Abstract
In the tumor-bearing host, T cells invariably fail to induce a clinically significant antitumor immune response. Although model systems support the existence of tumor peptide antigens, the molecular interactions critical for antigen presentation by the tumor cell remain unresolved. Here, we demonstrate that human follicular lymphoma cells are highly inefficient at presenting alloantigen despite their strong expression of major histocompatibility complex and low-to-intermediate expression of some adhesion and B7 costimulatory molecules. Activation of follicular lymphoma cells via CD40 induces or up-regulates both adhesion and B7 costimulatory molecules essential to repair this defect. More importantly, once primed, alloreactive T cells efficiently recognize unstimulated follicular lymphoma cells. Thus, correction of defective tumor immunity requires not only expression of major histocompatibility complex but also sufficient expression of multiple adhesion and costimulatory molecules.
Full text
PDF

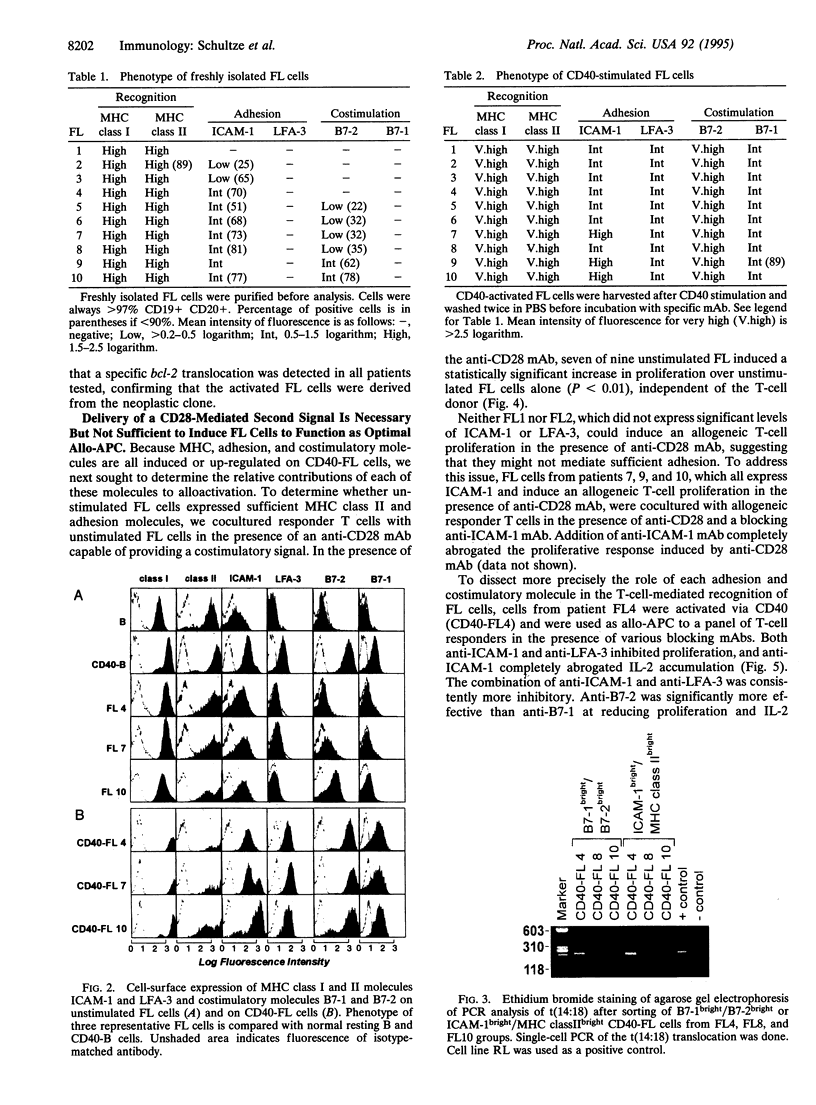
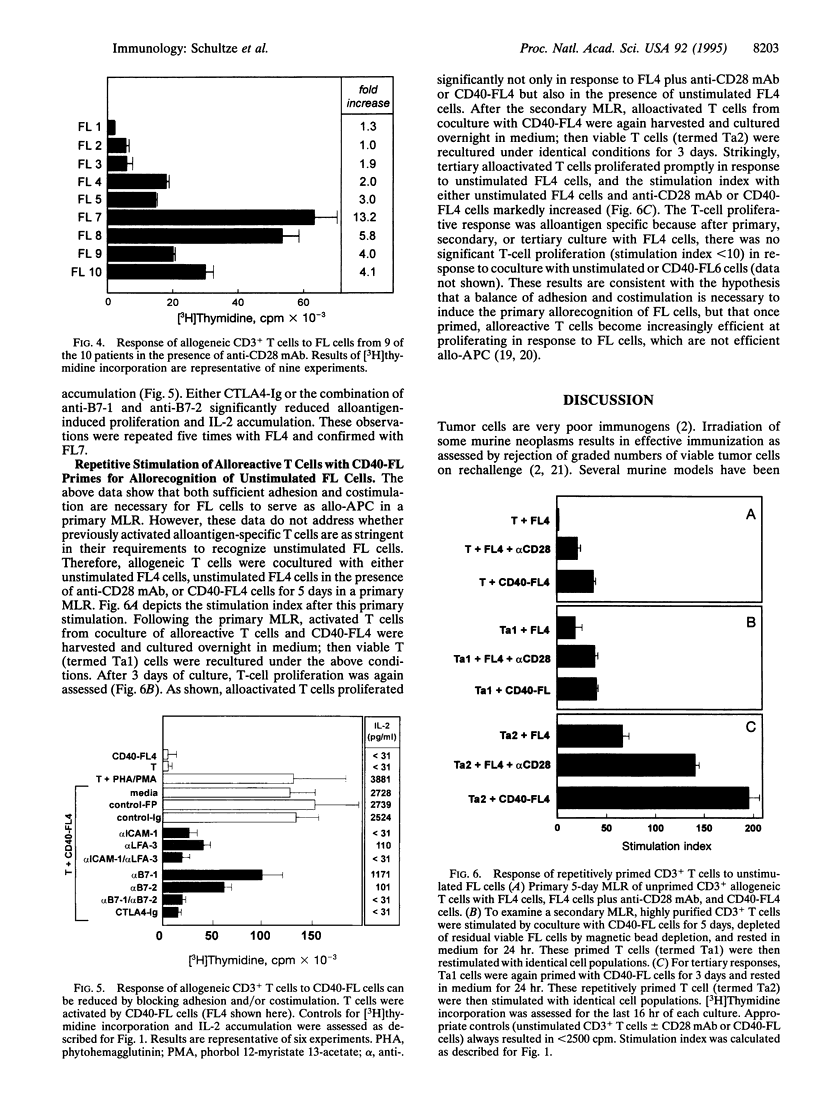

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banchereau J., Bazan F., Blanchard D., Brière F., Galizzi J. P., van Kooten C., Liu Y. J., Rousset F., Saeland S. The CD40 antigen and its ligand. Annu Rev Immunol. 1994;12:881–922. doi: 10.1146/annurev.iy.12.040194.004313. [DOI] [PubMed] [Google Scholar]
- Baskar S., Glimcher L., Nabavi N., Jones R. T., Ostrand-Rosenberg S. Major histocompatibility complex class II+B7-1+ tumor cells are potent vaccines for stimulating tumor rejection in tumor-bearing mice. J Exp Med. 1995 Feb 1;181(2):619–629. doi: 10.1084/jem.181.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon T., Cerottini J. C., Van den Eynde B., van der Bruggen P., Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- Boussiotis V. A., Freeman G. J., Gray G., Gribben J., Nadler L. M. B7 but not intercellular adhesion molecule-1 costimulation prevents the induction of human alloantigen-specific tolerance. J Exp Med. 1993 Nov 1;178(5):1753–1763. doi: 10.1084/jem.178.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis V. A., Freeman G. J., Gribben J. G., Daley J., Gray G., Nadler L. M. Activated human B lymphocytes express three CTLA-4 counterreceptors that costimulate T-cell activation. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11059–11063. doi: 10.1073/pnas.90.23.11059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boussiotis V. A., Freeman G. J., Griffin J. D., Gray G. S., Gribben J. G., Nadler L. M. CD2 is involved in maintenance and reversal of human alloantigen-specific clonal anergy. J Exp Med. 1994 Nov 1;180(5):1665–1673. doi: 10.1084/jem.180.5.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Ashe S., Brady W. A., Hellström I., Hellström K. E., Ledbetter J. A., McGowan P., Linsley P. S. Costimulation of antitumor immunity by the B7 counterreceptor for the T lymphocyte molecules CD28 and CTLA-4. Cell. 1992 Dec 24;71(7):1093–1102. doi: 10.1016/s0092-8674(05)80059-5. [DOI] [PubMed] [Google Scholar]
- Chen L., Linsley P. S., Hellström K. E. Costimulation of T cells for tumor immunity. Immunol Today. 1993 Oct;14(10):483–486. doi: 10.1016/0167-5699(93)90262-J. [DOI] [PubMed] [Google Scholar]
- Croft M., Bradley L. M., Swain S. L. Naive versus memory CD4 T cell response to antigen. Memory cells are less dependent on accessory cell costimulation and can respond to many antigen-presenting cell types including resting B cells. J Immunol. 1994 Mar 15;152(6):2675–2685. [PubMed] [Google Scholar]
- Dranoff G., Jaffee E., Lazenby A., Golumbek P., Levitsky H., Brose K., Jackson V., Hamada H., Pardoll D., Mulligan R. C. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dranoff G., Mulligan R. C. Gene transfer as cancer therapy. Adv Immunol. 1995;58:417–454. doi: 10.1016/s0065-2776(08)60624-0. [DOI] [PubMed] [Google Scholar]
- Freedman A. S., Boyd A. W., Berrebi A., Horowitz J. C., Levy D. N., Rosen K. J., Daley J., Slaughenhoupt B., Levine H., Nadler L. M. Expression of B cell activation antigens on normal and malignant B cells. Leukemia. 1987 Jan;1(1):9–15. [PubMed] [Google Scholar]
- Freeman G. J., Gribben J. G., Boussiotis V. A., Ng J. W., Restivo V. A., Jr, Lombard L. A., Gray G. S., Nadler L. M. Cloning of B7-2: a CTLA-4 counter-receptor that costimulates human T cell proliferation. Science. 1993 Nov 5;262(5135):909–911. doi: 10.1126/science.7694363. [DOI] [PubMed] [Google Scholar]
- Gribben J. G., Freedman A. S., Neuberg D., Roy D. C., Blake K. W., Woo S. D., Grossbard M. L., Rabinowe S. N., Coral F., Freeman G. J. Immunologic purging of marrow assessed by PCR before autologous bone marrow transplantation for B-cell lymphoma. N Engl J Med. 1991 Nov 28;325(22):1525–1533. doi: 10.1056/NEJM199111283252201. [DOI] [PubMed] [Google Scholar]
- Linsley P. S., Brady W., Grosmaire L., Aruffo A., Damle N. K., Ledbetter J. A. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J Exp Med. 1991 Mar 1;173(3):721–730. doi: 10.1084/jem.173.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Matulonis U. A., Dosiou C., Lamont C., Freeman G. J., Mauch P., Nadler L. M., Griffin J. D. Role of B7-1 in mediating an immune response to myeloid leukemia cells. Blood. 1995 May 1;85(9):2507–2515. [PubMed] [Google Scholar]
- Ostrand-Rosenberg S. Tumor immunotherapy: the tumor cell as an antigen-presenting cell. Curr Opin Immunol. 1994 Oct;6(5):722–727. doi: 10.1016/0952-7915(94)90075-2. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M. Cancer vaccines. Immunol Today. 1993 Jun;14(6):310–316. doi: 10.1016/0167-5699(93)90051-L. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M. New strategies for enhancing the immunogenicity of tumors. Curr Opin Immunol. 1993 Oct;5(5):719–725. doi: 10.1016/0952-7915(93)90127-e. [DOI] [PubMed] [Google Scholar]
- Pardoll D. M. Tumour antigens. A new look for the 1990s. Nature. 1994 Jun 2;369(6479):357–357. doi: 10.1038/369357a0. [DOI] [PubMed] [Google Scholar]
- Ranheim E. A., Kipps T. J. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993 Apr 1;177(4):925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein H., Gerdes J., Mason D. Y. The normal and malignant germinal centre. Clin Haematol. 1982 Oct;11(3):531–559. [PubMed] [Google Scholar]
- Tao M. H., Levy R. Idiotype/granulocyte-macrophage colony-stimulating factor fusion protein as a vaccine for B-cell lymphoma. Nature. 1993 Apr 22;362(6422):755–758. doi: 10.1038/362755a0. [DOI] [PubMed] [Google Scholar]
- Topalian S. L. MHC class II restricted tumor antigens and the role of CD4+ T cells in cancer immunotherapy. Curr Opin Immunol. 1994 Oct;6(5):741–745. doi: 10.1016/0952-7915(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Townsend S. E., Allison J. P. Tumor rejection after direct costimulation of CD8+ T cells by B7-transfected melanoma cells. Science. 1993 Jan 15;259(5093):368–370. doi: 10.1126/science.7678351. [DOI] [PubMed] [Google Scholar]
- Urashima M., Chauhan D., Uchiyama H., Freeman G. J., Anderson K. C. CD40 ligand triggered interleukin-6 secretion in multiple myeloma. Blood. 1995 Apr 1;85(7):1903–1912. [PubMed] [Google Scholar]
- Van Seventer G. A., Shimizu Y., Horgan K. J., Shaw S. The LFA-1 ligand ICAM-1 provides an important costimulatory signal for T cell receptor-mediated activation of resting T cells. J Immunol. 1990 Jun 15;144(12):4579–4586. [PubMed] [Google Scholar]






