Abstract
Non-cutaneous melanomas (NCM) are diverse and relatively uncommon. They often differ from cutaneous melanomas in their epidemiology, genetic profile and biological behaviour. Despite the growing body of evidence regarding the utility of positron emission tomography (PET)/CT in cutaneous melanoma, the data on its use in NCM are scarce. In this review, we will summarize the existing literature and present cases from our experience with NCM to illustrate current knowledge on the potential role and limitations of fluorine-18 fludeoxyglucose PET/CT in NCM.
Non-cutaneous melanomas (NCM) are classified according to origin: ocular, mucosal or unknown primary. Ocular melanomas may arise from the uvea or conjunctiva. Mucosal melanomas may originate from mucosal surfaces in the head and neck (oral cavity, nasal and paranasal sinuses) and gastrointestinal and genitourinary tracts. NCM are relatively rare, with ocular and mucosal melanomas accounting for only 5.5% and 1.3% of all melanomas in North America, respectively. The incidence of mucosal melanoma may vary according to the population studied (range, 0.2–10.0%) and is higher in Asian populations. By contrast, uveal melanomas are more common in Caucasians. Table 1 compares the prevalence, genetic features and prognosis of cutaneous melanomas and NCM.1,2 Staging and management of NCM varies by location and differs from cutaneous melanoma. In NCM, primary therapy consists of local resection, often with adjuvant radiotherapy. There may be a role for chemotherapy and immunotherapy; however, this approach has largely been extrapolated from experience with cutaneous tumours.
Table 1.
| Patient/tumour characteristics | Cutaneous | Non-cutaneous |
|---|---|---|
| Age (years) | 55 | 67 |
| Ultraviolet light association | Yes | No clear association |
| Incidence over time | Increasing | Stable |
| Distant metastases at presentation | 12% | Ocular, 3%; mucosal, 23% |
| Staging scheme | UIACC/American Joint Committee on Cancer and TNM | No single validated system |
| Genetic profile | ||
| C-Kit mutations | 1.7% | 15.6% (mucosal) |
| BRAF mutations | Common | Rare |
| 5-year survival | 80% | Ocular, 74.6%; mucosal, 23%; unknown primary, 29.1% |
BRAF, v-raf murine sarcoma viral oncogene homologue B; C-Kit, receptor tyrosine kinase for stem cell factor; UIACC, Union for International Cancer Control.
ROLE OF POSITRON EMISSION TOMOGRAPHY-CT IN CUTANEOUS MELANOMA
Melanoma is usually fluorine-18 fludeoxyglucose (18F-FDG) avid, and its efficacy in detection of lymph node metastases has been investigated. Although it cannot replace sentinel lymph node staging, reported detection rate of lymph node metastases is dependent on the size of a node, and for nodes measuring 6–10 mm, it is approximately 83%. Positron emission tomography (PET)-CT has been shown to detect more visceral and non-visceral metastases than CT.1 Meta-analyses show that PET-CT had better sensitivity and specificity in Stage III/IV melanoma than did standard clinical diagnostic imaging, resulting in more accurate staging. This results in a change in patient management in 15–64% of cases.
A previous study demonstrated a treatment change following PET-CT in 48.6% of patients with an overall accuracy of 98.7%, in comparison with PET alone (88.8%) and CT alone (69.7%). It has also been shown to be more accurate in the detection of skin and subcutaneous metastases when compared with whole-body MRI. A major clinical impact has also been demonstrated in cases of recurrent melanoma in up to 41% of patients.2 The role of PET in directing immunotherapy has yet to be established. Early reports suggest a role for the evaluation of metabolic response, for example in dendritic cell injections.
POSITRON EMISSION TOMOGRAPHY-CT IN NON-CUTANEOUS MELANOMA
Despite the evidence regarding the utility of PET-CT in cutaneous melanoma, the data on its use in NCM are scarce. In this review, we will explore the existing literature on each NCM subtype and illustrate our experience on the potential role and limitations of 18F-FDG PET/CT in NCM.
Mucosal melanoma
Mucosal melanomas may involve the head and neck (55.4%), female genital tract (18%), anorectal region (23.8%) or urinary tract (2.8%).3
Anorectal melanoma
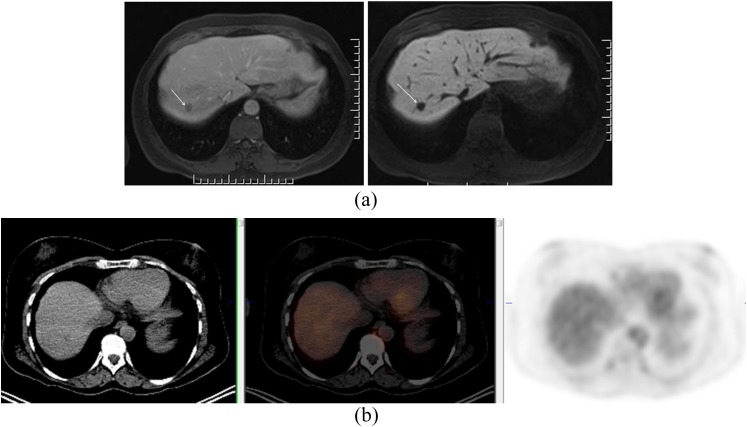
Anorectal malignant melanomas (ARMM) represent only 0.05% of all colorectal malignant tumours. The mean age at manifestation is 60–70 years usually with a female pre-dominance; except in India, where it is more common in males. The preferred staging modality has been MRI, and few reports exist that document the use of PET-CT in staging (Figure 1). A recent 45-year review in the Journal of the American College of Surgeons aiming to aggregate the clinical data and treatment options for anorectal melanoma suggested exploring the use of 18F-FDG PET/CT in the staging work-up of ARMM, despite the lack of experience to date.4
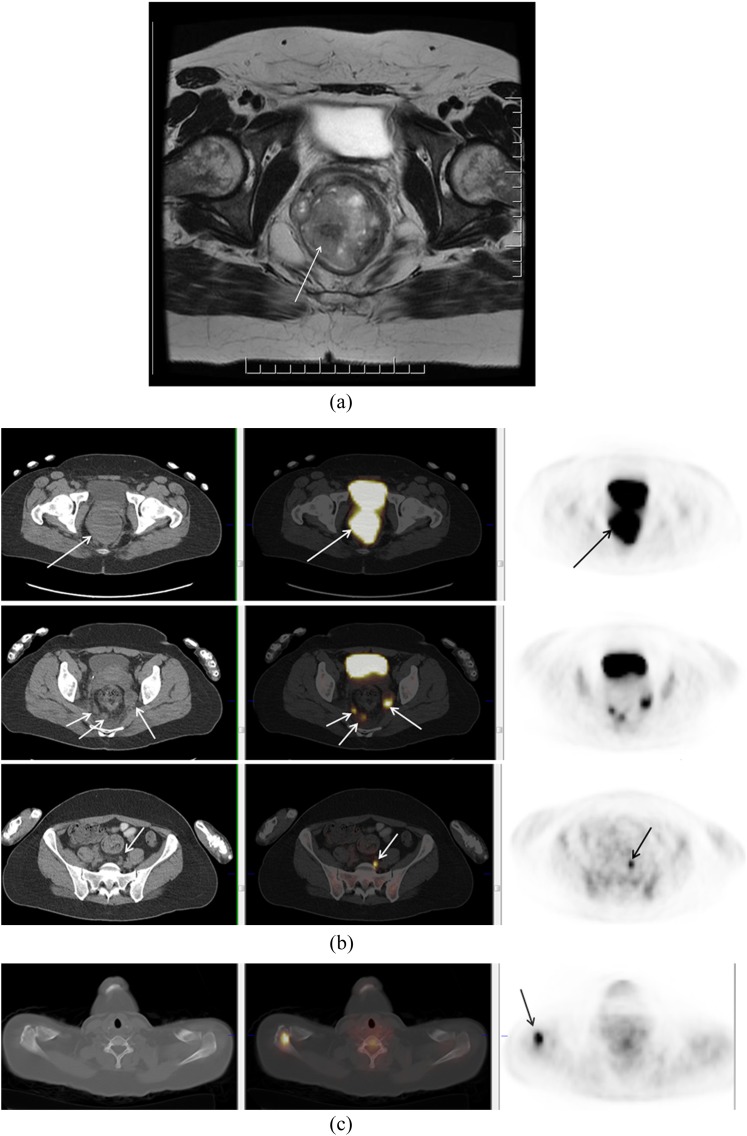
Figure 1.
A 37-year-old female who presented with rectal bleeding. Biopsy of the rectal mass showed mucosal melanoma. A large tumour was identified on MRI (a). On fluorine-18 fludeoxyglucose positron emission tomography (PET)/CT, primary tumour and multiple nodal metastases in mesorectal space and left common iliac chain were identified (b). PET/CT also demonstrated a Segment 7 liver metastasis and a deposit in the right acromion (c).
Sinonasal melanomas
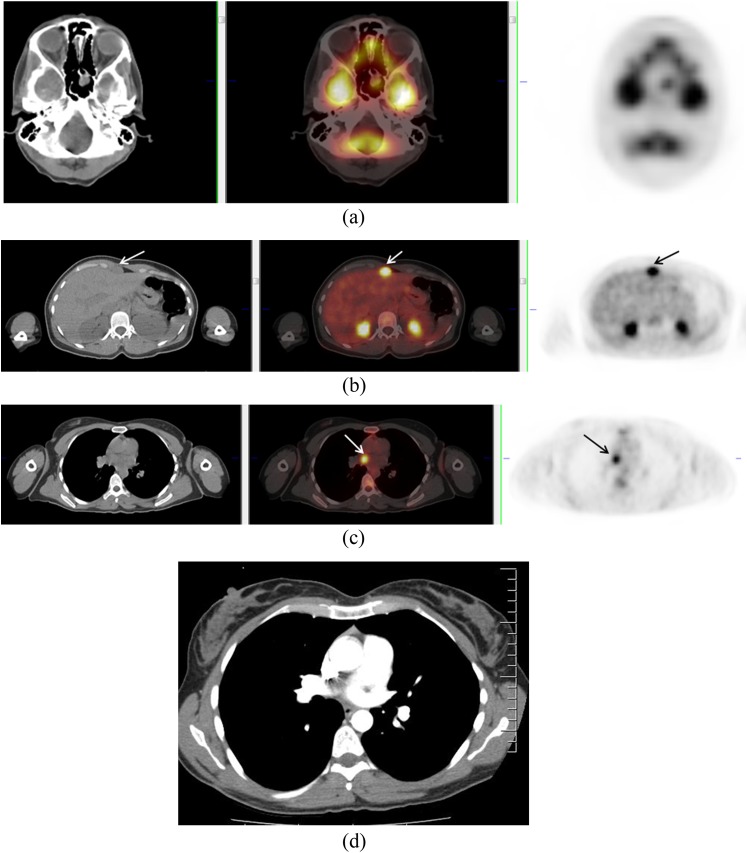
In general, sinonasal malignant melanoma (SNMM) affects older patients. A 5-year survival depends on its location and is approximately 37% for patients diagnosed with nasal cavity disease, 24% for patients with maxillary sinus tumours and 18% for patients with ethmoid sinus disease. A retrospective analysis of 18F-FDG PET/CT staged and followed 10 patients with SNMM and showed that PET/CT depicted the tumours and identified all metastases with the exception of 1 cerebral metastatic deposit.5 In our experience, PET may help detect unexpected metastases (Figure 2). Owing to the low prevalence of this disease, no large-scale trials on use of PET in staging are available.
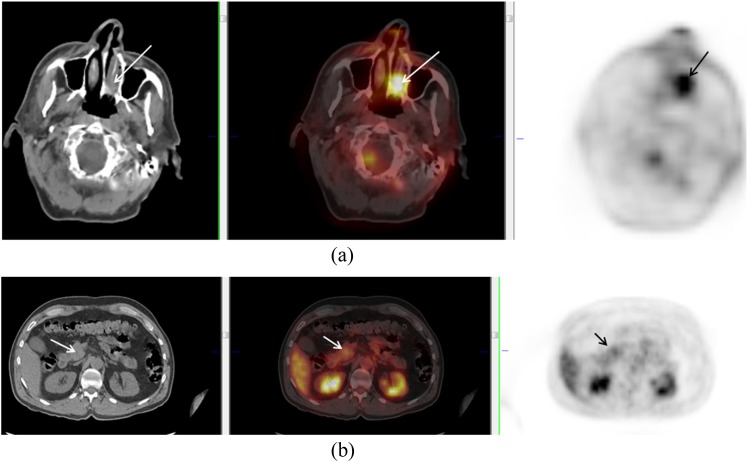
Figure 2.
A 70-year-old male with left nasal melanoma (arrows) (a). Positron emission tomography/CT showed uptake in a mass at the head of the pancreas (arrows) (b). Biopsy confirmed metastatic deposit from nasal melanoma.
Vulvovaginal melanoma
Vaginal melanomas comprise <2% of melanomas in females and tend to have a more aggressive course than cutaneous melanoma. In the USA, the 5-year survival is 60% and 25% for vulvar and vaginal melanoma, respectively. Staging is via the American Joint Committee on Cancer (AJCC) system, which incorporates tumour depth and surgical stage. MRI may be used to aid staging, and some case reports6 have been described.
Groin node status is an important prognostic factor in vulvovaginal melanoma (Figure 3). Metastases to the lung (100%), liver (57%) and peritoneum (43%) have been described in a small series of patients with vaginal melanoma (Figure 4). Despite the importance of staging and the propensity for distant metastases, there is limited literature on imaging features, especially regarding the use of PET-CT.7
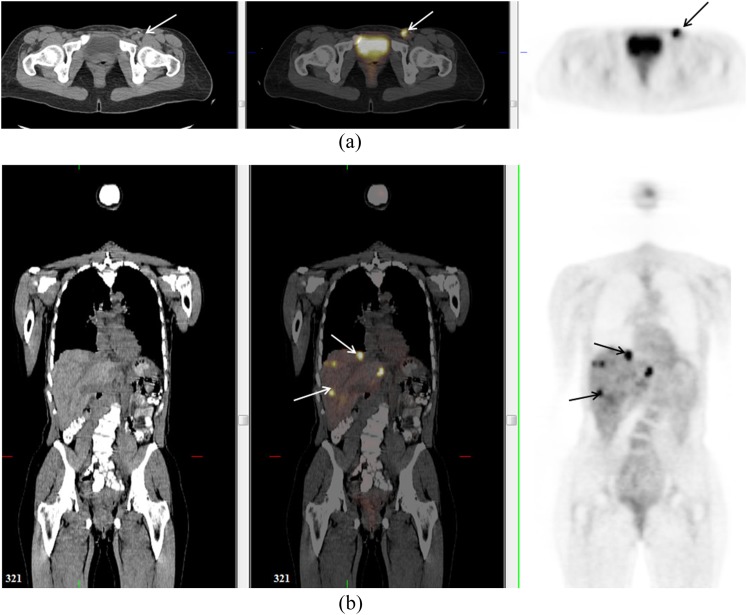
Figure 3.
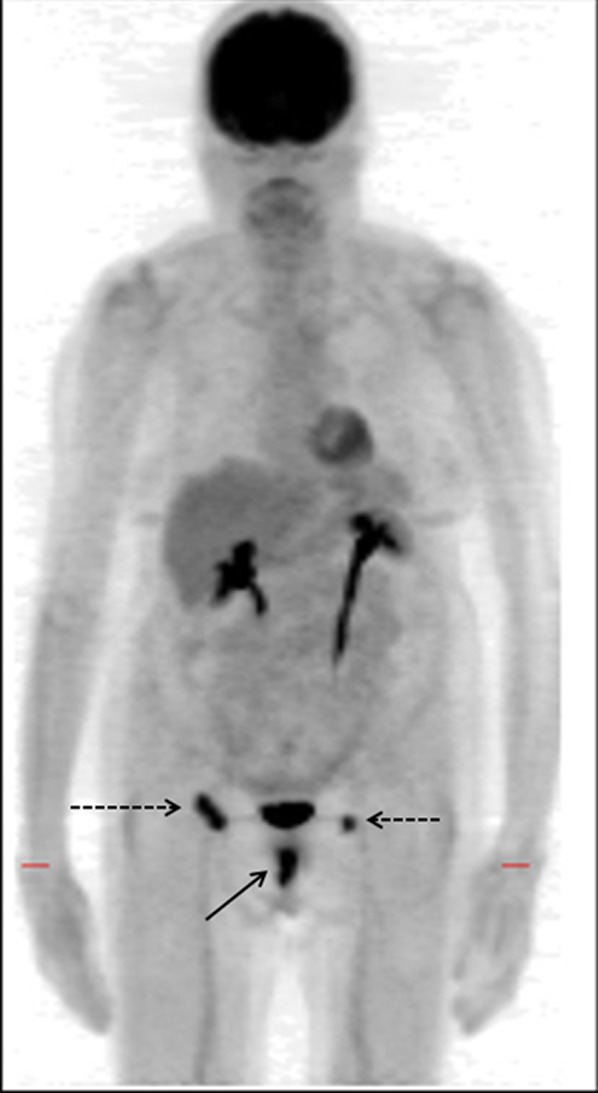
A 79-year-old female with vaginal melanoma (solid arrow). Positron emission tomography demonstrated uptake in primary lesion and bilateral inguinal nodes (dotted arrows).
Figure 4.
A 48-year-old female with vaginal melanoma. Positron emission tomography (PET)/CT confirmed uptake in left inguinal node (arrow) (a) but also detected previously unsuspected lesions in the liver (arrows) (b). These were not visualized on staging CT prior to PET but were seen on contrast-enhanced CT 4 months later (not shown).
Uveal and choroidal melanoma
Routine staging of uveal melanoma includes physical examination and ophthalmic ultrasonography to determine local extent of disease, and liver enzymes and chest radiography for distant staging. The most commonly used classification scheme is the AJCC TNM system. PET-CT is not a routine staging examination and identification of primary tumour by PET is limited. PET may identify up to 33% of T2 and 75% of T3 choroidal melanomas.
Whole-body PET-CT may have a role in predicting survival, with high tumour standardized uptake value associated with decreased time to metastasis. Existing literature on use of PET-CT for detection of distant metastases demonstrates conflicting results. A study reporting 52 patients screened with PET-CT at baseline showed unexpected metastases in only two cases (3.8%), both of which had hepatic metastases and normal liver enzymes.8 By contrast, a smaller series of 12 patients referred for restaging, staging prior to therapy or assessment of suspected metastases demonstrated vital metastases in all patients, including hepatic, osseous, lymphatic, pulmonary, adrenal and muscular deposits. In that article, PET-CT also ruled out pulmonary metastases in two patients with suspicious findings on CT and chest radiography.9
The use of liver function tests (LFTs) to detect liver metastases has been shown to have high specificity and predictive values but low sensitivity. Hepatic metastases without abnormal enzymes were also described in a case series by Kurli et al.10 Approximately 59% of patients with liver metastases are asymptomatic. Liver ultrasonography, often used for staging, has a sensitivity of only 78%. Data comparing PET with conventional imaging in detection of liver metastases are also contradictory. A small series including 27 liver metastases in 13 patients with uveal melanoma compared PET detection of liver metastases with CT or MR and reported a 59% false-negative rate for PET11 (Figures 5 and 6). This contradicts a large series of 333 patients who showed high sensitivity and positive-predictive values.12 However, this series included mostly early-stage disease with only 7 of 333 patients having distant metastases on PET-CT, all of which had T3 or T4 disease. Furthermore, the negative-predictive value (NPV) of PET in this study is uncertain. Direct head-to-head comparisons of PET and MR or CT are needed to clarify the performance of each modality in this patient population.
Figure 5.
A 47-year-old female with uveal melanoma. Coronal positron emission tomography-CT image demonstrated multiple fluorine-18 fludeoxyglucose avid bilateral hilar nodes. The pattern raised suspicion for an inflammatory aetiology, such as sarcoidosis, and transbronchial biopsy was performed. This was positive for non-necrotizing granulomatous inflammation.
Figure 6.
A 54-year-old female with uveal melanoma. Positron emission tomography (PET)/CT was negative; however, MR showed hepatic deposits. Gadolinium ethoxybenzyl dimeglumine (Primovist®; Bayer-Schering, Berlin, Germany)–enhanced MR (a) in the portal venous phase (left image) and in the hepatobiliary phase (right image) showing metastasis in Segment 7 (arrow). PET-CT at that level shows no abnormality (b).
Unknown primary site
Melanomas with unknown primary have traditionally been grouped with NCM as genetic analysis showed differences between these tumours and cutaneous melanoma. Recent studies have, however, suggested that they share genetic features with cutaneous melanoma.13 1-year survival appears similar to cutaneous melanoma in small cohorts. The role of PET in this patient population is yet to be determined (Figure 7).
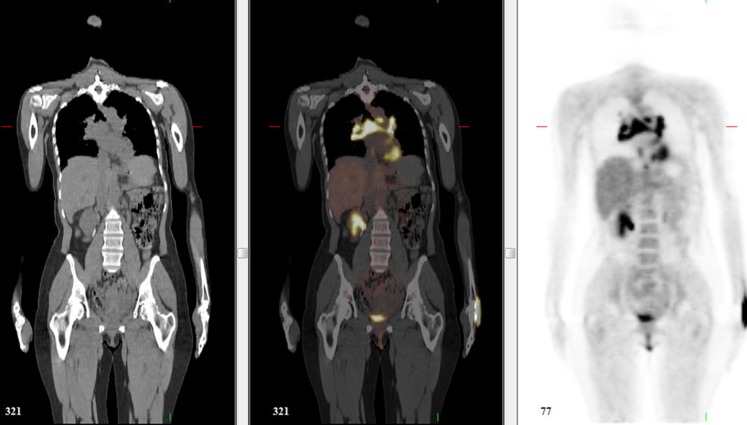
Figure 7.
A 33-year-old female with melanoma from unknown primary. Positron emission tomography (PET)/CT demonstrated multiple deposits including in the sphenoid sinus (a), subcutaneous fat (not shown) and anterior to the left lobe of the liver (b). PET also demonstrated uptake of fluorine-18 fludeoxyglucose at the level of the right pulmonary artery (c), with no corresponding abnormality on concurrent contrast-enhanced CT chest (d). Follow-up CT 2 months later (not shown) confirmed a soft-tissue mass at that location.
RESTAGING/RESPONSE ASSESSMENT
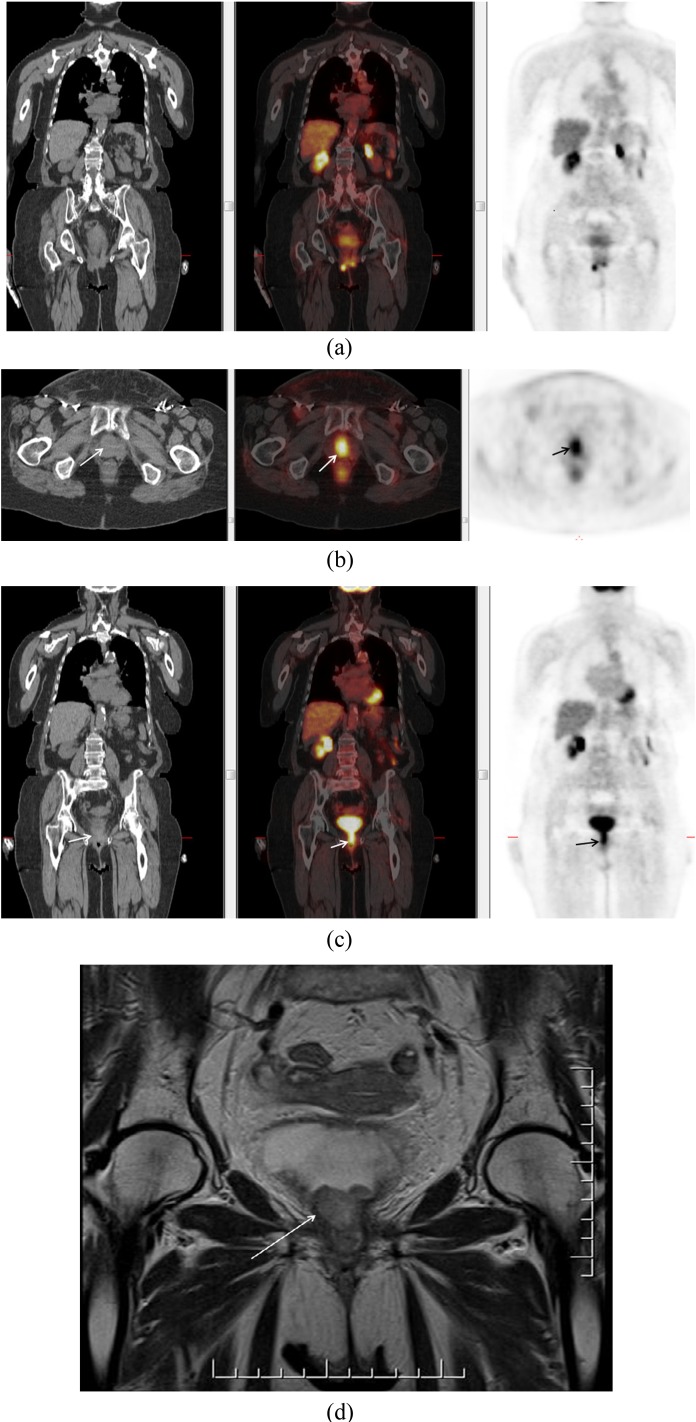
There is also little data available regarding the use of PET-CT in the restaging or response assessment of non-cutaneous melanoma. Small series postulate that it shows utility in management, particularly in predicting the absence of disease given high negative-predictive values. Others have suggested that PET-CT may have a promising role in response assessment to chemoembolization of hepatic metastases, although MR may be more useful for detection of smaller metastases (<1 cm) following therapy.14 PET may have a role in restaging patients before contemplating surgical salvage therapy in the setting of presumed localized tumour recurrence (Figure 8).
Figure 8.
A 75-year-old female who had a previous resection of vaginal melanoma. She presented with right vulvar nodule, and a positron emission tomography (PET)/CT was ordered to assess for distant disease prior to local resection. Coronal PET image shows fluorine-18 fludeoxyglucose avid nodules in bilateral vulva (a) and an infiltrative soft-tissue tumour extending along the urethra to the bladder base (b, c) (axial and coronal PET/CT images through the urethra) (arrows). Coronal T2 weighted MR image confirmed intermediate signal intensity mass extending to bladder base (arrow) (d). The patient was deemed unsuitable for resection and received systemic therapy.
CONCLUSION
Data on the use of 18F-FDG PET/CT in NCM are limited, likely due to the low prevalence of this disease and the varied biology of the distinct subtypes. Furthermore, current literature is often conflicting. Data on larger cohorts such as that published by Freton et al12 on choroidal melanoma should be interpreted with caution given the very small number of patients with metastatic disease. Although the current evidentiary review and our limited experience suggest that there may be a role for PET in the management of patients with NCM, large-scale confirmatory studies should be pursued. As for other uncommon diseases, a national or international registry may be needed to accrue sufficient number of patients to determine whether PET should be used as an adjunct or in place of conventional imaging strategies.
REFERENCES
- 1.Reinhardt MJ, Joe AY, Jaeger U, Huber A, Matthies A, Bucerius J, et al. Diagnostic performance of whole body dual modality 18F-FDG PET/CT imaging for N- and M-staging of malignant melanoma: experience with 250 consecutive patients. J Clin Oncol 2006; 24: 1178–87. doi: 10.1200/JCO.2005.03.5634 [DOI] [PubMed] [Google Scholar]
- 2.Subesinghe M, Marples M, Scarsbrook AF, Smith JT. Clinical impact of (18)F-FDG PET-CT in recurrent stage III/IV melanoma: a tertiary centre Specialist Skin Cancer Multidisciplinary Team (SSMDT) experience. Insights Imaging 2013; 4: 701–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patrick RJ, Fenske NA, Messina JL. Primary mucosal melanoma. J Am Acad Dermatol 2007; 56: 828–34. doi: 10.1016/j.jaad.2006.06.017 [DOI] [PubMed] [Google Scholar]
- 4.Falch C, Stojadinovic A, Hann-von-Weyhern C, Protic M, Nissan A, Faries MB, et al. Anorectal malignant melanoma: extensive 45-year review and proposal for a novel staging classification. J Am Coll Surg 2013; 217: 324–35. doi: 10.1016/j.jamcollsurg.2013.02.031 [DOI] [PubMed] [Google Scholar]
- 5.Haerle SK, Soyka MB, Fischer DR, Murer K, Strobel K, Huber GF, et al. The value of 18F-FDG-PET/CT imaging for sinonasal malignant melanoma. Eur Arch Otorhinolaryngol 2012; 269: 127–33. doi: 10.1007/s00405-011-1664-1 [DOI] [PubMed] [Google Scholar]
- 6.Moon WK, Kim SH, Han MC. MR findings of malignant melanoma of the vagina. Clin Radiol 1993; 48: 326–8. [DOI] [PubMed] [Google Scholar]
- 7.Oudoux A, Rousseau T, Bridji B, Resche I, Rousseau C. Interest of F-18 fluorodeoxyglucose positron emission tomography in the evaluation of vaginal malignant melanoma. Gynecol Oncol 2004; 95: 765–8. doi: 10.1016/j.ygyno.2004.08.044 [DOI] [PubMed] [Google Scholar]
- 8.Finger PT, Kurli M, Reddy S, Tena LB, Pavlick AC. Whole body PET/CT for initial staging of choroidal melanoma. Br J Ophthalmol 2005; 89: 1270–4. doi: 10.1136/bjo.2005.069823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klingenstein A, Haug AR, Nentwich MM, Tiling R, Schaller UC. Whole-body F-18-fluoro-2-deoxyglucose positron emission tomography/computed tomography imaging in the follow-up of metastatic uveal melanoma. Melanoma Res 2010; 20: 511–16. doi: 10.1097/CMR.0b013e3283403d6c [DOI] [PubMed] [Google Scholar]
- 10.Kurli M, Chin K, Finger PT. Whole-body 18 FDG PET/CT imaging for lymph node and metastatic staging of conjunctival melanoma. Br J Ophthalmol 2008; 92: 479–82. doi: 10.1136/bjo.2007.124339 [DOI] [PubMed] [Google Scholar]
- 11.Strobel K, Bode B, Dummer R, Veit-Haibach P, Fischer DR, Imhof L, et al. Limited value of 18F-FDG PET/CT and S-100B tumour marker in the detection of liver metastases from uveal melanoma compared to liver metastases from cutaneous melanoma. Eur J Nucl Med Mol Imaging 2009; 36: 1774–82. doi: 10.1007/s00259-009-1175-0 [DOI] [PubMed] [Google Scholar]
- 12.Freton A, Chin KJ, Raut R, Tena LB, Kivelä T, Finger PT. Initial PET/CT staging for choroidal melanoma: AJCC correlation and second nonocular primaries in 333 patients. Eur J Ophthalmol 2012; 22: 236–43. doi: 10.5301/ejo.5000049 [DOI] [PubMed] [Google Scholar]
- 13.Egberts F, Bergner I, Krüger S, Haag J, Behrens HM, Hauschild A, et al. Metastatic melanoma of unknown primary resembles the genotype of cutaneous melanomas. Ann Oncol 2014; 25: 246–50. doi: 10.1093/annonc/mdt411 [DOI] [PubMed] [Google Scholar]
- 14.Orcurto V, Denys A, Voelter V, Schalenbourg A, Schnyder P, Zografos L, et al. (18)F-fluorodeoxyglucose positron emission tomography/computed tomography and magnetic resonance imaging in patients with liver metastases from uveal melanoma: results from a pilot study. Melanoma Res 2012; 22: 63–9. [DOI] [PubMed] [Google Scholar]