, Miranda H, Shuler K, Dennis M, Sandercock PA, et al. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Ann Neurol 2014; 75: 67–76. doi: 10.1002/ana.24026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thomalla G, Cheng B, Ebinger M, Hao Q, Tourdias T, Wu O, et al. DWI-FLAIR mismatch for the identification of patients with acute ischaemic stroke within 4.5 h of symptom onset (PRE-FLAIR): a multicentre observational study. Lancet Neurol 2011; 10: 978–86. [DOI] [PubMed] [Google Scholar]
- 62.Aoki J, Kimura K, Iguchi Y, Shibazaki K, Iwanaga T, Watanabe M, et al. Intravenous thrombolysis based on diffusion-weighted imaging and fluid-attenuated inversion recovery mismatch in acute stroke patients with unknown onset time. Cerebrovasc Dis 2011; 31: 435–41. [DOI] [PubMed] [Google Scholar]
- 63.McDonald JS, Fan J, Kallmes DF, Cloft HJ. Pretreatment advanced imaging in patients with stroke treated with IV thrombolysis: evaluation of a multihospital data base. AJNR Am J Neuroradiol 2014; 35: 478–81. doi: 10.3174/ajnr.A3797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kharitonova T, Thoren M, Ahmed N, Wardlaw J, von Kummer R, Thomassen L, et al. Disappearing hyperdense middle cerebral artery sign in ischaemic stroke patients treated with intravenous thrombolysis: clinical course and prognostic significance. J Neurol Neurosurg Psychiatry 2008; 80: 273–8. [DOI] [PubMed] [Google Scholar]
- 65.Kharitonova T, Ahmed N, Thorén M, Wardlaw JM, von Kummer R, Glahn J, et al. Hyperdense middle cerebral artery sign on admission CT scan—prognostic significance for ischaemic stroke patients treated with intravenous thrombolysis in the safe implementation of thrombolysis in Stroke International Stroke Thrombolysis Register. Cerebrovasc Dis 2009; 27: 51–9. [DOI] [PubMed] [Google Scholar]
- 66.Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke 2007; 38: 967–73. [DOI] [PubMed] [Google Scholar]
- 67.Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. Neuroradiology 2007; 49: 93–102. doi: 10.1007/s00234-006-0183-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nogueira R, Liebeskind D, Gupta R, Levy E, Rai A, Barreto A, et al. Preliminary data for the dawn trial (DWI/PWI and CTP assessment in the triage of wake-up and late presenting strokes undergoing neurointervention): imaging based endovascular therapy for proximal anterior circulation occlusions beyond 8 h from last seen well in 193 stroke patients. J NeuroIntervent Surg 2009; 1: 85. [Google Scholar]
- 69.Ma H, Parsons MW, Christensen S, Campbell BC, Churilov L, Connelly A, et al. A multicentre, randomized, double-blinded, placebo-controlled Phase III study to investigate EXtending the time for Thrombolysis in Emergency Neurological Deficits (EXTEND). Int J Stroke 2012; 7: 74–80. [DOI] [PubMed] [Google Scholar]
- 70.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999; 30: 317–20. [DOI] [PubMed] [Google Scholar]
- 71.Kaufmann TJ, Huston J, 3rd, Mandrekar JN, Schleck CD, Thielen KR, Kallmes DF. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 2007; 243: 812–19. doi: 10.1148/radiol.2433060536 [DOI] [PubMed] [Google Scholar]
- 72.Dawkins AA, Evans AL, Wattam J, Romanowski CA, Connolly DJ, Hodgson TJ, et al. Complications of cerebral angiography: a prospective analysis of 2,924 consecutive procedures. Neuroradiology 2007; 49: 753–9. doi: 10.1007/s00234-007-0252-y [DOI] [PubMed] [Google Scholar]
- 73.Knauth M, von Kummer R, Jansen O, Hähnel S, Dörfler A, Sartor K. Potential of CT angiography in acute ischemic stroke. AJNR Am J Neuroradiol 1997; 18: 1001–10. [PMC free article] [PubMed] [Google Scholar]
- 74.Lev MH, Farkas J, Rodriguez VR, Schwamm LH, Hunter GJ, Putman CM, et al. CT angiography in the rapid triage of patients with hyperacute stroke to intraarterial thrombolysis: accuracy in the detection of large vessel thrombus. J Comput Assist Tomogr 2001; 25: 520–8. [DOI] [PubMed] [Google Scholar]
- 75.Chappell ET, Moure FC, Good MC. Comparison of computed tomographic angiography with digital subtraction angiography in the diagnosis of cerebral aneurysms: a meta-analysis. Neurosurgery 2003; 52: 624–31. [DOI] [PubMed] [Google Scholar]
- 76.Koelemay MJ, Nederkoorn PJ, Reitsma JB, Majoie CB. Systematic review of computed tomographic angiography for assessment of carotid artery disease. Stroke 2004; 35: 2306–12. doi: 10.1161/01.STR.0000141426.63959.cc [DOI] [PubMed] [Google Scholar]
- 77.Debrey SM, Yu H, Lynch JK, Lövblad KO, Wright VL, Janket SJ, et al. Diagnostic accuracy of magnetic resonance angiography for internal carotid artery disease: a systematic review and meta-analysis. Stroke 2008; 39: 2237–48. doi: 10.1161/STROKEAHA.107.509877 [DOI] [PubMed] [Google Scholar]
- 78.Tomanek AI, Coutts SB, Demchuk AM, Hudon ME, Morrish WE, Sevick RJ, et al. MR angiography compared to conventional selective angiography in acute stroke. Can J Neurol Sci 2006; 33: 58–62. [DOI] [PubMed] [Google Scholar]
- 79.Klingebiel R, Siebert E, Diekmann S, Wiener E, Masuhr F, Wagner M, et al. 4-D Imaging in cerebrovascular disorders by using 320-slice CT: feasibility and preliminary clinical experience. Acad Radiol 2009; 16: 123–9. doi: 10.1016/j.acra.2008.11.004 [DOI] [PubMed] [Google Scholar]
- 80.Eddleman CS, Jeong HJ, Hurley MC, Zuehlsdorff S, Dabus G, Getch CG, et al. 4D radial acquisition contrast-enhanced MR angiography and intracranial arteriovenous malformations: quickly approaching digital subtraction angiography. Stroke 2009; 40: 2749–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Willems PW, Brouwer PA, Barfett JJ, terBrugge KG, Krings T. Detection and classification of cranial dural arteriovenous fistulas using 4D-CT angiography: initial experience. AJNR Am J Neuroradiol 2011; 32: 49–53. doi: 10.3174/ajnr.A2248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qureshi AI. New grading system for angiographic evaluation of arterial occlusions and recanalization response to intra-arterial thrombolysis in acute ischemic stroke. Neurosurgery 2002; 50: 1405–14. [DOI] [PubMed] [Google Scholar]
- 83.Mori E, Tabuchi M, Yoshida T, Yamadori A. Intracarotid urokinase with thromboembolic occlusion of the middle cerebral artery. Stroke 1988; 19: 802–12. [DOI] [PubMed] [Google Scholar]
- 84.Wardlaw JM, von Kummer RI, Carpenter T, Parsons M, Lindley R, Cohen G, et al. Protocol for the perfusion and angiography imaging sub-study of the Third International Stroke Trial (IST-3) of alteplase treatment within six hours of acute ischemic stroke. Int J Stroke Jan 2013. Epub ahead of print. doi: 10.1111/j.1747-4949.2012.00946.x [DOI] [PubMed] [Google Scholar]
- 85.Fugate JE, Klunder AM, Kallmes DF. What is meant by “TICI”? AJNR Am J Neuroradiol 2013; 34: 1792–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zaidat OO, Yoo AJ, Khatri P, Tomsick TA, von Kummer R, Saver JL, et al. Recommendations on angiographic revascularization grading standards for acute ischemic stroke: a consensus statement. Stroke 2013; 44: 2650–63. doi: 10.1161/STROKEAHA.113.001972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wintermark M, Albers GW, Broderick JP, Demchuk AM, Fiebach JB, Fiehler J, et al. Acute stroke imaging research roadmap II. Stroke 2013; 44: 2628–39. doi: 10.1161/STROKEAHA.113.002015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Del GA, D'Amico D, Sobesky J, Wellwood I. Accuracy of the spot sign on computed tomography angiography as a predictor of haematoma enlargement after acute spontaneous intracerebral haemorrhage: a systematic review. Cerebrovasc Dis 2014; 37: 268–76. [DOI] [PubMed] [Google Scholar]
- 89.Schramm P, Schellinger PD, Klotz E, Kallenberg K, Fiebach JB, Külkens S, et al. Comparison of perfusion computed tomography and computed tomography angiography source images with perfusion-weighted imaging and diffusion-weighted imaging in patients with acute stroke of less than 6 hours' duration. Stroke 2004; 35: 1652–8. [DOI] [PubMed] [Google Scholar]
- 90.Camargo EC, Furie KL, Singhal AB, Roccatagliata L, Cunnane ME, Halpern EF, et al. Acute brain infarct: detection and delineation with CT angiographic source images versus nonenhanced CT scans. Radiology 2007; 244: 541–8. doi: 10.1148/radiol.2442061028 [DOI] [PubMed] [Google Scholar]
- 91.Coutts SB, Lev MH, Eliasziw M, Roccatagliata L, Hill MD, Schwamm LH, et al. ASPECTS on CTA source images versus unenhanced CT: added value in predicting final infarct extent and clinical outcome. Stroke 2004; 35: 2472–6. doi: 10.1161/01.STR.0000145330.14928.2a [DOI] [PubMed] [Google Scholar]
- 92.Aviv RI, Shelef I, Malam S, Chakraborty S, Sahlas DJ, Tomlinson G, et al. Early stroke detection and extent: impact of experience and the role of computed tomography angiography source images. Clin Radiol 2007; 62: 447–52. [DOI] [PubMed] [Google Scholar]
- 93.Alsop DC. ASL perfusion imaging: concepts and applications. ISMRM 2014. [Cited 30 June 2014] Available from: http://afni.nimh.nih.gov/sscc/staff/rwcox/ISMRM_2006/Syllabus%202006%20-%203340/files/M_09.pdf
- 94.Huang YC, Liu HL, Lee JD, Yang JT, Weng HH, Lee M, et al. Comparison of arterial spin labeling and dynamic susceptibility contrast perfusion MRI in patients with acute stroke. PLoS One 2013; 8: e69085. doi: 10.1371/journal.pone.0069085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bivard A, Krishnamurthy V, Stanwell P, Levi C, Spratt NJ, Davis S, et al. Arterial spin labeling versus bolus-tracking perfusion in hyperacute stroke. Stroke 2014; 45: 127–33. doi: 10.1161/STROKEAHA.113.003218 [DOI] [PubMed] [Google Scholar]
- 96.Bivard A, Levi C, Spratt N, Parsons M. Perfusion CT in acute stroke: a comprehensive analysis of infarct and penumbra. Radiology 2013; 267: 543–50. doi: 10.1148/radiol.12120971 [DOI] [PubMed] [Google Scholar]
- 97.Dani KA, Thomas RG, Chappell FM, Shuler K, MacLeod MJ, Muir KW, et al. Computed tomography and magnetic resonance perfusion imaging in ischemic stroke: definitions and thresholds. Ann Neurol 2011; 70: 384–401. doi: 10.1002/ana.22500 [DOI] [PubMed] [Google Scholar]
- 98.Wintermark M, Reichhart M, Cuisenaire O, Maeder P, Thiran JP, Schnyder P, et al. Comparison of admission perfusion computed tomography and qualitative diffusion- and perfusion-weighted magnetic resonance imaging in acute stroke patients. Stroke 2002; 33: 2025–31. [DOI] [PubMed] [Google Scholar]
- 99.Kucinski T, Naumann D, Knab R, Schoder V, Wegener S, Fiehler J, et al. Tissue at risk is overestimated in perfusion-weighted imaging: MR imaging in acute stroke patients without vessel recanalization. AJNR Am J Neuroradiol 2005; 26: 815–19. [PMC free article] [PubMed] [Google Scholar]
- 100.Dani KA, Thomas RG, Chappell FM, Shuler K, Muir KW, Wardlaw JM. Systematic review of perfusion imaging with computed tomography and magnetic resonance in acute ischemic stroke: heterogeneity of acquisition and postprocessing parameters: a translational medicine research collaboration multicentre acute stroke imaging study. Stroke 2012; 43: 563–6. [DOI] [PubMed] [Google Scholar]
- 101.Latchaw RE, Yonas H, Hunter GJ, Yuh WT, Ueda T, Sorensen AG, et al. Guidelines and recommendations for perfusion imaging in cerebral ischemia: a scientific statement for healthcare professionals by the writing group on perfusion imaging, from the Council on Cardiovascular Radiology of the American Heart Association. Stroke 2003; 34: 1084–104. [DOI] [PubMed] [Google Scholar]
- 102.Saver JL. Time is brain—quantified. Stroke 2006; 37: 263–6. [DOI] [PubMed] [Google Scholar]
- 103.Rowat AM, Wardlaw JM, Dennis MS, Warlow CP. Patient positioning influences oxygen saturation in the acute phase of stroke. Cerebrovasc Dis 2001; 12: 66–72. [DOI] [PubMed] [Google Scholar]
- 104.Wardlaw JM, Mielke O. Early signs of brain infarction at CT: observer reliability and outcome after thrombolytic treatment—systematic review. Radiology 2005; 235: 444–53. [DOI] [PubMed] [Google Scholar]
- 105.Agarwal S, Jones PS, Alawneh JA, Antoun NM, Barry PJ, Carrera E, et al. Does perfusion computed tomography facilitate clinical decision making for thrombolysis in unselected acute patients with suspected ischaemic stroke? Cerebrovasc Dis 2011; 32: 227–33. [DOI] [PubMed] [Google Scholar]
- 106.van Seeters T, Biessels GJ, Niesten JM, van der Schaaf IC, Dankbaar JW, Horsch AD, et al. Reliability of visual assessment of non-contrast CT, CT angiography source images and CT perfusion in patients with suspected ischemic stroke. PLoS One 2013; 8: e75615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wintermark M, Fischbein NJ, Smith WS, Ko NU, Quist M, Dillon WP. Accuracy of dynamic perfusion CT with deconvolution in detecting acute hemispheric stroke. AJNR Am J Neuroradiol 2005; 26: 104–12. [PMC free article] [PubMed] [Google Scholar]
- 108.Finlayson O, John V, Yeung R, Dowlatshahi D, Howard P, Zhang L, et al. Interobserver agreement of ASPECT score distribution for noncontrast CT, CT angiography, and CT perfusion in acute stroke. Stroke 2013; 44: 234–6. doi: 10.1161/STROKEAHA.112.665208 [DOI] [PubMed] [Google Scholar]
- 109.Girot M, Leclerc X, Gauvrit JY, Verdelho A, Pruvo JP, Leys D. Cerebral magnetic resonance imaging within 6 hours of stroke onset: inter- and intra-observer reproducibility. Cerebrovasc Dis 2003; 16: 122–7. [DOI] [PubMed] [Google Scholar]
- 110.Wong KS, Lam WW, Liang E, Huang YN, Chan YL, Kay R. Variability of magnetic resonance angiography and computed tomography angiography in grading middle cerebral artery stenosis. Stroke 1996; 27: 1084–7. [DOI] [PubMed] [Google Scholar]
- 111.Shrier DA, Tanaka H, Numaguchi Y, Konno S, Patel U, Shibata D. CT angiography in the evaluation of acute stroke. AJNR Am J Neuroradiol 1997; 18: 1011–20. [PMC free article] [PubMed] [Google Scholar]
- 112.Sunshine JL, Tarr RW, Lanzieri CF, Landis DM, Selman WR, Lewin JS. Hyperacute stroke: ultrafast MR imaging to triage patients prior to therapy. Radiology 1999; 212: 325–32. doi: 10.1148/radiology.212.2.r99au52325 [DOI] [PubMed] [Google Scholar]
- 113.Srinivasan A, Goyal M, Lum C, Nguyen T, Miller W. Processing and interpretation times of CT angiogram and CT perfusion in stroke. Can J Neurol Sci 2005; 32: 483–6. [DOI] [PubMed] [Google Scholar]
- 114.Löve A, Siemund R, Andsberg G, Cronqvist M, Holtas S, Bjorkman-Burtscher I. Comprehensive CT evaluation in acute ischemic stroke: impact on diagnosis and treatment decisions. Stroke Res Treat 2011; 2011: 726573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Smith-Bindman R, Lipson J, Marcus R, Kim KP, Mahesh M, Gould R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Arch Intern Med 2009; 169: 2078–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.González RG, Furie KL, Goldmacher GV, Smith WS, Kamalian S, Payabvash S, et al. Good outcome rate of 35% in IV-tPA-treated patients with computed tomography angiography confirmed severe anterior circulation occlusive stroke. Stroke 2013; 44: 3109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sylaja PN, Dzialowski I, Puetz V, Eliasziw M, Hill MD, Krol A, et al. Does intravenous rtPA benefit patients in the absence of CT angiographically visible intracranial occlusion? Neurol India 2009; 57: 739–43. doi: 10.4103/0028-3886.59469 [DOI] [PubMed] [Google Scholar]
- 118.Kane I, Sandercock P, Wardlaw J. Magnetic resonance perfusion diffusion mismatch and thrombolysis in acute ischaemic stroke: a systematic review of the evidence to date. J Neurol Neurosurg Psychiatry 2007; 78: 485–91. doi: 10.1136/jnnp.2006.100347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mishra NK, Albers GW, Davis SM, Donnan GA, Furlan AJ, Hacke W, et al. Mismatch-based delayed thrombolysis: a meta-analysis. Stroke 2010; 41: e25–33. [DOI] [PubMed] [Google Scholar]
- 120.Yassi N, Parsons MW, Christensen S, Sharma G, Bivard A, Donnan GA, et al. Prediction of poststroke hemorrhagic transformation using computed tomography perfusion. Stroke 2013; 44: 3039–43. doi: 10.1161/STROKEAHA.113.002396 [DOI] [PubMed] [Google Scholar]
- 121.Campbell BC, Christensen S, Parsons MW, Churilov L, Desmond PM, Barber PA, et al. Advanced imaging improves prediction of hemorrhage after stroke thrombolysis. Ann Neurol 2013; 73: 510–19. doi: 10.1002/ana.23837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cortijo E, Garcia-Bermejo P, Calleja AI, Pérez-Fernández S, Gómez R, Del Monte JM, et al. Intravenous thrombolysis in ischemic stroke with unknown onset using CT perfusion. Acta Neurol Scand 2013; 129: 178–83. doi: 10.1111/ane.12160 [DOI] [PubMed] [Google Scholar]