Abstract
Objective:
To analyse discrepant breast cancer detection in digital breast tomosynthesis (DBT) and digital mammography (DM).
Methods:
From a previous detection study comparing DBT and DM, 26 discrepant cases were extracted, 19 detected by DBT only and 7 by DM only. An expert panel of three radiologists reviewed these cases and documented the level of discrepancy, lesion visibility, radiographic pattern and lesion conspicuity and assessed the reason for non-detection. Differences between groups were tested using the Wilcoxon rank sum test, the Kruskal–Wallis test and visual grading characteristics.
Results:
The proportion of lesion periphery in fatty tissue was statistically significantly larger, and there were significantly more spiculated masses in DBT compared with DM in the DBT only group (p = 0.018; p = 0.015). The main reasons for missing a lesion were poor lesion visibility when using DM and interpretative error when using DBT.
Conclusion:
Lesion visualization is superior with DBT, particularly of spiculated tumours. A major reason for non-detection in DBT seems to be interpretative error, which may be due to lack of experience.
Advances in knowledge:
Our findings suggest that DBT is better than DM in visualizing breast cancer and that non-detection when using DBT is related to interpretative error regarding clearly visible lesions.
Although digital mammography (DM) is the standard technique for imaging examination of symptomatic females, as well as for screening, it is a well-established fact that the technique has important limitations in terms of breast cancer detection, especially in dense breasts, where the sensitivity has been reported as being as low as 30–60%.1,2 The main reasons are the obscuring effect of fibroglandular tissue and certain cancer growth patterns, for example, invasive lobular carcinoma (ILC) that sometimes grows diffusely in the breast in a single-file pattern and produces little desmoplastic response.3 In recent years, digital breast tomosynthesis (DBT) has developed into a promising three-dimensional (3D) breast-imaging technique that takes advantage of multiple exposures at different angles, which enables reconstruction of thin slices into a 3D volume and reduces the degrading effect of superimposed tissue.4,5 Data indicate that DBT is an important adjunct to conventional DM, as well as being a promising screening modality, with about 30% higher cancer detection rate than that of ordinary screening, when read in combination with DM.5–8
In an experimental clinical series in our institution, comparing the accuracy of one-view DBT with that of two-view DM, sensitivities of approximately 90% and approximately 79%, respectively, for cancer detection were found.9 In brief, the study included 185 symptomatic or asymptomatic females with subtle or negative findings on DM, but suspicious lesions on ultrasonography, yielding 89 females with 95 cancer lesions and 96 females with normal or benign findings. The females underwent standard assessment and one-view DBT. Five breast radiologists interpreted DBT and DM images independently in accordance with free-response receiver operating characteristic methodology,10 classifying findings in accordance with the American College of Radiology's Breast Imaging Reporting and Data System (BI-RADS).11 Cases of discrepant detection in DBT and DM form the basis of the current study.
When introducing a new diagnostic method, it is important to evaluate not only its accuracy but also to define its advantages and limitations in terms of imaging characteristics.12 The aim of this study was to reassess possible reasons for discrepant breast cancer detection in DBT vs DM by analysing detectability parameters and radiographic lesion characteristics, with the DBT and DM images displayed side by side.
METHODS AND MATERIALS
Study population
Discrepant cases were obtained from a previous detection study comparing one-view DBT and two-view DM, described above and in more detail elsewhere.9 A discrepant case had a cancer lesion classified as BI-RADS 3, 4 or 5 by at least two out of five readers in one modality and BI-RADS 1 or 2 in the other modality by the same two readers.
For 24 females with 25 cancer lesions in 24 breasts there had been discrepant interpretations in DBT vs DM, leading to a total of 26 discrepant cases (Table 1). 19 lesions were classified as BI-RADS 3, 4 or 5 in DBT and BI-RADS 1 or 2 in DM (DBT only group). Of these cancers, 18 were invasive (ductal carcinoma, n = 8; lobular carcinoma, n = 9; and tubular carcinoma, n = 1), and the mean age of the females were 63 years (range, 43–80 years).
Table 1.
Study population characteristics
| Parameter | DBT Only group | DM Only group |
|---|---|---|
| Mean age (range) (years) | 63 (43–80) | 62 (50–70) |
| Mean tumour size (range) (mm) | 19 (7–90) | 27 (9–90) |
| Lesion type, n (%) | ||
| Invasive ductal carcinoma | 8 (42) | 2 (29) |
| Invasive lobular carcinoma | 9 (47) | 3 (43) |
| Invasive tubular carcinoma | 1 (5) | 1 (14) |
| Ductal carcinoma in situ | 0 | 1 (14) |
| Others | 1 (5)a | 0 |
| Total | 19 (100) | 7 (100) |
| Histological grade of invasive cancers, n (%) | ||
| Grade 1 | 7 (39)b | 4 (67) |
| Grade 2 | 7 (39) | 2 (33) |
| Grade 3 | 4 (22) | 0 |
| Total | 18 (100) | 6 (100) |
DBT, digital breast tomosynthesis; DM, digital mammography.
Intracystic papillary cancer.
Of the patients with histological grade 1 cancers, four were symptomatic and three asymptomatic.
Seven lesions were classified as BI-RADS 3, 4 or 5 in DM and BI-RADS 1 or 2 in DBT (DM only group). Of these cancers, six were invasive (ductal carcinoma, n = 2; lobular carcinoma, n = 3; and tubular carcinoma, n = 1), and the mean age of the females were 62 years (range, 50–70 years). A case of ILC was represented in both discrepant groups. One female was represented in both discrepant groups with two different lesions (multifocal ILC). Of the 26 discrepant cases, there were no benign lesions.
Image display
The graphical user interface ViewDEX13 was used to display the DBT and DM images side by side on a Sun Microsystems Ultra™ 24 Workstation (Oracle America Inc., Santa Clara, CA) using two 5-megapixel flat-panel monitors (SMD21500; EIZO GmbH, Karlsruhe, Germany), calibrated in accordance with Digital Imaging and Communications in Medicine Part 14. The images were reviewed under low ambient light conditions (<50 lux).
Discrepancy analysis
An expert panel comprising two breast radiologists with 7 and 38 years' experience of mammography, respectively, and one resident in radiology reassessed discrepant cases by consensus, with the DM and DBT images displayed side by side. The expert panel were aware that they were reviewing verified abnormal cases and localized the lesions in the paired DBT and DM images for each case-specific evaluation. The histopathology results were available during the review process. The DM views were scored separately and the highest-ranking DM view was used for statistical analysis.
The discrepancy analysis included the following steps:
• The level of discrepancy was measured as the difference between the number of readers who classified a lesion as BI-RADS 3, 4 or 5 in one modality and the number of readers who classified the same lesion as BI-RADS 1 or 2 in the other modality.
• Lesion visibility was evaluated by classifying breast density using DM in accordance with BI-RADS:14 (1) fatty, (2) scattered fibroglandular densities, (3) heterogeneously dense and (4) dense. The proportion of lesion periphery in tissue with pre-dominately fatty attenuation (in DBT and DM), was estimated using the following four categories: (1) <25%; (2) 25–50%; (3) 50–75%; and (4) >75%, as illustrated in Figure 1.
• The radiographic pattern of the lesion was assigned to one of the following six groups: (1) spiculated mass, (2) well-circumscribed mass, (3) mass with indistinct margins, (4) architectural distortion, (5) microcalcifications and (6) no findings, which for DM meant no findings in either view.
• Lesion conspicuity was graded as (1) not visible, (2) subtle, (3) intermediate and (4) high.
• Finally, the expert panel tried to find a major reason for the discrepancy, describing the reason for misclassification of a lesion in one modality, the evaluation being similar to that used by Lewin et al.14 They used three major reason categories: (1) problems related to poor lesion visibility; (2) non-conspicuous radiographic appearance (e.g. lack of edge characteristics and non-conspicuous radiographic pattern); and (3) interpretative error regarding visible lesions.
Figure 1.
Four examples of breast cancers bordering on tissues with different density. Inserts show estimated proportion of lesion periphery bordering on tissue with pre-dominately fatty attenuation: (a) <25%, (b) 25–50%, (c) 50–75% and (d) >75%.
Statistical analysis
Differences between the discrepant groups were analysed using the Wilcoxon rank sum test when the samples were related (lesion periphery in tissue with pre-dominately fatty attenuation and radiographic pattern) and the Kruskal–Wallis test when the samples were independent (breast density), and using the independent samples test when analysing the level of discrepancy. Lesion conspicuity was analysed by using visual grading characteristics (VGC), a non-parametric rank-invariant statistical method for image quality evaluation,15 comparing DBT and the highest-ranking DM view. Statistical analysis was performed using SPSS® Statistics software v. 20.0.0 (IBM Corporation, Armont, NY).
RESULTS
Level of discrepancy
The level of discrepancy was significantly higher in the DBT only group than in the DM only group (p = 0.045), meaning there was a greater difference between the number of readers who classified a lesion as BI-RADS 3, 4 or 5 in DBT and how many readers classified the same lesion as BI-RADS 1 or 2 in DM.
Lesion visibility
There was no significant difference in breast density between the discrepant groups. Although the numbers were small in the DM only group, there was a trend towards higher breast density (BI-RADS categories 3 and 4) in the DBT only group, with 11 vs 2 cases. Notably, there were also two cases of fatty breasts in the DBT only group (Table 2). The discrepant cancers in the DBT only group had a significantly larger proportion of lesion periphery in tissue with pre-dominately fatty attenuation in DBT compared with concurrent DM (p = 0.018). The discrepant cancers in the DM only group displayed the same proportion of lesion periphery in tissue with pre-dominately fatty attenuation in DBT and DM (except for one case with a higher proportion of visible lesion periphery in DBT).
Table 2.
Lesion visibility of discrepant cancers, related to breast density and to lesion periphery in tissue with pre-dominately fatty attenuation in digital breast tomosynthesis (DBT) only group and digital mammography (DM) only group, respectively
| Breast density |
Periphery in fat |
||||||
|---|---|---|---|---|---|---|---|
| Breast density | DBT only group (n) | DM only group (n) | Periphery in fat | DBT only groupa |
DM only group |
||
| DBT (n) | DM (n) | DBT (n) | DM (n) | ||||
| Fatty | 2 | 0 | <25% | 3 | 8 | 1 | 1 |
| Scattered densities | 6 | 5 | 25–50% | 7 | 6 | 2 | 2 |
| Heterogeneously dense | 8 | 2 | 50–75% | 4 | 3 | 2 | 2 |
| Dense | 3 | 0 | >75% | 5 | 2 | 2 | 1 |
One case was excluded from the DM only group, owing to lack of a mass, and therefore had no definable lesion periphery (ductal carcinoma in situ).
Radiographic pattern
The most common radiographic cancer pattern revealed by DBT was a spiculated mass [ILC, n = 7; invasive ductal carcinoma, n = 5; and invasive tubular carcinoma, n = 1] (Table 3). Six out of seven spiculated tumours were grade 1, while grade 3 tumours presented as spiculated masses or masses with an indistinct border. Three of the four grade 3 tumours were not visible in DM. Typical cases in the DBT only group are illustrated in Figure 2, in which spiculated masses revealed by DBT are not visible on concurrent DM.
Table 3.
Radiographic pattern of cancers subject to discrepant detection in digital breast tomosynthesis (DBT) and concurrent digital mammography (DM) in the DBT only group and DM only group
| Radiographic pattern | DBT only group |
DM only group |
||
|---|---|---|---|---|
| DBT (n) | DM (n) | DBT (n) | DM (n) | |
| Spiculated mass | 13a | 2a | 5 | 5 |
| Indistinct mass | 3 | 2 | 0 | 0 |
| Well-circumscribed mass | 1 | 1 | 0 | 0 |
| Architectural distortion | 2 | 3 | 1 | 1 |
| Microcalcifications | 0 | 0 | 1 | 1 |
| No finding | 0 | 11 | 0 | 0 |
This difference was statistically significant (p = 0.0153).
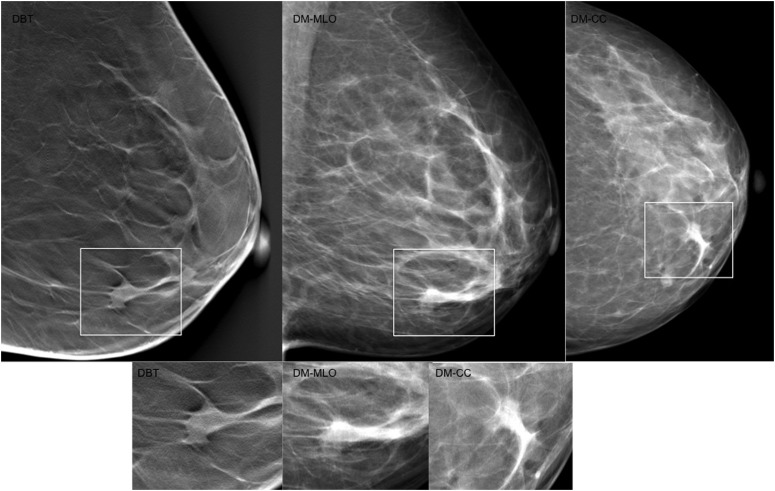
Figure 2.
Discrepant lesions classified as Breast Imaging Reporting and Data System (BI-RADS) 3, 4 or 5 by all readers in digital breast tomosynthesis (DBT) and classified as BI-RADS 1 or 2 by all readers in digital mammography (DM), with a radiographic pattern of a spiculated mass in DBT but no findings in concurrent DM. (a) A case of an symptomatic 76-year-old female with a 10-mm invasive lobular carcinoma, grade 2. (b) A case of an asymptomatic 49-year-old female with a 10-mm invasive tubular carcinoma, grade 1. (c) A case of a symptomatic 66-year-old female with a 15-mm invasive ductal carcinoma, grade 1. (d) A case of a 45-year-old female with a palpable mass, in whom histology showed an 18-mm invasive ductal carcinoma, grade 1.
The discrepant cancers in the DM only group displayed similar radiographic patterns in DBT and DM. Of the seven discrepant cases in the DM only group, five were spiculated masses (ILC, n = 2; invasive ductal carcinoma, n = 2; and invasive tubular carcinoma, n = 1) and the remaining two presented as architectural distortion (ILC, n = 1) and microcalcifications (ductal carcinoma in situ, n = 1), respectively. The latter was the only case of microcalcifications in all the discrepant cases. Retrospectively, it was deemed to be equally visible in both DM and DBT (Figure 3a).
Figure 3.
Cancers classified as Breast Imaging Reporting and Data System (BI-RADS) 3, 4 or 5 in digital mammography (DM) and classified as BI-RADS 1 or 2 in digital breast tomosynthesis (DBT) by at least two readers, most likely because of interpretative error. (a) A case of a 59-year-old female with a cluster of microcalcifications in DBT and DM, classified as BI-RADS 3, 4 or 5 by four readers in DM and classified as BI-RADS 1 or 2 by two readers in DBT. Histology showed a 45 × 25-mm ductal carcinoma in situ, grade 3. (b) A case of a 63-year-old female with a spiculated lesion in DBT and DM, classified as BI-RADS 3, 4 or 5 by all readers in DM and classified as BI-RADS 1 or 2 by two readers in DBT. The lesion was deemed to be less conspicuous in DBT than in DM, but still clearly visible. Microscopy revealed a 10-mm invasive tubular carcinoma, grade 1. (c) A case of a 66-year-old female with a spiculated, retromamillary lesion in DBT and DM craniocaudal (CC) view, classified as BI-RADS 3, 4 or 5 by all readers in DM and classified as BI-RADS 1 or 2 by two readers in DBT. Microscopy revealed a 10-mm invasive ductal carcinoma, grade 2. (d) A case of a 50-year-old female with architectural distortion in DBT and DM mediolateral oblique view, but without any findings in the CC view, classified as BI-RADS 1 or 2 by three readers in both DBT and DM. Histology showed a large multicentric invasive lobular carcinoma within two areas (90 × 40 mm and 10 × 5 mm). MLO, mediolateral oblique.
Lesion conspicuity
Visual grading analysis [area under the VGC curve (AUCVGC)] showed that lesions appeared more conspicuous in DBT compared with DM (AUCVGC = 0.083; confidence interval, 0.73–0.93). Of all the discrepant cancers, there was only one that was deemed to be more conspicuous in the DM image than in the DBT image, especially in the craniocaudal view (Figure 3b).
Major reason for discrepancy
As shown in Table 4, the main reason for missing a cancer in DM was related to poor lesion visibility, which in turn was caused by tissue overlap. On the other hand, the main reason for missing a cancer in DBT seemed to be related to interpretative error, since all lesions were clearly visible in DBT, as illustrated in the four cases in Figure 3. Only one case in the DM only group was missed because of a non-characteristic radiographic appearance in DBT (Figure 4). No lesions were missed in DBT as a result of poor lesion visibility. One case—the largest of the discrepant cancers—was represented in both discrepant groups, but for different reasons. With DM the reason for missing the lesion was poor visibility, whereas with DBT the probable reason was interpretative error (Figure 3d).
Table 4.
Number of discrepant cancers and probable reasons for misclassification in digital breast tomosynthesis (DBT) and digital mammography (DM)
| Reason for misclassification | Classified as BI-RADS 3, 4 or 5 in DBT and as BI-RADS 1 or 2 in DM (DBT only group) | Classified as BI-RADS 3, 4 or 5 in DM and as BI-RADS 1 or 2 in DBT (DM only group) |
|---|---|---|
| Visibility | 13 (ILC, n = 6; IDC, n = 6; Tub, n = 1) | 0 |
| Radiographic appearance | 3 (ILC, n = 1; IDC, n = 2) | 1 (IDC) |
| Interpretative error | 3 (ILC, n = 2; intracystic papillary cancer, n = 1) | 6 (ILC, n = 3; IDC, n = 1; Tub = 1; ductal carcinoma in situ, n = 1) |
BI-RADS, Breast Imaging Reporting and Data System; IDC, invasive ductal carcinoma; ILC, invasive lobular carcinoma; Tub, invasive tubular carcinoma.
Figure 4.
A 62-year-old female with a spiculated lesion in digital breast tomosynthesis (DBT) and digital mammography (DM) images, classified as Breast Imaging Reporting and Data System (BI-RADS) 3, 4 or 5 by all readers in DM but classified as BI-RADS 1 or 2 by four readers in DBT, most likely because of uncharacteristic radiographic appearance. Histology showed a 9-mm invasive ductal carcinoma, grade 1. It was deemed to be equally conspicuous in both DM and DBT, but of all the discrepant cases, it was still the most difficult cancer to detect in DBT. This could be explained by the perilesional breast parenchyma, which was organized in coarse bundles, making it more difficult for the lesion to stand out from the background. CC, craniocaudal; MLO, mediolateral oblique.
DISCUSSION
In a side-by-side comparison of DBT and concurrent DM, we analysed cancers that had been classified as BI-RADS 3, 4 or 5 by at least two out of five readers in one modality and BI-RADS 1 or 2 in the other modality by the same two readers. We found that the main reason for missing a cancer in DM was poor visibility due to dense breast parenchyma, tissue overlap and a radiographically non-conspicuous lesion. The main reason for missing a cancer in a DBT image was interpretative error.
We quantified lesion visualization by using several parameters, for example, proportion of lesion periphery in tissue with pre-dominately fatty attenuation and lesion conspicuity. The improved conspicuity in DBT was not only due to the reduction of tissue overlap, since lesions were also more conspicuous in fatty breasts. This can partly be due to the in-plane artefact, typically seen with the filtered back-projection reconstruction algorithm, generating a dark halo around lesions, thus enhancing the lesion-to-background contrast, resulting in increased lesion conspicuity (e.g. Figure 4).16
Does DM have any advantages over DBT? Of the 26 discrepant cases, there was only one lesion that was more conspicuous in the DM image (Figure 3b). The most difficult cancer to detect in DBT images was a 9-mm invasive ductal carcinoma grade 1, illustrated in Figure 4, which was missed by four readers in DBT and detected by all readers in DM, although it was retrospectively deemed to be equally conspicuous in both modalities. As suggested, this problem could be due to the surrounding parenchyma having a similar appearance, thus making the cancer less conspicuous.
ILC is often difficult to detect in DM.17,18 ILC is the second most common microscopic type of invasive breast carcinoma. In DM, an ILC may present as a spiculated tumour, but it may sometimes grow in single cell rows, diffusely infiltrating the tissue without forming a tumour mass, presenting radiographically as an area of architectural distortion or an area of asymmetry, and sometimes even being radiographically occult. In a recent study by Skaane et al,19 the sensitivity when using DBT was over 8% higher than when using DM, and the type of lesions that accounted for the increase was ILCs. In our study, DBT had advantages in terms of detection of ILCs, with a significantly larger proportion of ILCs in the DBT only group compared with the proportion of ILCs in the previous detection study, from which the discrepant detections were extracted (47% vs 20%; p = 0.025; χ2 test). In this study, the ILCs appeared mostly as spiculated masses in DBT, whereas in DM there were not usually any radiographic findings at all.
In the DM only group, all cancers were clearly visible in DBT, yet they had been missed. Failure to detect salient pathological features in radiographic images is an inevitable part of being a radiologist.20,21 This effect is reduced in breast cancer screening programmes by use of double reading. The discrepant cases in this study could have been missed in a double-reading setting, as they were missed by at least two readers. Hypothetically, analysing a 3D volume might induce a different search pattern from a top–down search instead of a more holistic salient driven bottom–up search. This, in addition to the more extensive set of images, could increase the constraint of interpreting 3D DBT volumes, thereby possibly leading to more interpretative errors. Interpretative error could also be related to the radiologist's level of experience. The DM experience of the five readers involved in the detection study from which we extracted the discrepant cases ranged from 3 to 25 years (mean, 16.6 years). In a recent publication by our group, it was suggested that optimum diagnostic performance in DBT requires experience in DM,22 while other studies have shown that DBT outperforms DM when readers have limited experience.23 In another study, no correlation was found between reader experience and performance when comparing DBT plus DM with DM.24 Thus far, there have been no studies analysing performance in terms of the reading of DBT images by readers who only have experience of DBT. We established that there was potential for improvement in the reading of DBT images, since the major reason for missing a cancer was interpretative error regarding clearly visible lesions.
A limitation of this study is the descriptive set-up of the discrepancy analysis, involving subjective reassessment of the images. Although the sample size was small, the extraction2 of discrepant cases was based on five radiologists reading and scoring DBT and DM images of 185 females (89 abnormal breasts with 95 malignant lesions). Also, this population was enriched with difficult cases, and the findings on DM were subtle or non-existent, giving DM an advantage over DBT. The use of enriched populations creates a logistic benefit when studying a disease with a low prevalence.25 However, the drawback is that the results are not entirely applicable to a screening population. Discrepancy studies from the screening trials with DBT will in a near future provide further information on this topic.
In conclusion, our study shows that DBT has advantages over DM in terms of detection of breast cancer, thanks to its better visualization of the lesions, particularly spiculated tumours. Missing a lesion in DM is mainly due to poor lesion visibility, caused not only by tissue overlap but also by the less conspicuous radiographic appearance of the lesions. Missing a lesion in DBT seems to be related to interpretative error regarding clearly visible lesions, a problem that may be reduced with increased experience.
FUNDING
The study was part financed by the Swedish Research Council and Lund University, Medical Faculty (ALF grant).
REFERENCES
- 1.Mandelson MT, Oestreicher N, Porter PL, White D, Finder CA, Taplin SH, et al. Breast density as a predictor of mammographic detection: comparison of interval- and screen-detected cancers. J Natl Cancer Inst 2000; 92: 1081–7. [DOI] [PubMed] [Google Scholar]
- 2.Carney PA, Miglioretti DL, Yankaskas BC, Kerlikowske K, Rosenberg R, Rutter CM, et al. Individual and combined effects of age, breast density, and hormone replacement therapy use on the accuracy of screening mammography. Ann Intern Med 2003; 138: 168–75. [DOI] [PubMed] [Google Scholar]
- 3.Lopez JK, Bassett LW. Invasive lobular carcinoma of the breast: spectrum of mammographic, US, and MR imaging findings. Radiographics 2009; 29: 165–76. doi: 10.1148/rg.291085100 [DOI] [PubMed] [Google Scholar]
- 4.Niklason LT, Christian BT, Niklason LE, Kopans DB, Castleberry DE, Opsahl-Ong BH, et al. Digital tomosynthesis in breast imaging. Radiology 1997; 205: 399–406. doi: 10.1148/radiology.205.2.9356620 [DOI] [PubMed] [Google Scholar]
- 5.Baker JA, Lo JY. Breast tomosynthesis: state-of-the-art and review of the literature. Acad Radiol 2011; 18: 1298–310. doi: 10.1016/j.acra.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 6.Houssami N, Skaane P. Overview of the evidence on digital breast tomosynthesis in breast cancer detection. Breast 2013; 22: 101–8. doi: 10.1016/j.breast.2013.01.017 [DOI] [PubMed] [Google Scholar]
- 7.Skaane P, Bandos AI, Gullien R, Eben EB, Ekseth U, Haakenaasen U, et al. Comparison of digital mammography alone and digital mammography plus tomosynthesis in a population-based screening program. Radiology 2013; 267: 47–56. [DOI] [PubMed] [Google Scholar]
- 8.Ciatto S, Houssami N, Bernardi D, Caumo F, Pellegrini M, Brunelli S, et al. Integration of 3D digital mammography with tomosynthesis for population breast-cancer screening (STORM): a prospective comparison study. Lancet Oncol 2013; 14: 583–9. doi: 10.1016/S1470-2045(13)70134-7 [DOI] [PubMed] [Google Scholar]
- 9.Svahn TM, Chakraborty DP, Ikeda D, Zackrisson S, Do Y, Mattsson S, et al. Breast tomosynthesis and digital mammography: a comparison of diagnostic accuracy. Br J Radiol 2012; 85: e1074–82. doi: 10.1259/bjr/53282892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chakraborty DP, Winter LH. Free-response methodology: alternate analysis and a new observer-performance experiment. Radiology 1990; 174: 873–81. doi: 10.1148/radiology.174.3.2305073 [DOI] [PubMed] [Google Scholar]
- 11.D'Orsi CJ SE, Mendelson EB, Morris EA. ACR BI-RADS® Atlas, Breast Imaging Reporting and Data System. Reston, VA: American College of Radiology; 2013. [Google Scholar]
- 12.Kopans DB. Digital breast tomosynthesis: a better mammogram. Radiology 2013; 267: 968–9. doi: 10.1148/radiol.13130086 [DOI] [PubMed] [Google Scholar]
- 13.Håkansson M, Svensson S, Zachrisson S, Svalkvist A, Båth M, Månsson LG. ViewDEX 2.0: a Java-based DICOM-compatible software for observer performance studies. Proc SPIE 2009; 7263: 72631G1–72631G10. [DOI] [PubMed] [Google Scholar]
- 14.Lewin JM, D'Orsi CJ, Hendrick RE, Moss LJ, Isaacs PK, Karellas A, et al. Clinical comparison of full-field digital mammography and screen-film mammography for detection of breast cancer. AJR Am J Roentgenol 2002; 179: 671–7. doi: 10.2214/ajr.179.3.1790671 [DOI] [PubMed] [Google Scholar]
- 15.Bath M, Mansson LG. Visual grading characteristics (VGC) analysis: a non-parametric rank-invariant statistical method for image quality evaluation. Br J Radiol 2007; 80: 169–76. [DOI] [PubMed] [Google Scholar]
- 16.Tingberg A. X-ray tomosynthesis: a review of its use for breast and chest imaging. Radiat Prot Dosimetry 2010; 139: 100–7. doi: 10.1093/rpd/ncq099 [DOI] [PubMed] [Google Scholar]
- 17.Andersson I. Invasive breast cancer. In: Radiologic–pathologic correlations from head to toe: understanding the manifestations of disease. Berlin: Springer-Verlag; 2005. [Google Scholar]
- 18.Garnett S, Wallis M, Morgan G. Do screen-detected lobular and ductal carcinoma present with different mammographic features? Br J Radiol 2009; 82: 20–7. doi: 10.1259/bjr/52846080 [DOI] [PubMed] [Google Scholar]
- 19.Skaane P, Gullien R, Bjørndal H, Eben EB, Ekseth U, Haakenaasen U, et al. Digital breast tomosynthesis (DBT): initial experience in a clinical setting. Acta Radiol 2012; 53: 524–9. doi: 10.1258/ar.2012.120062 [DOI] [PubMed] [Google Scholar]
- 20.Berlin L. Accuracy of diagnostic procedures: has it improved over the past five decades? AJR Am J Roentgenol 2007; 188: 1173–8. doi: 10.2214/AJR.06.1270 [DOI] [PubMed] [Google Scholar]
- 21.Mello-Thoms C, Dunn S, Nodine CF, Kundel HL, Weinstein SP. The perception of breast cancer: what differentiates missed from reported cancers in mammography? Acad Radiol 2002; 9: 1004–12. [DOI] [PubMed] [Google Scholar]
- 22.Svahn T, Lång K, Andersson I, Zackrisson S. Differences in radiologists' experiences and performance in breast tomosynthesis. In: Maidment A, ed. Proceedings of the 11th International Workshop, IWDM; 8–11 July 2012. Philadelphia, PA: Springer Berlin Heidelberg; 2012. pp. 377–85. [Google Scholar]
- 23.Wallis MG, Moa E, Zanca F, Leifland K, Danielsson M. Two-view and single-view tomosynthesis versus full-field digital mammography: high-resolution X-ray imaging observer study. Radiology 2012; 262: 788–96. doi: 10.1148/radiol.11103514 [DOI] [PubMed] [Google Scholar]
- 24.Andrew P, Smith ER, Niklason L. Clinical performance of breast tomosynthesis as a function of radiologist experience level. In: Krupinski EA, ed. Proceedings of the 9th International Workshop, IWDM 2008; 20–23 July 2008. Tucson, AZ: Springer Berlin Heidelberg, 2008. pp. 61–6. [Google Scholar]
- 25.Pinsky PF, Gallas B. Enriched designs for assessing discriminatory performance—analysis of bias and variance. Stat Med 2012; 31: 501–15. [DOI] [PubMed] [Google Scholar]