Abstract
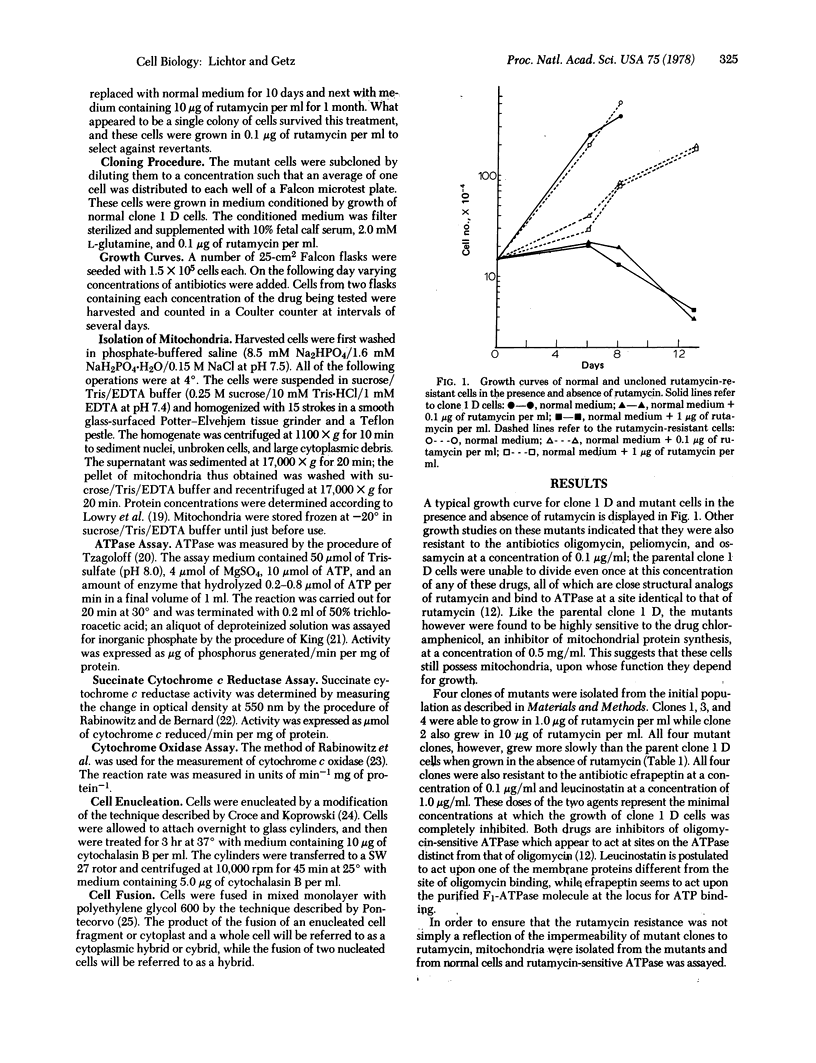
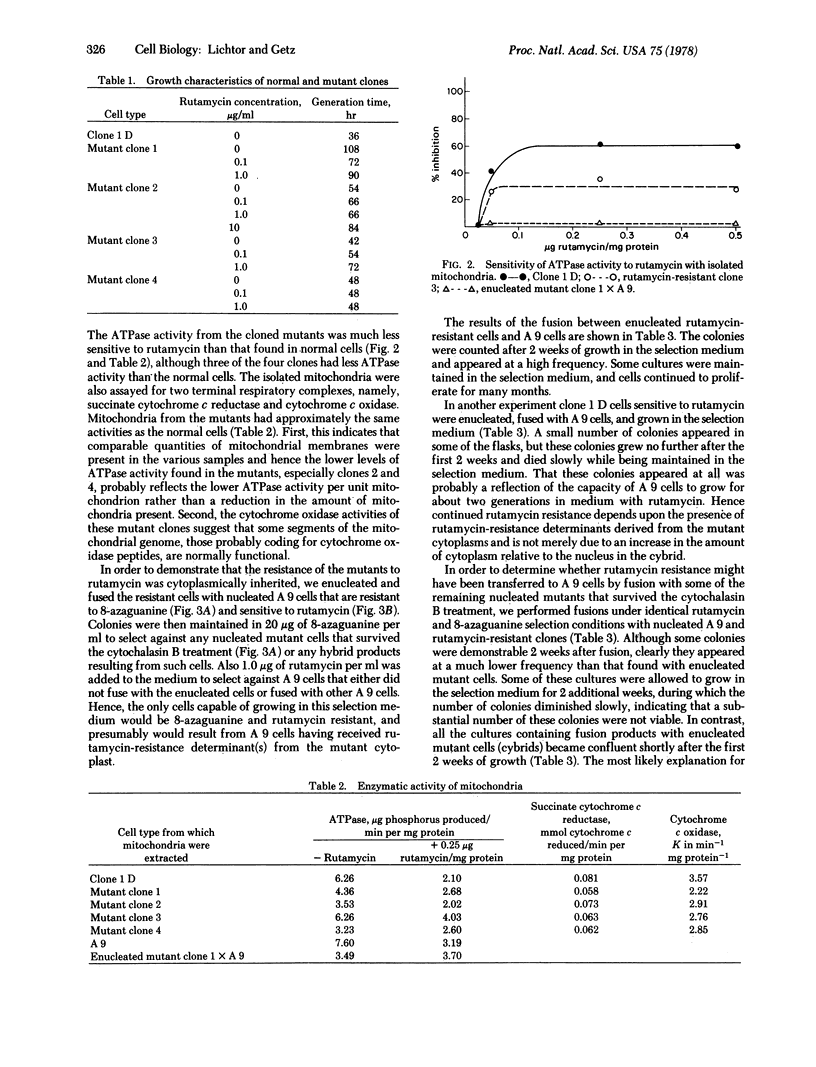
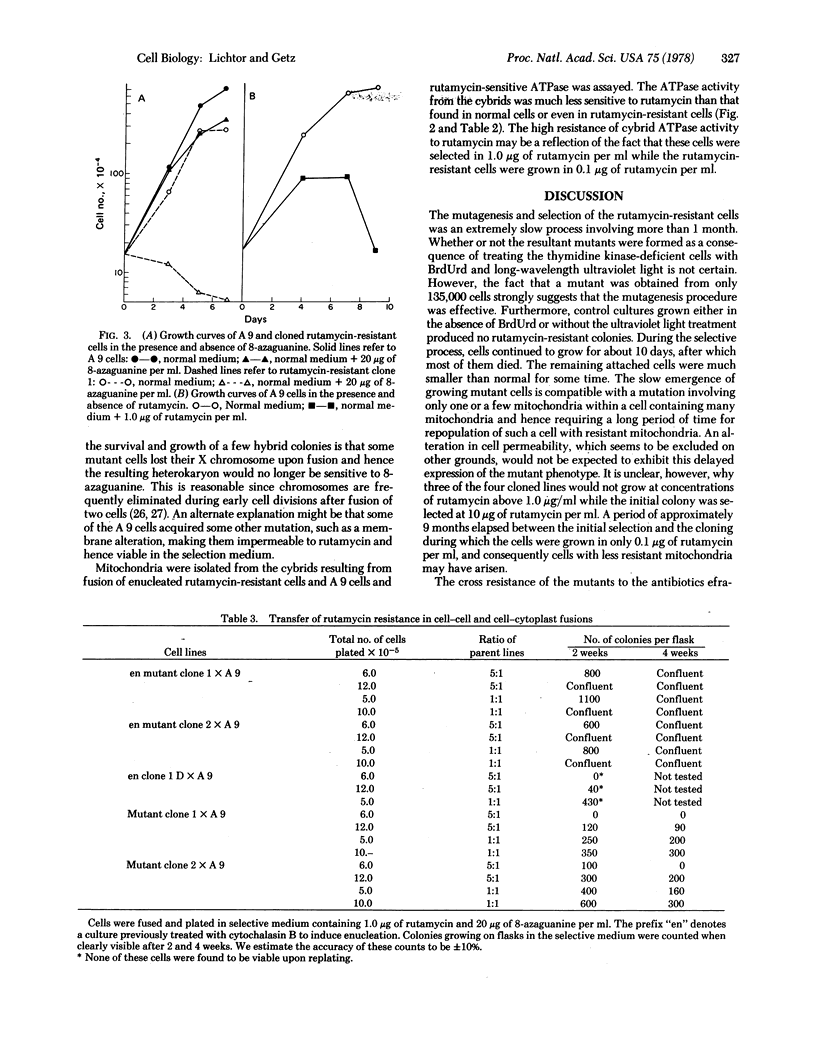
Mouse fibroblasts resistant to the drug rutamycin were isolated by selectively introducing BrdUrd into the mitochondrial genome of a line of mouse fibroblasts (clone 1 D) lacking a cytoplasmic thymidine kinase enzyme. The ATPase (ATP phosphohydrolase; EC 3.6.1.3) activity of mitochondria isolated from these cells was resistant to rutamycin. The rutamycin-resistant mutants were enucleated with cytochalasin B and fused with mouse A 9 cells resistant to 8-azaguanine and sensitive to rutamycin. Cytoplasmic hybrids, or cybrids, were selected as cells resistant to rutamycin and 8-azaguanine, and appeared at a high frequency. Other fusions between rutamycin-resistant nucleated cells and A 9 produced colonies at a much lower frequency. Finally, fusions between enucleated clone 1 D cells and A 9 cells produced no rutamycin-resistant colonies. These results indicate that rutamycin resistance is a cytoplasmically inherited characteristic in this cell line.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berk A. J., Clayton D. A. Mechanism of mitochondrial DNA replication in mouse L-cells: asynchronous replication of strands, segregation of circular daughter molecules, aspects of topology and turnover of an initiation sequence. J Mol Biol. 1974 Jul 15;86(4):801–824. doi: 10.1016/0022-2836(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Bunn C. L., Wallace D. C., Eisenstadt J. M. Cytoplasmic inheritance of chloramphenicol resistance in mouse tissue culture cells. Proc Natl Acad Sci U S A. 1974 May;71(5):1681–1685. doi: 10.1073/pnas.71.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu E. H., Sun N. C., Chang C. C. Induction of auxotrophic mutations by treatment of Chinese hamster cells with 5-bromodeoxyuridine and black light. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3459–3463. doi: 10.1073/pnas.69.11.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M., Koprowski H. Enucleation of cells made simple and rescue of SV40 by enucleated cells made even simpler. Virology. 1973 Jan;51(1):227–229. doi: 10.1016/0042-6822(73)90382-6. [DOI] [PubMed] [Google Scholar]
- Ebner E., Mason T. L., Schatz G. Mitochondrial assembly in respiration-deficient mutants of Saccharomyces cerevisiae. II. Effect of nuclear and extrachromosomal mutations on the formation of cytochrome c oxidase. J Biol Chem. 1973 Aug 10;248(15):5369–5378. [PubMed] [Google Scholar]
- Gijzel W. P., Strating M., Kroon A. M. The biogenesis of mitochondria during proliferation and muturation of the intestinal epithelium of the rat. Effects of oxytetracycline. Cell Differ. 1972 Aug;1(3):191–198. doi: 10.1016/0045-6039(72)90028-0. [DOI] [PubMed] [Google Scholar]
- King E. J. The colorimetric determination of phosphorus. Biochem J. 1932;26(2):292–297. doi: 10.1042/bj0260292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovác L., Weissová K. Oxidative phosphorylation in yeast. 3. ATPase activity of the mitochondrial fraction from a cytoplasmic respiratory-deficient mutant. Biochim Biophys Acta. 1968 Jan 15;153(1):55–59. doi: 10.1016/0005-2728(68)90145-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lardy H., Reed P., Lin C. H. Antibiotic inhibitors of mitochondrial ATP synthesis. Fed Proc. 1975 Jul;34(8):1707–1710. [PubMed] [Google Scholar]
- Mitchell C. H., England J. M., Attardi G. Isolation of chloramphenicol-resistant variants from a human cell line. Somatic Cell Genet. 1975 Jul;1(3):215–234. doi: 10.1007/BF01538447. [DOI] [PubMed] [Google Scholar]
- Perlman P. S., Mahler H. R. Formation of yeast mitochondria. 3. Biochemical properties of mitochondria isolated from a cytoplasmic petite mutant. J Bioenerg. 1970 Jul;1(2):113–138. doi: 10.1007/BF01515977. [DOI] [PubMed] [Google Scholar]
- Pontecorvo G. Production of mammalian somatic cell hybrids by means of polyethylene glycol treatment. Somatic Cell Genet. 1975 Oct;1(4):397–400. doi: 10.1007/BF01538671. [DOI] [PubMed] [Google Scholar]
- RABINOWITZ M., DE BERNARD B. Studies on the electron transport system. X. Preparation and spectral properties of a particulate DPNH and succinate cytochrome c reductase from heart muscle. Biochim Biophys Acta. 1957 Oct;26(1):22–29. doi: 10.1016/0006-3002(57)90049-5. [DOI] [PubMed] [Google Scholar]
- Rabinowitz M., Getz G. S., Casey J., Swift H. Synthesis of mitochondrial and nuclear DNA in anerobically grown yeast during the development of mitochondrial function in response to oxygen. J Mol Biol. 1969 May 14;41(3):381–400. doi: 10.1016/0022-2836(69)90283-6. [DOI] [PubMed] [Google Scholar]
- Rao P. N., Johnson R. T. Premature chromosome condensation: a mechanism for the elimination of chromosomes in virus-fused cells. J Cell Sci. 1972 Mar;10(2):495–513. doi: 10.1242/jcs.10.2.495. [DOI] [PubMed] [Google Scholar]
- Schatz G. Impaired binding of mitochondrial adenosine triphosphatase in the cytoplasmic "petite" mutant of Saccharomyces cerevisiae. J Biol Chem. 1968 May 10;243(9):2192–2199. [PubMed] [Google Scholar]
- Schwartz A. G., Cook P. R., Harris H. Correction of a genetic defect in a mammalian cell. Nat New Biol. 1971 Mar 3;230(1):5–8. doi: 10.1038/newbio230005a0. [DOI] [PubMed] [Google Scholar]
- Siegel R. L., Jeffreys A. J., Sly W., Craig I. W. Isolation and detailed characterization of human cell lines resistant to D-threo-chloramphenicol. Exp Cell Res. 1976 Oct 15;102(2):298–310. doi: 10.1016/0014-4827(76)90045-8. [DOI] [PubMed] [Google Scholar]
- Spolsky C. M., Eisenstadt J. M. Chloramphenicol-resistant mutants of human HeLa cells. FEBS Lett. 1972 Sep 15;25(2):319–324. doi: 10.1016/0014-5793(72)80514-3. [DOI] [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. 3. Function and synthesis of the oligomycin sensitivity-conferring protein of yeast mitochondria. J Biol Chem. 1970 Apr 10;245(7):1545–1551. [PubMed] [Google Scholar]
- Tzagoloff A. Assembly of the mitochondrial membrane system. I. Characterization of some enzymes of the inner membrane of yeast mitochondria. J Biol Chem. 1969 Sep 25;244(18):5020–5026. [PubMed] [Google Scholar]
- Tzagoloff A., Meagher P. Assesmbly of the mitochondrial membrane system. VI. Mitochondrial synthesis of subunit proteins of the rutamycin-sensitive adenosine triphosphatase. J Biol Chem. 1972 Jan 25;247(2):594–603. [PubMed] [Google Scholar]
- Tzagoloff A., Rubin M. S., Sierra M. F. Biosynthesis of mitochondrial enzymes. Biochim Biophys Acta. 1973 Feb 12;301(1):71–104. doi: 10.1016/0304-4173(73)90013-x. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Bunn C. L., Eisenstadt J. M. Cytoplasmic transfer of chloramphenicol resistance in human tissue culture cells. J Cell Biol. 1975 Oct;67(1):174–188. doi: 10.1083/jcb.67.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]



