Abstract
Objective:
The aim of this study was to find a new radiation protector, platelet factor 4 (PF4) and to identify its effect on haemopoietic microenvironment in vitro and in vivo.
Methods:
Radiation damage on bone marrow mesenchymal stem cells ex and in vitro was set up as models. Growth curve analysis, clonogenic survival assay, FACSCalibur™ (BD Immunocytometry Systems, San Jose, CA), 5-ethynyl-2′-deoxyuridine immunofluorescence staining and quantitative reverse transcription–polymerase chain reaction were employed to assess the characterization of bone marrow mesenchymal stem cells (BMSCs), proliferation, apoptosis, cell cycle and gene expression.
Results:
A dose- and time-dependent enhancement of cell viability and survival was observed for PF4 treatment along with 500 cGy γ-radiation in vitro. The same phenomena were noted in vivo, including enhancement of adherence and proliferation ability while inhibition of cell apoptosis, which were associated with a short-term decrease in the G0/G1 ratio owing to S phase arrest. These were accompanied with enhanced Bcl-2 expression and p53/p21 loss.
Conclusion:
These results uncover that PF4 might be a novel therapeutic approach, which could reduce DNA damage and increase survival of BMSCs, in part, by inhibiting p53/p21 axis and facilitating DNA damage repair.
Advances in knowledge:
This study explores the feasibility of a new radioprotector and hence may be clinically important.
Radio- and chemotherapy have been extensively used in malignancy. These treatments inevitably lead to damage of normal tissues and cells especially haematopoietic stem/progenitor cells (HSPCs), which provide all of the downstream components of the blood. The recovery of haematopoiesis from ionizing irradiation (IR) is critically dependent on the repopulation of resident HSPCs and their microenvironment.1 Bone marrow mesenchymal stem cells (BMSCs), consist a heterogeneous population of cells and form a unique bone marrow niche,2 not only provide the structural and functional support for HSPCs but also protect them from both direct and indirect cell death in physiological and pathological conditions.3 Since HSPCs are more sensitive to radiation, mesenchymal stem cells (MSCs) luckily have an intrinsic radiation-resistant phenotype.4,5
Although effective radiation countermeasure approaches were initiated six decades ago, very few have unanimous endorsement by health professionals. Radiation protectors [such as WR-2721, Toll-like receptor ligands, flavonoids, Ex-RAD® (Onconova Therapeutics Inc., Newton, PA) and vitamin E] are prophylactic agents that are administered before exposure to prevent radiation-induced cellular and molecular damage. Radiation mitigators (such as cytokines, growth factors, 5-androstenediol and CLT-008) are drugs administered shortly after irradiation that accelerate recovery or repair of radiation injury.6,7 WR-2721 was found to be the most effective radioprotective agent for the haematopoietic syndrome, but its toxic side effects have stood in its way.7,8 The search for agents that protect against the acute and late effects of ionizing radiation injury in a safe, effective and non-toxic manner will undoubtedly continue into the future and affect other areas of radiation research.
Platelet factor 4 (PF4), a founding member of the chemokine C-X-C family, plays multiple roles in haematopoiesis. It has been reported that PF4 inhibits the growth of HSPCs; promotes the adhesion of HSPCs to endothelial cells; reduces the chemo sensitivity of bone marrow cells to several cytotoxic agents; and significantly accelerates the recovery of mice haematopoiesis from a total body irradiation (TBI).9–14 However, there have been no previous reports investigating whether PF4 affects the haemopoietic microenvironment, particularly BMSCs, an essential HSPC niche component.
In this study, we utilized IR injury models to investigate the effects of PF4 on primary human BMSCs (hBMSCs) in vitro and on mouse BMSCs (mBMSCs) in vivo. The phenomenological and mechanistic data provided evidence that there is a dose-dependent radioprotective effect of PF4 on BMSCs, which was associated with modulating the expression of four genes related to short-term cell cycle arrest and long-term cellular apoptosis decrease. These findings significantly contribute to the search for ideal radioprotective agents for use in a variety of radiation scenarios, which will undoubtedly continue into the future and influence other areas of radiation research.
METHODS AND MATERIALS
Animals
7- to 8-week-old C57BL/6 male mice were maintained under specific pathogen-free conditions. The animal husbandry, experiments and welfare were conducted in accordance with the detailed rules for the administration of animal experiments for medical research purposes issued by the Ministry of Health of China and were approved by the Animal Experiment Administration Committee of the Fourth Military Medical University, Xi'an, China.
Cell culture of bone marrow mesenchymal stem cells
hBMSCs were obtained after informed consent from unaffected bone sites of patients who underwent surgery for osteoarthritis approved by the Fourth Military Medical University in China. Single-cell suspension was obtained by passing through an 18-gauge needle and filtered with a nylon membrane, followed by erythrolysis in buffered 0.14-M NH4Cl. Cells were cultured in l-glutamine Dulbecco's minimum essential medium (LDMEM; Gibco®, Life Technologies Ltd, Grand Island, NY) containing 15% foetal bovine serum (FBS; Gibco) in a humidified atmosphere of 5% CO2 at 37 °C. Cells at passages 3–5 were used.
mBMSC expansion was performed as described previously.15 Briefly, the mononuclear cells (MNC) from tibias and femurs were collected and quantified, and suspended in DF12 medium (Hyclone™, Logan, UT) supplemented with 15% FBS. For cell adhesion experiment, MNC were seeded in 24-well plates at a density of 5 × 105 ml−1 in complete LDMEM and were trypsinized to calculate the ratio of adherent cells at a 4-h time interval until 24 h.
Bone marrow mesenchymal stem cell differentiation assay
To induce MSC differentiation, MSCs were grown in mineralization-inducing media containing 100 μM ascorbic acid, 10 mM β-glycerophosphate and 100 nM dexamethasone. Alizarin red staining was performed as described previously.16 To induce adipogenic differentiation, MSCs were grown in adipogenic-inducing media containing 0.5 mM isobutylmethylxanthine, 1 μM dexamethasone, 200 μM indomethacin and 10 μM recombinant human insulin (Sigma, St Louis, MO). Media were changed every 3 days. After 2 weeks of culture in vitro; Oil-Red-O staining was performed to detect the lipid droplets using an Oil-Red-O stain kit according to the manufacturer's instruction (DBS, Pleasanton, CA).
Ionizing radiation
Mice or cells were exposed to various doses of γ-radiation at a dose rate of 285.13 ± 5.00 cGy min−1 and an irradiation distance of 80 ± 3 cm using an experimental 60Co-γ irradiator (Fourth Military Medical University).
Platelet factor 4 treatment
hBMSCs were grown to a confluence of 80% and incubated with various concentrations of PF4 (Sigma) or equivalent heated PF4 (negative control) for 12 h or 10 μM WR-2721 (positive control) for 30 min before or after irradiation. The WR-2721 was kindly provided by Tsutomu V. Kagiya from Kyoto University, Kyoto, Japan.
According to our previous studies,11 two intraperitoneal (i.p.) injections of PF4 (40 μg kg−1) or equivalent heated PF4 were administered at 6-h intervals, and 20 h after the second injection, the mice received 500-cGy γ-radiation TBI. As a positive control, mice were subjected to an i.p. injection of WR-2721 (1 mM kg−1) 30 min prior to irradiation. Mice were sacrificed by cervical dislocation 3 days after TBI.
Growth curve analysis
Cells were seeded in 24-well plates (Nunc, Roskilde, Denmark) at a concentration of 10,000 cells per well. Individual cultures were harvested daily for 10 days, and cell counts were performed in duplicate in a haemocytometer using trypan blue exclusion.
Clonogenic survival assay
BMSCs were seeded at a density of 100 cells per well in 6-well plates (Nunc). Cells were cultured for 14 days until colonies were clearly visible. The number, size and frequency of colony-forming unit fibroblasts (CFU-F) were assessed using a microscope. A CFU-F was defined as >50 cells. Colonies were washed with phosphate-buffered saline (PBS), fixed in 4% paraformaldehyde for 30 min and stained with Wright–Giemsa dye solution for 10 min as previously described.17 All colony images are representative of one of three independent experiments.
Carboxyfluorescein diacetate succinimidyl ester
To assay cell proliferation, hBMSCs were incubated with carboxyfluorescein diacetate succinimidyl ester (CFSE; Sigma) at 37 °C for 15 min, followed by incubation in 3-ml ice-cold LDMEM for 5 min on ice. Cells were then washed with cold LDMEM and seeded in a 6-well plate (1 × 106 cells per well) in complete medium. 10 days after IR, cells were collected and analysed by flow cytometry.
Flow cytometry
Single-cell suspensions (3–5 × 105) were incubated with appropriate antibodies on ice for 30 min and then analysed by using a FACSCalibur™ (BD Immunocytometry Systems, San Jose, CA) after washing. Dead cells were excluded by propidium iodide staining. Biotinylated antihuman and antimouse monoclonal antibodies for CD29, CD44, CD34 and CD45 were from BD PharMingen (San Jose, CA). Data were analysed with the CellQuest™software (BD Biosciences, San Jose, CA).
Immunofluorescence
For 5-ethynyl-2′-deoxyuridine (EdU) incorporation experiments, cells were seeded in 24-well plates. EdU (RiboBio, Guangzhou, China) was added at a 50-μM final concentration 24 h before harvesting the cells. Immunofluorescence staining was performed according to protocol.
Quantitative reverse transcription–polymerase chain reaction
Total RNA was extracted by using the TRIzol® reagent (Invitrogen™). cDNA was prepared by using a reverse transcription (RT) system (Takara, Dalian, China). Quantitative real time polymerase chain reaction (PCR) was performed in triplicates by using a kit (SYBR® Premix EX Taq™; Takara) and the ABI Prism® 7500 Real-Time PCR System, with β-actin as an internal control. Primers are listed in Supplementary Table A.
Statistical analysis
The statistical analysis was performed with the SPSS® v. 12.0 (SPSS Inc., Chicago, IL) program. The experiments were performed in triplicate, and the results were expressed as mean ± standard deviation. Mouse survival was analysed by using the Kaplan–Meier analysis. The comparisons between groups were undertaken using the unpaired Student's t-test. Differences with p < 0.05 were considered significant.
RESULTS
Characterization of bone marrow mesenchymal stem cell
FACSCalibur was employed to identify BMSC by their expression of various molecules, including CD29 and CD44, and by the absence of markers such as CD34 and CD45 (Figure 1a). The identity of the cells was further confirmed based on their differentiation potential. The BMSCs were differentiated at passages 3 into adipocytes and osteoblasts for 14 days, which were determined based on Oil Red and von Kossa staining, respectively (Figure 1b).
Figure 1.
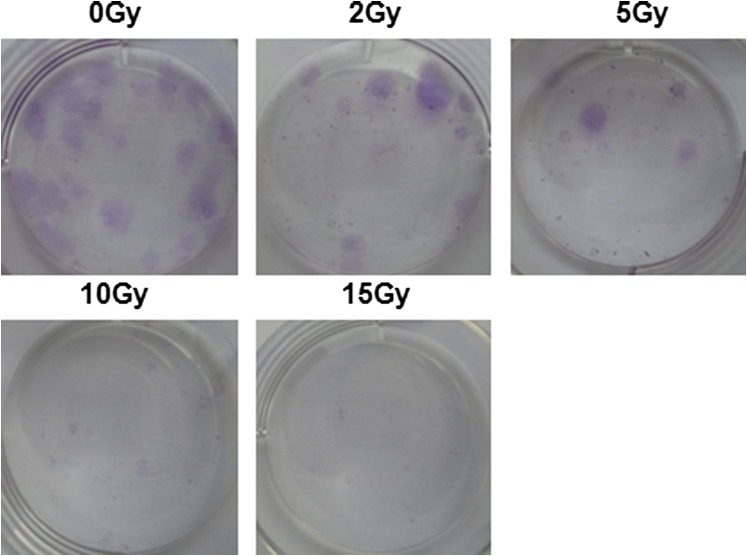
Characterization of bone marrow mesenchymal stem cells (BMSCs). (a) Histogram of cell surface markers determined by flow cytometry, showing that BMSCs were negative for CD45 and CD34 and positive for CD44 and CD29. (b) BMSCs differentiated into adipocytes and osteoblasts for 14 days and subsequently stained with Oil Red and von Kossa staining, respectively. (c) In vitro irradiation survival curves of human BMSCs (hBMSCs). Cells were grown in vitro and irradiated from 0 to 15 Gy alone. (d) The colony-forming unit fibroblasts (CFU-F) in (c) were counted on the 14th day after ionizing irradiation and presented as CFU-F per 100 cultured cells. (e) Effect of platelet factor (PF4) in irradiation killing of hBMSCs in vitro. Cells were irradiated after 12 h incubation in different doses of PF4. (f) Cumulative administration of PF4. Cells were irradiated after different cumulative administrations of PF4 in vitro. Bars, means ± standard deviation. *p < 0.05; **p < 0.01; #p < 0.001; n = 5.
To characterize MSCs in more detail, the radiation survival curve of the cells was first carried out. Result, shown in Figure 1c, demonstrated significant radioresistance of primary hBMSCs in vitro (D0 = 9.1 ± 0.56 Gy). This observation was further supported by CFU-F assay (Figure 1d and Supplementary Figure A).
Platelet factor 4–protected bone marrow mesenchymal stem cells from irradiation
To assess the therapeutic efficacy of PF4 on irradiated hBMSCs, we evaluated the administration using trypan blue staining and confirmed a significant increase of cell survival over the dose range of 0.01–10 μg ml−1 PF4 (Figure 1e). However, cumulative administration had no synergistic effect on exposed cells (Figure 1f). Collectively, the observation of radiation survival curves showed that pre-incubation of PF4 dose dependently repressed cell death and pre-incubating 1 μg ml−1 PF4 for 12 h prior to 500 cGy irradiation was an optimal system.
To further evaluate the effect of PF4 on cell viability and morphology, microscopy was employed to observe hBMSCs dynamically for a month after exposure. The exponential growth phases appeared on the 10th day in PF4 pre-treated cells, while on the 18th day in radiated cells. Furthermore, the former demonstrated a classical elongated spindle shape and whirlpool growth with larger size, exhibited more integral boundaries and a larger nucleus with fewer secretary granules and fragmented cells.
Platelet factor 4 promoted the proliferation of bone marrow mesenchymal stem cells
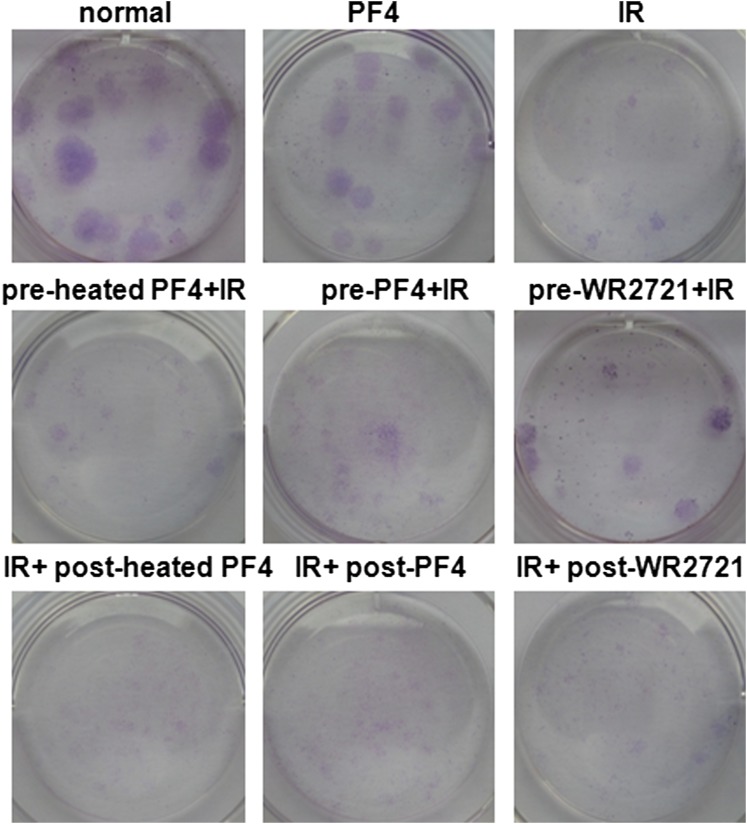
To examine the role of PF4 in the proliferation of hBMSCs, cells were incubated with PBS, heated PF4, PF4 or WR-2721 before or after irradiation. The clonogenic survival assay showed that pre-incubation with PF4 and WR-2721 but not post-incubation could promote the proliferation of hBMSCs after IR (Figure 2a and Supplementary Figure B). This was confirmed by the observation that pre-incubation with PF4 and WR-2721 displayed significant staining of MSCs with EdU under a fluorescence microscope (Figure 2b,c). Moreover, we found by FACSCalibur that lower expression of CFSE was detected in PF4 and WR-2721 pre-treated cells (Figure 2d). These results demonstrated that PF4 as well as WR-2721 could efficiently improve the proliferation of MSCs after IR.
Figure 2.
Platelet factor 4 (PF4)-promoted human bone marrow mesenchymal stem cells (hBMSCs) proliferation in vitro. (a) Clonogenic survival assay. Cells were incubated with equivalent phosphate-buffered saline, heated PF4, PF4 (1 μg ml−1) or WR-2721 (10 μM) before or after 500 cGy ionizing irradiation and were counted on the 14th day after ionizing irradiation (IR). (b) Representative images of 500-cGy irradiated hBMSCs stained with 5-ethynyl-2′-deoxyuridine (EdU). (c) Quantification of EdU+ cells in (b). (d) Carboxyfluorescein diacetate succinimidyl ester (CFSE) proliferation assay. CFSE-labelled hBMSCs were stimulated with PF4 (1 μg ml−1) or WR-2721 (10 μM) before or after 500 cGy IR. Bars, means ± standard deviation. *p < 0.050; **p < 0.010; #p < 0.001; n = 5. CFU, colony-forming unit.
Platelet factor 4 repressed the apoptosis of human bone marrow mesenchymal stem cells
To further evaluate the role of PF4 in hBMSCs, we employed FACSCalibur to analyse cell apoptosis at various time points after IR. Since we had confirmed that administration of PF4 or WR-2721 post IR appeared not to affect the proliferation of MSCs, pre-incubation with heated PF4 as negative control and WR-2721 as positive control were retained in the next experiments. As shown in Figure 3a,b, early apoptosis of irradiated hBMSCs was two-fold higher than that of PF4 pre-treated exposed cells 2 days after exposure, and this disparity increased notably at the fourth day (Figure 3c,d). Moreover, the results showed that PF4 significantly reduced the percentage of late apoptotic and dead cells while increased the percentage of living cells from the fourth day up to the sixth day (Figure 3e,f). Collectively, these data suggested that PF4 repressed the apoptosis induced by irradiation in vitro.
Figure 3.
Platelet factor 4 (PF4)–repressed human bone marrow mesenchymal stem cell (hBMSC) apoptosis and induced S phase arrest in vitro. (a–f) hBMSCs were pre-incubated with 1 μg ml−1 of PF4 for 12 h or 10 μM WR-2721 for 30 min before 500- and 0-cGy ionizing irradiation. Cell apoptosis was analysed by FACSCalibur™ (BD Immunocytometry Systems, San Jose, CA) on the second day (a, b); on the fourth day (c, d); and on the sixth day (e, f). (g) Short-term S phase arrest regulation by PF4. Cell cycle was analysed by FACSCalibur at 20 h. (h) Qualitative measurement of (g). Bars, means ± standard deviation. *p < 0.05; **p < 0.01; #p < 0.001; n = 5. IR, ionizing irradiation; PI, propidium iodide.
Platelet factor 4–induced short-term S phase arrest in human bone marrow mesenchymal stem cells
We had previously observed that PF4 could block bone marrow cells in the S phase in vivo (Gao Ying, China, 2010, personal communication), so cell cycle assays were performed by FACSCalibur at 20 h and 4 days after exposure. Consistently, the level of S phase increased remarkably in PF4-treated hBMSCs, whichever cells were irradiated or not (Figure 3g,h). Moreover, it is interesting that this effect maintained only in the early stage after exposure, and we could not observe the S phase arrest on the fourth day (data not shown). In addition, an accumulation of G0/G1 phase was detected in irradiated cells, which demonstrated that IR induced a G1 phase arrest in hBMSCs. These in vitro data demonstrated that PF4 inhibited apoptosis and accelerated proliferation of irradiated MSCs, most likely by suppressing the cell cycle process in a short term.
Platelet factor 4–protected bone marrow mesenchymal stem cells in vivo
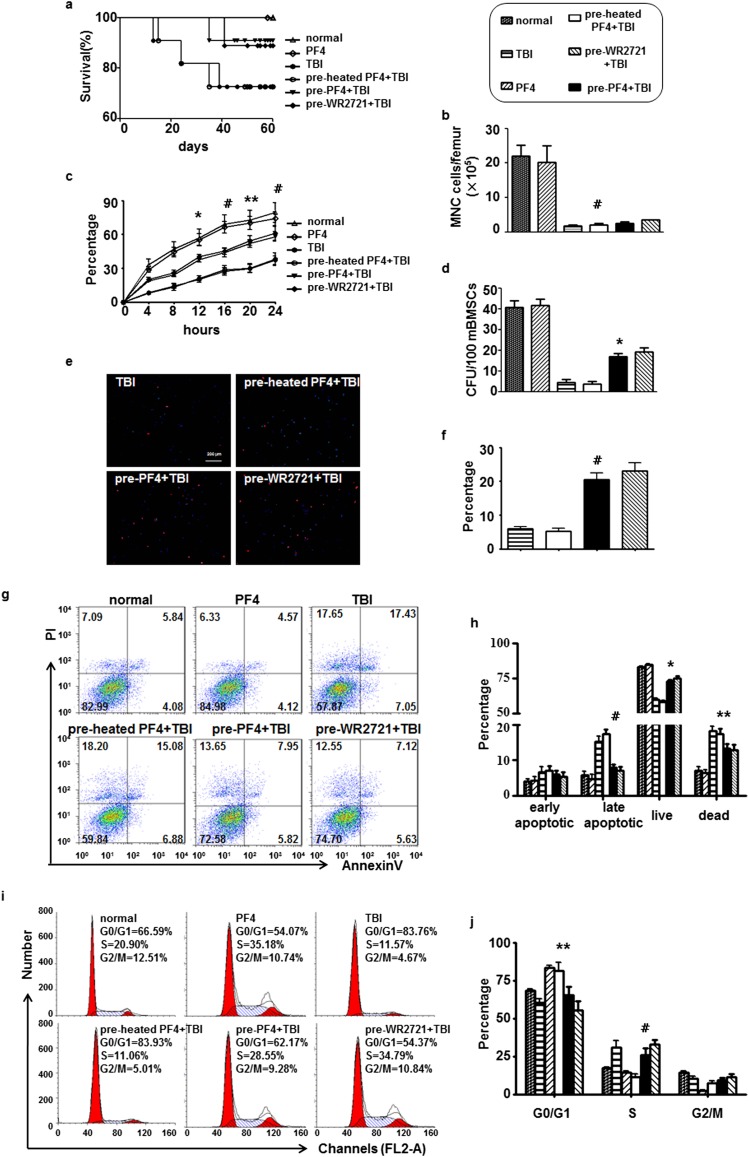
To confirm whether there was a similar haematopoietic protection of PF4 in vivo, we performed several experiments on sublethally irradiated C57BL/6J mice. On the 60th day post irradiation, the survivals of the PF4-pre-treated mice were 90.9% compared with 72.7% of survivals in the heated PF4-pre-treated mice (Figure 4a). Until the mice sacrificed on the third day after TBI, the total MNC showed a significant decrease by 90%, but there is no significant discrepancy between PF4 treated or not treated mice (Figure 4b). According to Drouet et al18 and Lee et al19 MNC do not recover in the early phase (<5 days) after TBI whatever treatment was performed.
Figure 4.
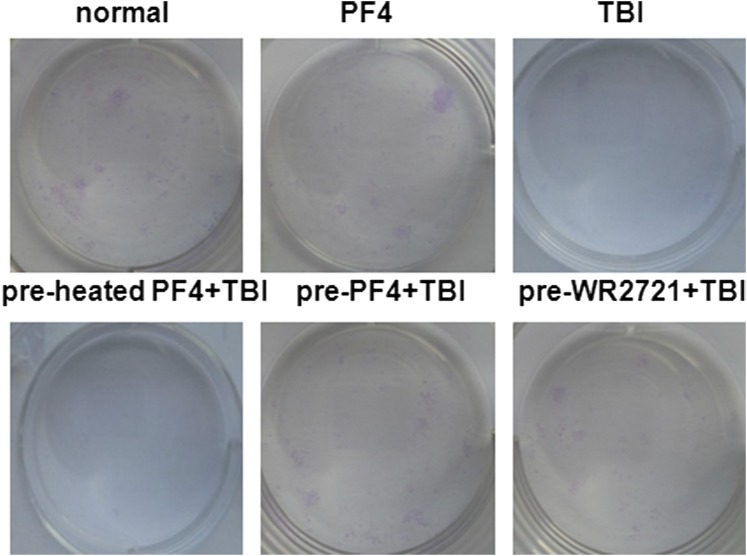
Platelet factor 4 (PF4)–protected bone marrow mesenchymal stem cells (BMSCs) in vivo. Two intraperitoneal injections of PF4 (40 μg kg−1) or equivalent heated PF4 were administered at 6-h intervals, and 20 h after the second injection, the mice received 500-cGy γ-radiation total body irradiation (TBI). WR-2721 (150 mg kg−1) was administered 30 min before TBI for a positive control. The mice were sacrificed by cervical dislocation 3 days after exposure. (a) The survival of the mice was plotted for 60 days. (b) The number of mononuclear cells (MNCs) in different treated mice on the third day after exposure. (c) The percentages of adherent MNC in different culture time. (d) Colony-forming unit fibroblasts (CFU-F) were counted at the 14th day after culture. 5-ethynyl-2′-deoxyuridine (EdU) immunofluorescent staining was performed on the tenth culture day. (e) Representative images of mouse BMSCs stained with EdU. (f) Quantification of EdU+ cells in (e). (g, h) Cell apoptosis was analysed by FACSCalibur™ (BD Immunocytometry Systems, San Jose, CA) on the fifth culture day. (i, j) Cell cycle was performed when isolated bone marrow cells were totally adherent. Bars, means ± standard deviation. *p < 0.05; **p < 0.01; #p < 0.001; n = 8. PI, propidium iodide.
We next examined the adhesion, proliferation and apoptosis of mBMSCs. The adherent MNC expanded in a time-dependent manner in the presence of PF4 (Figure 4c). The colony-forming assay showed that pre-treated with PF4 but not inactive heated-PF4 could facilitate the proliferation of mBMSCs after TBI (Figure 4d and Supplementary Figure C). A similar situation was observed in the EdU immunofluorescent staining (Figure 4e,f). We subsequently assessed the apoptosis of mBMSCs by FACSCalibur on the fifth culture day, and the result showed that there were decreased late apoptotic and dead cells in PF4-treated mice (Figure 4g,h). These data indicated that PF4 could effectively promote mBMSC recovery from TBI in vivo, probably through facilitating the proliferation and repressing the apoptosis of cells.
To determine whether this radioprotective effect was also owing to a regulation of the cell cycle in vivo, we determined their percentages when cells were totally adherent. A significant accumulation of S phase and decrease of G0/G1 phase were observed in PF4 pre-administrated mice compared with the control groups (Figure 4i,j). Collectively, these in vivo data suggested that PF4, as a radiation protector, could have similar effects on BMSCs as well as in vitro.
Platelet factor 4–modulated cell cycle and apoptosis-related gene expression in human bone marrow mesenchymal stem cells
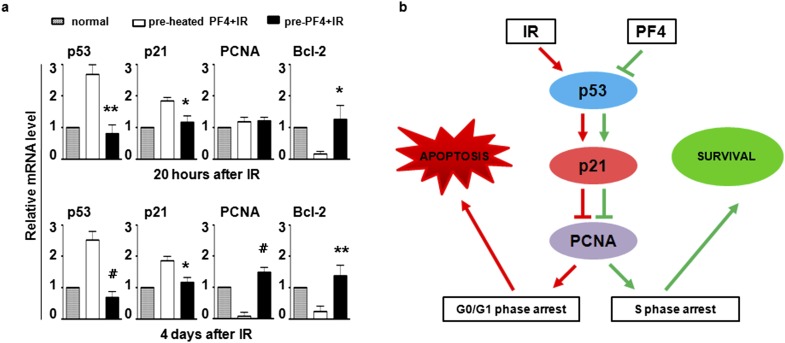
To define the cellular and molecular basis for these phenomena, we employed quantitative RT-PCR (qRT-PCR) to examine the expression of seven genes that were critical to S phase arrest and cell apoptosis. Compared with the heated PF4-pre-incubated cells, three genes [Cyclin D1, cyclin-dependent kinase 4 (CDK4) and retinoblastoma (Rb)] were essentially unchanged or slightly changed but showed no statistical significance, the genes related to DNA damage [tumour protein p53 (p53) and cyclin-dependent kinase inhibitor 1 (p21WAF1/CIP1)] were downregulated, while proliferating cell nuclear antigen (PCNA) known as a DNA repair gene was upregulated in the PF4 pre-incubated cells (Figure 5a). These data indicated that PF4 may regulate the expression of some important proteins of cell cycle to reduce DNA damage.
Figure 5.
Platelet factor 4 (PF4)–modulated cell cycle and apoptosis-related gene expression. (a) Human bone marrow mesenchymal stem cells were pre-incubated with PF4 or equivalent heated PF4 for 12 h before 500 cGy ionizing irradiation, and gene expression was analysed by quantitative reverse transcription–polymerase chain reaction at 20 h and 4 days after ionizing irradiation (IR). The unradiated cells were used as a control. (b) Schematic diagram of potential signaling triggered by PF4. Bars, means ± standard deviation. *p < 0.05; **p < 0.01, #p < 0.001; n = 8. PCNA, proliferating cell nuclear antigen.
DISCUSSION
The search for ideal protective agents for use in a variety of radiation scenarios has continued for more than six decades, a number of compounds are now available for use in a variety of radiation contexts.7 However, the transition of agents from animal testing to use in clinics has been slow. These limitations emphasized a need to identify efficacious novel agents that are somaticly occurring with low toxicity and those that provide a long window of protection on the survivability of normal tissue.
We have previously demonstrated that a somatic cytokine, PF4, accelerated the recovery of haematopoiesis from TBI mice.11,20 However, its effect on haematopoietic microenvironment remains to be established. In this study, we developed the BMSC irradiative injury models to uncover the therapeutic efficacy of PF4 on bone marrow (BM) niches and to further identify the potential mechanism ex and in vivo. The phenomenological evidence for the radioprotective effect of PF4 on BMSCs is firstly supported by the dose-dependent enhancement of cell viability and cell survival from irradiation, while withdrawing PF4 after IR could retain its antiradiative ability. We also observed a plateau phase, indicating that PF4 might specifically bind to some receptor on BMSCs and be easy to saturate. By monitoring the dynamic changes of proliferation, apoptosis and cell cycle, we further found that PF4 could promote the proliferation of BMSCs while inhibit their apoptosis, which may be associated with blocking cells in the S phase, which is much more radioresistant.21 Accordingly, pre-administration of PF4 in vivo protected BMSCs from radiation injury and maintained cell proliferation most likely through a short-term S phase arrest.
We have tried to unveil the potential molecular mechanisms underlying the PF4-mediated antiradiation of MSCs. A series of evidence had shown that the cyclin-dependent kinase inhibitor p21WAF1/CIP1 downstream of PF4, is either up- or downregulated in different cells.22,23 Our results from qRT-PCR using in vitro cultured hBMSCs showed that the expression of p21WAF1/CIP1 is upregulated in irradiated cells and downregulated in a PF4-dependent manner, and its upstream gene p53 and downstream gene PCNA were changed accordingly. These observations provided that PF4, a chemokine, could interfere with the cell cycle by inhibiting p53/p21 axis24 and facilitate the S phase DNA replication and DNA damage repair by late activating PCNA25 (Figure 5b).
Moreover, the PF4-mediated downregulation of p21WAF1/CIP1 might not depend on Cyclin D1/CDK4 pathways (data not shown). Gentilini et al23 reported that PF4-dependent downregulation of cyclin E/CDK2 activity was associated with increased binding of p21WAF1/CIP1 on human umbilical vein endothelial cells, which may be a breach and needs further study. On the other hand, we observed an additive effect of PF4 that Bcl-2, an important anti-apoptotic gene, was upregulated, which matched the results of our previous study that showed PF4 could downregulate the expression of Bax protein in vivo.20 These data provided proof-of-principle of a mechanistic lead for the potential anti-apoptotic effects of PF4 via the caspase signalling pathway.
FUNDING
This work was supported by grants from the key project funds of Shanxi science and technology program (2010K01163) and the Natural Science Foundation of China (81100354). This study was implemented in the Graduates Innovation Center of Fourth Military Medical University.
REFERENCES
- 1.Harfouche G, Martin MT. Response of normal stem cells to ionizing radiation: a balance between homeostasis and genomic stability. Mutat Res 2010; 704: 167–74. doi: 10.1016/j.mrrev.2010.01.007 [DOI] [PubMed] [Google Scholar]
- 2.Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010; 466: 829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greenberger JS, Epperly M. Bone marrow-derived stem cells and radiation response. Semin Radiat Oncol 2009; 19: 133–9. doi: 10.1016/j.semradonc.2008.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh S, Kloss FR, Brunauer R, Schimke M, Jamnig A, Greiderer-Kleinlercher B, et al. Mesenchymal stem cells show radioresistance in vivo. J Cell Mol Med 2012; 16: 877–87. doi: 10.1111/j.1582-4934.2011.01383.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicolay NH, Sommer E, Lopez R, Wirkner U, Trinh T, Sisombath S, et al. Mesenchymal stem cells retain their defining stem cell characteristics after exposure to ionizing radiation. Int J Radiat Oncol Biol Phys 2013; 87: 1171–8. [DOI] [PubMed] [Google Scholar]
- 6.Singh VK, Ducey EJ, Brown DS, Whitnall MH. A review of radiation countermeasure work ongoing at the Armed Forces Radiobiology Research Institute. Int J Radiat Biol 2012; 88: 296–310. [DOI] [PubMed] [Google Scholar]
- 7.Weiss JF, Landauer MR. History and development of radiation-protective agents. Int J Radiat Biol 2009; 85: 539–73. doi: 10.1080/09553000902985144 [DOI] [PubMed] [Google Scholar]
- 8.Prasanna PG, Stone HB, Wong RS, Capala J, Bernhard EJ, Vikram B, et al. Normal tissue protection for improving radiotherapy: where are the gaps? Transl Cancer Res 2012; 1: 35–48. [PMC free article] [PubMed] [Google Scholar]
- 9.Thachil J. The prothrombotic potential of platelet factor 4. Eur J Intern Med 2010; 21: 79–83. doi: 10.1016/j.ejim.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 10.Kowalska MA, Rauova L, Poncz M. Role of the platelet chemokine platelet factor 4 (PF4) in hemostasis and thrombosis. Thromb Res 2010; 125: 292–6. doi: 10.1016/j.thromres.2009.11.023 [DOI] [PubMed] [Google Scholar]
- 11.Zhu BG, Yang L, Tian Q. Radiation protection effect of platelet factor 4 on hematopoiesis of acute radiation injured mice. Zhonghua Xue Ye Xue Za Zhi 2000; 21: 327–8. [Google Scholar]
- 12.Lambert MP, Xiao L, Nguyen Y, Kowalska MA, Poncz M. The role of platelet factor 4 in radiation-induced thrombocytopenia. Int J Radiat Oncol Biol Phys 2011; 80: 1533–40. doi: 10.1016/j.ijrobp.2011.03.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambert MP, Rauova L, Bailey M, Sola-Visner MC, Kowalska MA, Poncz M. Platelet factor 4 is a negative autocrine in vivo regulator of megakaryopoiesis: clinical and therapeutic implications. Blood 2007; 110: 1153–60. doi: 10.1182/blood-2007-01-067116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maurer AM, Zhou B, Han ZC. Roles of platelet factor 4 in hematopoiesis and angiogenesis. Growth Factors 2006; 24: 242–52. [DOI] [PubMed] [Google Scholar]
- 15.Wang YC, Wang SH, Wei YN, Du DW, Xu H, Gao CC, et al. Notch-RBP-J signaling is required by bone marrow stromal cells for the treatment of acute graft versus host disease. Stem Cell Res 2013; 11: 721–35. doi: 10.1016/j.scr.2013.04.009 [DOI] [PubMed] [Google Scholar]
- 16.Ye L, Fan Z, Yu B, Chang J, Al Hezaimi K, Zhou X, et al. Histone demethylases KDM4B and KDM6B promotes osteogenic differentiation of human MSCs. Cell Stem Cell 2012; 11: 50–61. doi: 10.1016/j.stem.2012.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sugrue T, Brown JA, Lowndes NF, Ceredig R. Multiple facets of the DNA damage response contribute to the radioresistance of mouse mesenchymal stromal cell lines. Stem Cells 2013; 31: 137–45. doi: 10.1002/stem.1222 [DOI] [PubMed] [Google Scholar]
- 18.Drouet M, Mourcin F, Grenier N, Leroux V, Denis J, Mayol JF, et al. Single administration of stem cell factor, FLT-3 ligand, megakaryocyte growth and development factor, and interleukin-3 in combination soon after irradiation prevents nonhuman primates from myelosuppression: long-term follow-up of hematopoiesis. Blood 2004; 103: 878–85. [DOI] [PubMed] [Google Scholar]
- 19.Lee MO, Song SH, Jung S, Hur S, Asahara T, Kim H, et al. Effect of ionizing radiation induced damage of endothelial progenitor cells in vascular regeneration. Arterioscler Thromb Vasc Biol 2012; 32: 343–52. [DOI] [PubMed] [Google Scholar]
- 20.Lin HL, Yang L, Wang XR. Effect of PF4 on 60CO γ ray irradiation-induced bone marrow cell apoptosis in mice. [In Chinese.] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2007; 23: 813–15. [PubMed] [Google Scholar]
- 21.Maier P, Wenz F, Herskind C. Radioprotection of normal tissue cells. Strahlenther Onkol March 2014. Epub ahead of print. doi: 10.1007/s00066-014-0637-x [DOI] [PubMed]
- 22.Liu YJ, Lu SH, Han ZC. Signal transduction of chemokine platelet factor 4 in human erythroleukemia cells. Int J Hematol 2002; 75: 401–6. [DOI] [PubMed] [Google Scholar]
- 23.Gentilini G, Kirschbaum NE, Augustine JA, Aster RH, Visentin GP. Inhibition of human umbilical vein endothelial cell proliferation by the CXC chemokine, platelet factor 4 (PF4), is associated with impaired downregulation of p21(Cip1/WAF1). Blood 1999; 93: 25–33. [PubMed] [Google Scholar]
- 24.Heber-Katz E, Zhang Y, Bedelbaeva K, Song F, Chen X, Stocum DL. Cell cycle regulation and regeneration. Curr Top Microbiol Immunol 2013; 367: 253–76. doi: 10.1007/82_2012_294 [DOI] [PubMed] [Google Scholar]
- 25.Wang SC. PCNA: a silent housekeeper or a potential therapeutic target? Trends Pharmacol Sci 2014; 35: 178–86. doi: 10.1016/j.tips.2014.02.004 [DOI] [PubMed] [Google Scholar]