Abstract
Background
Preliminary evidence suggests intravenous ketamine has rapid effects on suicidal cognition, making it an attractive candidate for depressed patients at imminent risk of suicide. In the first randomized controlled trial of ketamine using an anesthetic control condition, we tested ketamine’s acute effects on explicit suicidal cognition and a performance-based index of implicit suicidal cognition (Implicit Association Test; IAT) previously linked to suicidal behavior.
Method
Symptomatic patients with treatment-resistant unipolar major depression (inadequate response to ≥3 antidepressants) were assessed using a composite index of explicit suicidal ideation (Beck Scale for Suicidal Ideation, Montgomery-Asberg Rating Scale suicide item, Quick Inventory of Depressive Symptoms suicide item) and the IAT to assess suicidality implicitly. Measures were taken at baseline and 24 hr following a single subanesthetic dose of ketamine (n = 36) or midazolam (n = 21), a psychoactive placebo agent selected for its similar, rapid anesthetic effects. Twenty four hours postinfusion, explicit suicidal cognition was significantly reduced in the ketamine but not the midazolam group.
Results
Fifty three percent of ketamine-treated patients scored zero on all three explicit suicide measures at 24 hr, compared with 24% of the midazolam group (χ2 = 4.6; P = .03). Implicit associations between self- and escape-related words were reduced following ketamine (P = .01; d = .58) but not midazolam (P = .68; d = .09). Ketamine-specific decreases in explicit suicidal cognition were largest in patients with elevated suicidal cognition at baseline, and were mediated by decreases in nonsuicide-related depressive symptoms.
Conclusions
Intravenous ketamine produces rapid reductions in suicidal cognition over and above active placebo. Further study is warranted to test ketamine’s antisuicidal effects in higher-risk samples.
Keywords: suicide/self-harm, depression, clinical trials, biological markers, mood disorders, antidepressants
INTRODUCTION
Suicide is a leading cause of death and injury worldwide.[1, 2] Currently, clinicians’ options are limited when faced with an acutely suicidal patient. Conventional treatments including antidepressants,[3] cognitive therapy,[4] and electroconvulsive therapy,[5] are effective but slow to act, leaving a substantial number of patients struggling with suicidal thoughts after weeks of treatment. Even inpatient hospitalization fails to prevent suicide in as many as 1,500 cases per year,[6] and there is an additional high risk of suicide during the period immediately following hospital discharge.[7] Incidence rates for suicidal behaviors remain largely unchanged over the last decade and a half in spite of increasing access to treatment,[1] highlighting the need for novel treatment approaches.
Ketamine, a glutamate N-methyl-d-aspartate (NMDA) receptor antagonist used more frequently as an anesthetic agent, has shown rapid antidepressant properties in patients with depression[8] and treatment-resistant depression (TRD),[9–11] defined as insufficient response to at least two adequate antidepressant trials. In an open study of ketamine in TRD, we previously reported reduced clinician-rated explicit suicidal cognition 24 hr after ketamine infusion.[12] Furthermore, suicidal cognition was continuously eradicated for 2 weeks in ketamine responders given thrice-weekly, repeat infusions. Similar additional reports in TRD,[13] bipolar disorder,[14] and depressed patients presenting to the emergency department with suicidal ideation[15] suggest that ketamine can reduce suicidal ideation within 40 min of infusion and reductions may be maintained for up to 10 days.
Effective treatment of suicidality is further constrained by inaccuracy of standard risk assessments. For example, 78% of inpatients going on to complete suicide explicitly denied suicidal ideation in their last communication to clinicians.[16] The Implicit Association Test (IAT; see Supporting Information for details) is a performance-based measure of cognition that is reliable,[17] resistant to attempts to “fake good,”[18] and has been shown to predict future behavior more accurately than explicit measures across a range of socially “taboo” cognitions (e.g., prejudicial attitudes).[19] Variants of the IAT developed to assess suicidal cognition have been shown to differentiate between suicidal and nonsuicidal individuals.[20,21] Furthermore, when assessed in the emergency department, the IAT predicts a sixfold increase in the odds of making a subsequent suicide attempt within the following 6 months, exceeding the predictive validity of other well-established risk factors as well as both patients’ and clinicians’ explicit predictions.[21] In our previous open-label trial, we reported that an IAT index of suicidal cognition was reduced 24 hr postketamine infusion.[12] The IAT may therefore offer a clinically relevant performance-based assessment of suicidal cognition that is both predictive of future behavior and sensitive to treatment change, although it has not previously been studied in controlled trials.
Given that major depression is associated with high placebo-responsiveness,[22] the current study aimed to provide a critical extension of previous findings through inclusion of a psychoactive placebo arm, using a randomized, controlled, double-blind design. While this psychoactive placebo-controlled design adds novel information to the literature on ketamine’s antisuicidal effects, the use of a placebo in patients at imminent risk of suicide presents ethical concerns. We therefore conducted this first actively controlled examination in the context of a TRD sample with heterogeneous levels of suicidal cognition, excluding individuals at imminent risk. We aimed to assess the differential effects of ketamine versus midazolam—a benzodiazepine anesthetic agent with no established antidepressant properties, selected to elicit transient psychoactive effects on a similar time course to ketamine—on explicit measures of suicidality and the IAT. Establishing ketamine-specific effects in the context of an RCT improves on previous designs by helping to isolate ketamine’s specific therapeutic benefit from the role of expectancy and practice effects on implicit and explicit suicide measures. To further the goal of personalized, mechanistic intervention for suicidality we also provide the first placebo-controlled exploration of ketamine-specific (1) predictors and (2) mechanisms of antisuicidal effects. Analyses were designed to explore novel moderators and mediators of ketamine’s antisuicidal properties from a range of variables previously associated with suicide risk (depression, hopelessness, state anxiety, implicit suicidal cognition) and ketamine-specific cognitive effects (dissociative symptoms, euphoric mood)[23] which might reflect glutamate-mediated psychological target engagement during the acute infusion period.
METHODS
TRD patients were recruited for a two-site double-blind randomized controlled trial (RCT) of ketamine’s antidepressant efficacy, described in detail elsewhere.[11] The current manuscript’s suicidality indices were included a priori as outcomes based on prior work.[12] Treatment resistance was defined as three or more failed, adequate antidepressant trials, as determined by the Antidepressant Treatment History Form.[24] DSM-IV-TR diagnoses of Major Depressive Disorder were established by SCID-I/P interview. Eligible participants were outpatients with moderate-to-severe depression (clinician-rated Inventory of Depressive Symptomatology score ≥32)[25] who were psychotropic medication-free for ≥1 week prior to infusion (4 weeks for fluoxetine). Patients whom research team psychiatrists deemed unsafe for study participation due to serious and imminent suicidality were excluded.
Additional inclusion criteria, detailed methods, antidepressant efficacy findings, and adverse events are described elsewhere.[11] Briefly, following an overnight hospital stay, patients were randomized in a 2:1 ratio to receive a single IV infusion of ketamine hydrochloride (0.5 mg/kg) or midazolam (0.045 mg/kg) over 40 min, followed by a second overnight hospital stay. Patients were assessed for depressive symptoms using the Montgomery-Asberg Depression Rating Scale (MADRS),[26] a 10-item clinician-administered measure that includes a single suicidality item rated on a 0–6 scale, and the Quick Inventory of Depressive Symptomatology-Self Report (QIDS-SR),[27] a 16-item self-report measure of depression symptoms that includes a single suicidality item rated on a 0–3 scale.
Fifty-seven participants completed measures of explicit suicidal cognition described below (ketamine: n = 36; midazolam: n = 21) at both baseline (1–4 days prior to infusion; mode = 1 day) and 24 hr post infusion. Fifty-four participants (ketamine: n = 35; midazolam: n = 19) completed implicit measures.1 Suicide measures were not collected in all participants due to unavailability of equipment/time constraints (n = 15) or patient refusal (n = 3). Patients with and without suicide assessment data did not differ significantly on demographics or clinical history (Ps > .2) or on proportion allocated to ketamine versus midazolam (χ2 = .54, P = .46). Total baseline MADRS scores were slightly higher in the current subsample as compared to the full RCT sample (t70 = 2.61, P = .01).
Table 1 presents clinical, demographic, and suicide risk-related characteristics of the primary suicidality sample (n = 57). All participants signed informed consent. Institutional Review Boards approved all procedures.
TABLE 1.
Baseline descriptive and clinical characteristics of primary sample
| Ketamine group (n = 36) | Midazolam group (n = 21) | |
|---|---|---|
| Age, mean (SD), y | 48.6 (11.4) | 43.8 (10.9) |
| Female, No. (%) | 20 (56%) | 10 (48%) |
| Non-Hispanic Caucasian, No. (%) | 22 (61%) | 16 (76%) |
| Mini mental status examination, mean (SD) | 29.6 (0.9) | 29.6 (0.5) |
| Median education level, y | 14 | 16 |
| Time since illness onset, mean (SD), y | 24.3 (12.4) | 21.5 (15.3) |
| Age-of-onset, mean (SD), y | 22.9 (9.6) | 20.4 (10.4) |
| Number of episodes, mean (SD) | 1.9 (1.7) | 2.3 (2.1) |
| Duration of current episode, mean (SD), y | 11.6 (14.3) | 7.8 (8.8) |
| Number of failed antidepressant trials in current episode, mean (SD) | 5.1 (1.9) | 5.4 (1.9) |
| History of suicide attempts, No. (%) | 10 (28%) | 8 (38%) |
| MADRS total score (baseline), mean (SD) | 33.3 (5.6) | 32.4 (4.8) |
| QIDS total score (baseline), mean (SD) | 16.6 (4.2) | 16.5 (4.7) |
| BSS, mean (SD) | 6.1 (6.8) | 6.2 (6.7) |
| BSS score ≥ 4, No. (%) | 19 (52.8%) | 11 (52.4%) |
| All suicidal indices = 0, No. (%) | 4 (11.1%) | 0 (0%) |
Note: Groups do not differ significantly on any variable according to unpaired t-tests (continuous variables; Ps > .13) and χ2 tests (categorical variables; Ps > .25). MADRS, Montgomery-Asberg depression rating scale (16); QIDS, quick inventory of depressive symptoms (self-report); BSS, Beck scale for suicide ideation; “All suicidal indices” includes MADRS and QIDS suicide items and BSS.
EXPLICIT MEASURES
The Beck Scale for Suicide Ideation (BSS), [28] a 21-item self-report measure that is reliable and highly correlated (r> = .90) with the clinician-rated Scale for Suicide Ideation,[29] was administered as the primary explicit suicidal cognition measure (summing items 1–19). To reduce Type I error while preserving power, a composite explicit suicidality index (SIcomposite) was calculated by summing z-scores on the BSS, MADRS suicidality item (MADRS-SI), and QIDS-SR suicidality item (QIDS-SI), as in previous work.[12]
IMPLICIT ASSOCIATION TEST (IAT)
Two variants of the IAT (IAT-Death, assessing the strength of association between words related to “Death” and “Me”; IAT-Escape, assessing associations between “Escape” and “Me”; see Supporting Information) were selected based on our previous findings and the hypothesis that individuals contemplating suicide would be characterized by greater self-identification with death (relative to life) and escape (relative to stay). Both associations are stronger in suicide attempters than nonattempters.[30] Briefly, the IAT assesses the relative speed with which individuals classify words when the same manual key response is required for two constructs (e.g., when “death” words and “me” words both require a right finger response, relative to when “life” words and “me” words both require a right finger response). “Escape = Me” and “Death = Me” D-scores were calculated for each participant, where D = [(mean RT during Escape = Me [or Death = Me] block) – (mean RT during Stay = Me [or Life = Me] block) ÷ (SD of RT across all trials)].
EXPLORATORY ANALYSIS: MODERATORS AND MEDIATORS
Potential moderators of the differential effects of ketamine versus midazolam on SIcomposite reduction included the following measures collected at baseline: SIcomposite, the MADRS total score after removing the suicidality item (MADRS-totalnonSI), history of suicide attempts (present vs. absent), and the two IAT D-scores.
Potential mediators of ketamine’s impact on suicidality included change scores in the following measures collected concurrently with SIcomposite (i.e., at baseline and 24 hr): MADRS-totalnonSI; the Beck Hopelessness Scale (BHS;[31] a widely used self-report instrument that has been shown to predict future suicidal acts;[32] the state form of the State-Trait Anxiety Inventory (STAI-S)[33] as a measure of anxious mood state, an acute predictor of suicidal behavior;[34] and IAT D-scores. Additionally, change scores from baseline to 40 min post infusion onset were used to assess behaviorally for potential glutamate-mediated, ketamine-specific “target engagement” (e.g., dissociative and/or euphoric feelings, which characterize ketamine but not midazolam infusion): the Clinician-Administered Dissociative States Scale (CADSS)[35] and a visual analogue scale (VAS) assessing “Euphoric” mood on a 0–10 scale. Finally, mediation by change scores in MADRS-totalnonSI as assessed at baseline and 4 hr post infusion was also explored to test temporal precedence of change in depression. Change scores for moderator/mediator analyses were calculated as 24-hr-baseline score.
STATISTICAL ANALYSIS
The primary outcome was the explicit SIcomposite score at 24 hr post infusion. Implicit outcomes were IAT Escape = Me and Death = Me D-scores. Twenty-four hour scores were compared across groups using ANCOVA with baseline values as a covariate. Due to positively skewed distributions on explicit measures, bootstrapping, an approach robust to violations of normality,[36] was used to derive two-tailed P-values for all analyses. χ2 tests were used to compare groups on categorical measures. Correlation analyses were used to explore relationships between explicit and implicit measures. Regression of SIcomposite change scores on interaction terms (group x moderator variable), controlling for the independent effects of group and the moderator variable, were used to identify baseline moderators differentially predicting change in explicit suicidality following ketamine versus midazolam. Mediation analyses were performed to identify measures statistically mediating the relationship between group and explicit suicidality reduction based on a standard 3-variable path model.[37] In this approach, M is considered a mediator of the relationship between X and Y if and only if: (1) X is related to Y (path c); (2) X is related to M (path a); (3) M is related to Y, controlling for X (path b); and (4) the effect of X on Y controlling for M is significantly different from the direct effect of X on Y (path a × b). Bootstrap tests were used to test the significance of each path using the Multilevel Mediation and Moderation Toolbox (http://wagerlab.colorado.edu/tools). For completeness, statistical trends at two-tailed P < .1 are reported in addition to significant effects (P < .05). Bonferonni correction was applied to all analyses of ketamin-eversus midazolam effects, but not to exploratory moderator/mediator analyses.
RESULTS
PRIMARY OUTCOMES
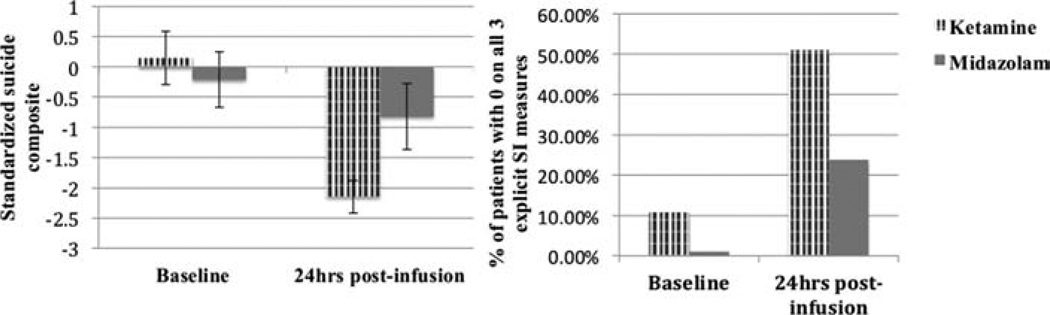
Tweny-four hours postinfusion, SIcomposite scores were reduced in the ketamine group compared to midazolam, adjusting for baseline values (F1,54 = 8.8, P = .01, between-groups Cohen’s d = 0.82, a large effect; Table 2; Fig. 1). Fifty-three percent of ketamine-treated patients scored zero on all three explicit suicide measures at 24 hr, compared with 24% of the midazolam group at 24 hr (χ2 = 4.6; P = .03) and 7% of all patients at baseline. 86.1% of ketamine-treated patients scored below a BSS score of 4 (sometimes considered a clinically meaningful cut-off[38]) at 24 hr compared to 61.9% of the midazolam group at 24 hr (χ2 = 4.4; P = .04) and 47.4% of all patients at baseline. In omnibus tests, there were no significant differential effects of ketamine versus midazolam on “Escape = Me” or “Death = Me” implicit associations (Ps > .5). However, replicating our previous finding, there was a decrease in “Escape = Me” associations from baseline to 24 hr postketamine (t34 = 2.5, P = .02, d = .58). This effect was not present in the midazolam group (P = 1.0; Table 2; between-groups d = .25).
TABLE 2.
Ketamine effects on explicit and implicit suicidality and related measures
| Ketamine (n = 36)a |
Midazolam (n = 21)a |
|||
|---|---|---|---|---|
| Measure | Mean (SD) | Within-groups effect size: Cohen’s d [95% CI] |
Mean (SD) | Within-groups effect size: Cohen’s d [95% CI] |
| SIcomposite | ||||
| Baseline | 0.15 (2.66) | d = 1.06 [0.57–1.55] | −0.21 (2.12) | d = 0.29 [0.01–0.57] |
| 24 hr post infusion | −2.23 (1.63)***§ | −0.91 (2.52)*§ | ||
| BSS | ||||
| Baseline | 6.11 (6.76) | d = 0.88 [0.45–1.30] | 6.19 (6.68) | d = 0.34 [0.05–0.63] |
| 24 hr post infusion | 1.13 (2.65)*** | 3.95 (6.46) | ||
| MADRS-SI | ||||
| Baseline | 1.61 (1.37) | d = 0.72 [0.27–1.17] | 1.48 (1.03) | d = 0.20 [−0.16 –0.58] |
| 24 hr post infusion | 0.72 (1.05)*** | 1.24 (1.26) | ||
| QIDS-SI | ||||
| Baseline | 0.97 (0.84) | d = 1.04 [0.56–1.51] | 0.76 (0.76) | d = 0.18 [−0.18–0.56] |
| 24 hr post infusion | 0.22 (0.54)*** | 0.62 (0.74) | ||
| BHS | ||||
| Baseline | 12.79 (4.92) | d = 0.90 [0.52–1.27] | 10.65 (5.85) | d = 0.49 [0.05–0.93] |
| 24 hr post infusion | 7.20 (6.48)*** | 7.65 (6.25)* | ||
| STAI-S | ||||
| Baseline | 58.97 (9.70) | d = 1.21 [0.74–1.69] | 57.50 (9.82) | d = 0.98 [0.37–1.60] |
| 24 hr post infusion | 41.91 (16.31)*** | 44.10 (15.93)*** | ||
| IAT: Escape = Me | ||||
| Baseline | −0.25 (0.40) | d = 0.58 [0.09–1.07] | −0.36 (0.34) | d = 0.09 [−0.47–0.66] |
| 24 hr post infusion | −0.48 (0.39)** | −0.39 (0.32) | ||
| IAT: Death = Me | ||||
| Baseline | −0.39 (0.45) | d = −0.06 [0.41–0.28] | −0.54 (0.47) | d = −0.37 [0.98–0.23] |
| 24 hr post infusion | −0.36 (0.48) | −0.39 (0.30) | ||
Note: SIcomposite, composite explicit suicidal ideation index; BSS, Beck scale for suicidal ideation (17); MADRS-SI = Montgomery-Asberg Depression Rating Scale (16) Suicidality Item; QIDS-SI, quick inventory of depressive symptoms suicidality item; BHS, Beck hopelessness scale; STAI-S, Spielberger state-trait anxiety inventory, state form; IAT, implicit association test (5).
Within-groups baseline and post-infusion scores compared by paired t-tests with bootstrapping significance test; for non-primary outcomes, multiple comparisons correction applied separately across explicit measures (5 measures) and implicit measures (2 measures); baseline-to-post infusion effect size (d) calculated from means, SDs, and pre-post correlation coefficients (20).
P ≤ .001 (primary outcome)
P < .05 (primary outcome)
P ≤ .002 unadjusted for multiple comparisons, P ≤ .01 adjusted
P = .01 unadjusted, P = .02 adjusted
P < .05 unadjusted, P < .25 adjusted.
Total n reduced to 54 for IAT, BHS, and STAI-S.
Figure 1.
Baseline and 24 hr post infusion scores (means with SEM) on the primary outcome measure (SIcomposite, standardized around baseline means) and percentage of patients scoring 0 on all three explicit suicide measures, as a function of intervention (ketamine vs. midazolam).
ASSOCIATIONS BETWEEN BASELINE IMPLICIT AND EXPLICIT MEASURES
At baseline, stronger “Escape = Me” implicit associations were associated with greater SIcomposite scores (r = .35; P = .006). Baseline “Death = Me” associations were not significantly associated with SIcomposite scores (r = .22; P = .10) or with “Escape = Me” scores (r = .21; P = .12).
EXPLORATORY ANALYSIS: MODERATORS OF OUTCOME
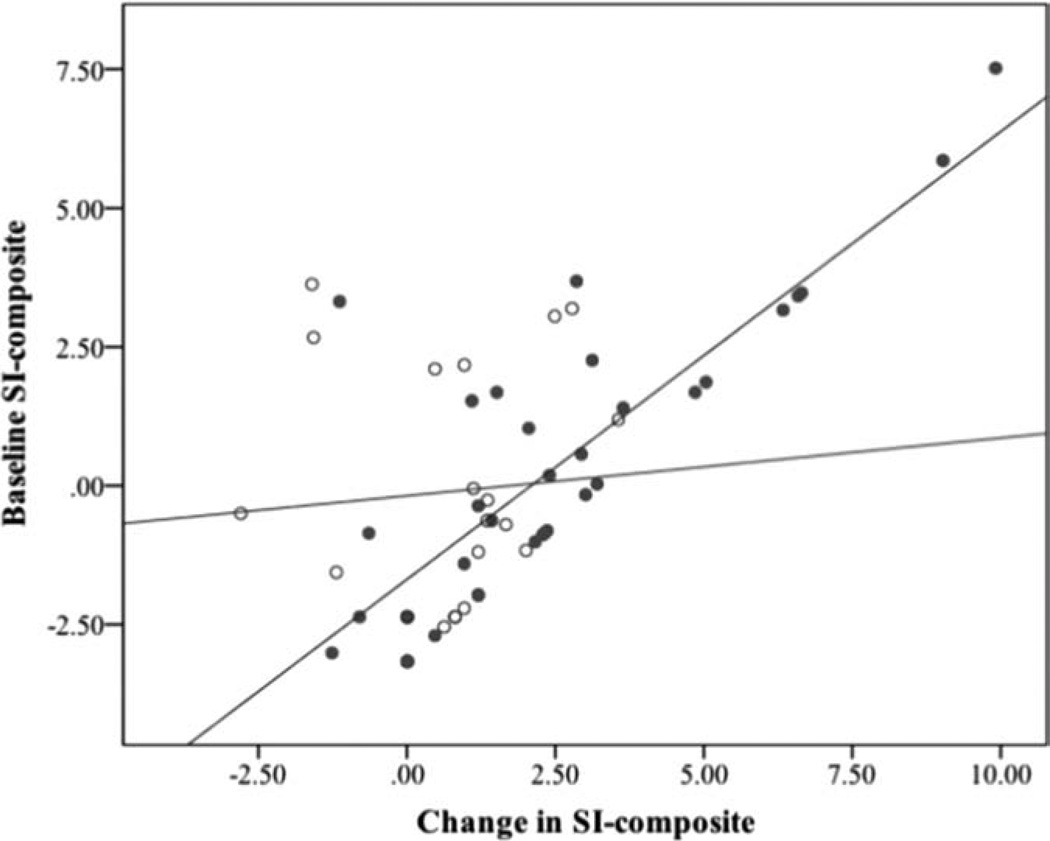
In regression analyses predicting change in SIcomposite, the differential effect of ketamine over midazolam was greatest for those with the highest baseline SIcomposite scores (baseline SIcomposite X group interaction: β = .66, P = .004; r = .82, P < .001 for baseline SIcomposite predicting change in SIcomposite in ketamine group; r = .08, P = .75 for baseline SIcomposite predicting change in SIcomposite in midazolam group; Fig. 2). By contrast, non-suicide-related depression symptom severity at baseline (MADRS-totalnonSI) did not moderate outcome nor predict SIcomposite change in either group (Ps > .21).
Figure 2.
Moderating effect of baseline SIcomposite scores on differential change in SIcomposite as a function of intervention group (ketamine vs. midazolam). Baseline SIcomposite scores explain 67% of variance in SIcomposite change in the ketamine group (blue/solid dots), and <1% of variance in the midazolam group (red/hollow circles), suggesting differential benefits of ketamine versus midazolam increase as baseline scores increase.
Similarly, history of suicide attempt differentially predicted larger decreases in SIcomposite in the ketamine but not the midazolam group (suicide attempt history X group interaction: β = .48, P = .02; r = .52, P = .001 for suicide attempt history predicting change in SIcomposite in ketamine group; r = .01, P = .99 for suicide attempt history predicting change in SIcomposite in midazolam group).
Larger implicit Escape = Me IAT D-scores also differentially predicted SIcomposite change in the ketamine but not the midazolam group (Escape = Me X group interaction: β = .43, P = .045; r = .38, P = .02 for baseline SIcomposite predicting change in SIcomposite in ketamine group; r = −.11, P = .66 for baseline SIcomposite predicting change in SIcomposite in midazolam group). Death = Me IAT D-scores also moderated outcomes, but showed an opposing pattern, inversely predicting outcome in the midazolam but not the ketamine group (Death = Me X group interaction: β = .37, P = .004; r = .14, P = .42 for baseline SIcomposite predicting change in SIcomposite in ketamine group; r = −.45, P = .05 for baseline SIcomposite predicting change in SIcomposite in midazolam group).
EXPLORATORY ANALYSIS: MEDIATORS OF OUTCOME
The relationship between group and change in SIcomposite was fully mediated by change in MADRS-totalnonSI score at both 4 hr and 24 hr (see Table 3 for all mediation statistics). Using change in VAS-Euphoric as a mediator, the mediation path was significant at the trend level. Change in hopelessness (BHS) was related to change in SIcomposite and marginally related to group, but the a × b mediation path was not significant. No other examined variables met any of the criteria for mediation (CADSS change, STAI-S change, IAT change).
TABLE 3.
Mediation statistics for mediators of relationship between treatment group and change in SIcomposite
| M: | Path a: Treatment->M | Path b: M->SIcomposite (controlling for treatment) | Path a ×b: Treatment->M-> SIcomposite |
|---|---|---|---|
| Measures collected concurrently with SIcomposite (baseline and 24 hr) | |||
| Change in MADRSnonSI | b = 8.8; t56 = 3.5; R2 = .18; P < 001 | b = .10; t53 = 3.6; R2 = .19; P < 001 | b = .90; t53 = 2.3; R2 = .09; P = .001 |
| Change in BHS | b = 2.6; t53 = 1.6; R2 = .11; P = .09 | b = .16; t53 = 3.0; R2 = .15; P = .002 | b = .43; t53 = 1.3; R2 = .03; P = .12 |
| Change in STAI-S | b = 3.7; t53 = .85; R2 = .01; P = .38 | b = .03; t53 = .88; R2 = .01; P = .42 | b = .16; t53 = .61; R2 = .01; P = .68 |
| Change in Escape = Me IAT | b = .13; t53 = 1.0; R2 = .02; P = .33 | b = .05; t53 = .09; R2 < .01; P = .96 | b = .01; t53 = .07; R2 < .01; P = .91 |
| Change in Death = Me IAT | b = .12; t53 = .76; R2 = .01; P = .44 | b = .58; t53 = .83; R2 = .01; P = .43 | b = .05; t53 = .34; R2 < .01; P = .31 |
| Measures temporally preceding change in SIcomposite | |||
| Change in MADRSnonSI baseline to 4 hr |
b = 6.1; t56 = 2.6; R2 = .11; P = .009 | b = .08; t56 = 2.1; R2 = .07; P = .02 | b = .48; t56 = 1.6; R2 = .04; P = .04 |
| Change in VAS-Euphoric: baseline to 40 min |
b = 2.2; t56 = 3.4; R2 = .17; P < 001 | b = .23; t56 = 2.0; R2 = .07; P = .06 | b = .52; t56 = 1.7; R2 = .05; P = .09 |
| Change in CADSS: baseline to 40 min |
b = 14.8; t56 = 5.9; R2 = .38; P < 001 | b = .04; t56 = .99; R2 = .02; P = .46 | b = .67; t56 = .98; R2 = .02; P = .49 |
Note: Paths with P < .05, unadjusted (derived via bootstrapping) appear in bold; paths with P < .10, unadjusted appear in bold italics; X = group (ketamine vs. midazolam), M = mediator (found in column 1), Y = change in composite suicidal ideation index (SIcomposite). Path c, which was held constant across all analyses (the effect of treatment group on change in SIcomposite), was also significant (b = 1.7; t56 = 2.6; P = .009); therefore mediation criteria are met when path’s a, b and a × b are all significant. MADRS-SI, Montgomery-Asberg Depression Rating Scale (16) Suicidality Item; MADRSnonSI, sum of all other MADRS items, sleep and appetite items carried forward from baseline; BHS, Beck hopelessness scale; STAI-S, Spielberger State-Trait Anxiety Inventory, State Form; IAT, implicit association test (5).
DISCUSSION
In the first RCT of ketamine in TRD to use a psychoactive placebo condition, ketamine-treated patients exhibited large, rapid reductions in explicit suicidal cognition, which were significantly greater than reductions observed in midazolam-treated patients. While only 7% of the TRD sample showed no evidence of suicidal cognition at baseline, ketamine eradicated all self-report and clinician-rated indications of suicidal ideation in 53% of patients, compared to 24% of patients receiving midazolam. Ketamine also reduced a range of secondary variables previously linked to increased risk of future suicidal acts, including implicit suicide-related associations[21] (specifically, Escape = Me associations), hopelessness,[32] and state anxiety,[34] although evidence for a specific benefit of ketamine over midazolam in these secondary analyses was mixed (Table 2), highlighting the importance of a controlled design in evaluating both antisuicidal efficacy and related mechanisms. Overall depression decrease mediated ketamine-specific anti-suicidal effects. Robust effects were observed in spite of factors constraining power in the current analyses, including low-to-moderate baseline levels of suicidality in the current sample and anxiolytic effects of midazolam, supporting the strength of ketamine’s impact on suicidality. In sum, results are consistent with the contention that ketamine’s rapid antidepressant actions could have life-saving potential.
Individuals with the highest levels of baseline suicidal cognition, assessed both explicitly and implicitly, as well as individuals endorsing a history of suicide attempt, showed the greatest differential effects of ketamine over midazolam, suggesting that ketamine may work most efficaciously in individuals at the highest risk of suicide (Fig. 2). Given that imminent suicide risk and urgent need for inpatient hospitalization were safety exclusions in this RCT, further studies in samples selected for high risk are clearly warranted and would provide an essential next step in validating preventative clinical applications in suicidal patients. Personalized medicine, a goal in psychiatric care that has yet to be realized, would be advanced by follow-up studies assessing whether these moderators of ketamine-specific anti-suicidal effects might also moderate response when multiple bona fide treatment conditions are used.
The mechanisms of ketamine’s antidepressant actions are the subject of ongoing inquiry, with preclinical and clinical reports suggesting roles for increased AMPA-to-NMDA receptor throughput,[39] enhanced synaptic connections in cortical and hippocampal neurons, and stimulation of brain-derived neurotrophic factor signaling.[40, 41] Posited mechanisms of suicidality include both psychological (state anxiety/agitation, hopelessness, impulsivity) and biological (reduced serotonin turnover, impaired prefrontal function) factors.[34, 42, 43] Our study bridges these literatures by providing initial data on mechanisms of ketamine’s antisuicidal effects. A recent report indicated that suicide attempts and high suicidal intent are specifically associated with increases in the inflammation marker quinolinic acid, an NMDA-receptor agonist, suggesting a potential direct pathway from ketamine’s NMDA antagonist action to reductions in suicidality.[44] However, using a range of clinical constructs with relevance to suicide, the most robust mediator of ketamine’s antisuicidal effects in the current cohort was reduction in overall (nonsuicide related) depressive symptoms. Thus, in the context of TRD, ketamine’s antisuicidal and antidepressant properties appear to be one and the same. Further studies in diagnostically heterogeneous cohorts are needed to assess whether ketamine also exhibits independent, suicide-specific effects, outside the context of depression symptom reduction.
Marginal evidence for a mediating role of psychological “target engagement” during infusion, as assessed by a one-item index of euphoric mood, was also found, suggesting downstream reductions in suicidality are more likely in individuals who experience a “high” during ketamine infusion. This relationship may indicate that suicidality reduction depends upon the efficiency of acute glutamate-mediated drug actions, which include transient euphoria.[23] Another common side effect potentially reflecting ketamine target engagement (dissociation) did not similarly mediate suicidality reduction, which could be due to restricted range, as dissociative effects were mild in the current study.[11] Alternatively, this pattern of findings could suggest that experiencing temporary euphoria during the infusion is specifically helpful in reducing the desire to die up to 24 hr later. The moderating effect of euphoria is unlikely to reflect enhanced placebo responses in individuals reporting “high” feelings, as the same association was not observed in the midazolam group in spite of reports of feeling “high” in the majority of patients (63%). Studies directly assessing drug binding/NMDA-receptor target engagement would help to clarify the nature of this potential mechanism.
Evidence for the clinical utility of the IAT as a performance-based index of suicidality was mixed. Fully consistent with our previous open-label trial,[12] Escape = Me associations were uniquely correlated with explicit suicidal cognition at baseline, while Death = Me associations were unrelated to suicidality. Notably, we also replicated a pattern of change in IAT performance observed in our previous report: change from pre-to-post-ketamine was evident specifically in Escape = Me associations but not Death = Me associations. Finally, while greater baseline Escape = Me scores predicted better outcome in the ketamine group, consistent with other indices of increased suicide risk, larger Death = Me scores predicted a different pattern: decreased likelihood of explicit suicidality reduction following midazolam, suggesting reduced placebo-responsiveness of suicidality. While Escape = Me associations consistently perform in accordance with hypotheses in our TRD studies, Death = Me associations have been specifically linked to prospective risk of suicide attempt,[21] and have greater face validity as a suicide assessment. These replicated findings could suggest that in the context of a highly chronic TRD sample with heterogeneous magnitudes of explicit suicidality, the desire to escape is more salient and/or more strongly effected by ketamine than the desire to die, or that Death = Me associations are insufficiently elevated at baseline to allow for detection of change. The observation of significant changes in Escape = Me associations in the ketamine but not the midazolam treatment group suggests that identical changes in our previous open-label study were not merely a practice effect; however, the lack of a significant treatment effect in omnibus tests does not permit strong inferences regarding ketamine-specific effects. This is likely due to insufficient power, given low baseline rates of strong Escape = Me associations and substantial variance routinely observed in performance-based measures (achieved power for treatment x time interaction = 0.50; n = 108 needed for power = 0.80). While explicit measures were more robust in the current research context, future work is nevertheless warranted to assess whether the IAT can improve assessment of suicide risk and treatment-related change in clinical settings, where motivation to conceal suicidal thoughts may be prevalent.[16]
Several limitations of the current study should be noted. Although ketamine’s large effect sizes on explicit symptom measures obviate the need for standard RCT sample sizes, power for implicit analyses may have been constrained. Generalizability of findings may be limited to patients meeting study enrollment criteria, including absence of imminent suicide risk; furthermore, only 30% of patients reported history of suicide attempt. It is therefore unclear how findings would apply to patients with more urgent suicide risk levels, particularly given that SI reduction is only an indirect index of suicide risk. Moderator and mediator analyses focused on self-report, clinician-rated, and behavioral measures, to the exclusion of biological variables. Future translational studies should aim to explore biological and psychological mechanisms in concert in order to delineate the full pathway from biological intervention to psychological outcome.
These limitations notwithstanding, the current study improves substantially on previous work, which has exclusively used saline placebo arms or open-label designs in small samples, and provides the first insights into moderators and psychological mechanisms of ketamine’s antisuicidal effects, which are strongly intertwined with overall antidepressant effects in TRD. These results add to a growing literature suggesting ketamine may have the potential to be life-saving for acutely suicidal patients. Given that current options in the clinical management of acute suicidality are severely limited, future studies are warranted to assess whether these rapid effects can be (1) extended to high-risk samples and (2) prolonged, e.g., through repeated ketamine infusions, or through synergistic combinations with more durable, but slower-to-act, strategies (cognitive therapy, conventional medications).
Supplementary Material
Acknowledgments
We thank Sarah Pillemer, Jeremy Joseph, and Daniel Mortenson for their assistance with this work. Dr. Charney and Icahn School of Medicine at Mount Sinai have been named on a use patent application of ketamine for the treatment of depression; if ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration for this indication, Dr. Charney and Icahn School of Medicine at Mount Sinai could benefit financially; Dr. Charney served on the 2012 Institute of Medicine Committee on DHS Workforce Resilience and was on the 2012 Editorial Board of CNS Spectrums. Dr. Mathew has been named as an inventor on a pending use patent of ketamine for depression; he has relinquished his claim to any royalties and will not benefit financially if ketamine is approved for this use; The other authors report no financial relationships with commercial interests. This work was supported with resources and the use of facilities at the Michael E. DeBakey VA Medical Center, Houston, TX. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.
Contract grant sponsor: Evotec, Janssen Pharmaceuticals, and Avanir, by NIMH Career Development Award 1K23 MH-094707, and by grant UL1 TR000067 from the NIH National Center for Advancing Translational Sciences (to J.W.M.); Contract grant sponsor: Icahn School of Medicine at Mount Sinai from AstraZeneca, Brainsway, Euthymics, Neosync, and Roche and CNS Response, Otsuka, Servier, and Sunovion (to D.V.I.); Contract grant sponsor: NIH, NIH/NIMH, NARSAD, and USAMRAA (to D.S.C.); Contract grant sponsor: AstraZeneca, Bristol-Myers Squibb, Naurex, Roche, Genentech, and NIMH grant RO1 MH-081870, by the Department of Veterans Affairs (VA), by a NARSAD Independent Investigator Award and funding from the Brown Foundation, Inc. (to S.J.M.); Contract grant sponsor: Career Development Award from NIMH (1K23MH100259) (to R.B.P.).
Footnotes
72 participants received study medication in the parent RCT.
FINANCIAL DISCLOSURES
Drs. Price, Chang, Al Jurdi, Soleimani, Iqbal, and Ms. Foulkes report no financial relationships with commercial interests. In the previous 36 months, Dr. Murrough has received research support from Evotec, Janssen Pharmaceuticals and Avanir. Dr. Iosifescu has received research funding through Mount Sinai School of Medicine from AstraZeneca, Brainsway, Euthymics, Neosync, and Roche; he has received consulting fees for CNS Response, Otsuka, and Servier. Dr. Charney has been named as an inventor on a pending use-patent of ketamine for the treatment of depression. If ketamine were shown to be effective in the treatment of depression and received approval from the Food and Drug Administration for this indication, Dr. Charney and Mount Sinai School of Medicine could benefit financially. Dr. Mathew has received consulting fees or research support from Allergan, AstraZeneca, Bristol-Myers Squibb, Cephalon, Inc., Corcept, Johnson & Johnson, Noven, Roche Pharmaceuticals, and Takeda.
REFERENCES
- 1.Nock MK, Borges G, Bromet EJ, et al. Suicide and suicidal behavior. Epidemiol Rev. 2008;30:133–154. doi: 10.1093/epirev/mxn002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kochanek K, Xu J, Murphy S, et al. Deaths: Preliminary Data for 2009. Natl Vital Stat Rep. 2009;59:1–51. [PubMed] [Google Scholar]
- 3.Szanto K, Mulsant BH, Houck P, et al. Occurrence and course of suicidality during short-term treatment of late-life depression. Arch Gen Psychiatry. 2003;60:610–617. doi: 10.1001/archpsyc.60.6.610. [DOI] [PubMed] [Google Scholar]
- 4.Brown GK, Ten Have T, Henriques GR, et al. Cognitive therapy for the prevention of suicide attempts: a randomized controlled trial. JAMA. 2005;294:563–570. doi: 10.1001/jama.294.5.563. [DOI] [PubMed] [Google Scholar]
- 5.Kellner CH, Fink M, Knapp R, et al. Relief of expressed suicidal intent by ECT: a consortium for research in ECT study. Am J Psychiatry. 2005;162:977–982. doi: 10.1176/appi.ajp.162.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Watts BV, Young-Xu Y, Mills PD, et al. Examination of the effectiveness of the Mental Health Environment of Care Checklist in reducing suicide on inpatient mental health units. Arch Gen Psychiatry. 2012;69:588–592. doi: 10.1001/archgenpsychiatry.2011.1514. [DOI] [PubMed] [Google Scholar]
- 7.Qin P, Nordentoft M. Suicide risk in relation to psychiatric hospitalization: evidence based on longitudinal registers. Arch Gen Psychiatry. 2005;62:427–432. doi: 10.1001/archpsyc.62.4.427. [DOI] [PubMed] [Google Scholar]
- 8.Berman RM, Cappiello A, Anand A, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47:351–354. doi: 10.1016/s0006-3223(99)00230-9. [DOI] [PubMed] [Google Scholar]
- 9.Mathew S, Murrough J, Aan Het Rot M, et al. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13:71–82. doi: 10.1017/S1461145709000169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zarate CA, Singh JB, Carlson PJ, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 11.Murrough JW, Iosifescu DV, Chang LC, et al. Anitdepressant efficacy of ketamine in treatment-resistant major depression: A two-site, randomized controlled trial. Am J Psychiatry. 2013;170:1134–1142. doi: 10.1176/appi.ajp.2013.13030392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Price RB, Nock MK, Charney DS, et al. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry. 2009;66:522–526. doi: 10.1016/j.biopsych.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Diazgranados N, Ibrahim L, Brutsche NE, et al. Rapid resolution of suicidal ideation after a single infusion of an NMDA antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–1611. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zarate Ca, Brutsche NE, Ibrahim L, et al. Replication of ketamine’s antidepressant efficacy in bipolar depression: a randomized controlled add-on trial. Biol Psychiatry. 2012;71:939–946. doi: 10.1016/j.biopsych.2011.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Larkin GL, Beautrais AL. A preliminary naturalistic study of low-dose ketamine for depression and suicide ideation in the emergency department. Int J Neuropsychopharmacol. 2011;14:1127–1131. doi: 10.1017/S1461145711000629. [DOI] [PubMed] [Google Scholar]
- 16.Busch KA, Fawcett J, Jacobs DG. Clinical correlates of inpatient suicide. J Clin Psychiatry. 2003;64:14–19. doi: 10.4088/jcp.v64n0105. [DOI] [PubMed] [Google Scholar]
- 17.Cunningham WA, Preacher KJ, Banaji MR. Implicit attitude measures: consistency, stability, and convergent validity. Psychol Sci. 2001;12:163–170. doi: 10.1111/1467-9280.00328. [DOI] [PubMed] [Google Scholar]
- 18.Banse R, Seise J, Zerbes N. Implicit attitudes towards homosexuality: reliability, validity, and controllability of the IAT. J Exp Psychol. 2001;48:145–160. doi: 10.1026//0949-3946.48.2.145. [DOI] [PubMed] [Google Scholar]
- 19.Greenwald AG, Poehlman TA, Uhlmann EL, et al. Understanding using the Implicit Association Test: III. Meta-analysis of predictive validity. J Pers Soc Psychol. 2009;97:17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- 20.Nock MK, Banaji MR. Prediction of suicide ideation and attempts among adolescents using a brief performance-based test. JConsult Clin Psychol. 2007;75:707–715. doi: 10.1037/0022-006X.75.5.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nock MK, Park JM, Finn CT, et al. Measuring the suicidal mind: implicit cognition predicts suicidal behavior. Psychol Sci. 2010;21:511–517. doi: 10.1177/0956797610364762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iovieno N, Papakostas GI. Correlation between different levels of placebo response rate and clinical trial outcome in major depressive disorder: a meta-analysis. J Clin Psychiatry. 2012;73:1300–1306. doi: 10.4088/JCP.11r07485. [DOI] [PubMed] [Google Scholar]
- 23.Krystal JH, Perry EB, Jr, Gueorguieva R, et al. Comparative and interactive human psychopharmacologic effects of ketamine and amphetamine: implications for glutamatergic and dopaminergic model psychoses and cognitive function. Arch Gen Psychiatry. 2005;62:985–994. doi: 10.1001/archpsyc.62.9.985. [DOI] [PubMed] [Google Scholar]
- 24.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 25.Rush AJ, Gullion CM, Basco MR, et al. The Inventory of Depressive Symptomatology (IDS): psychometric properties. Psychol Med. 1996;26:477–486. doi: 10.1017/s0033291700035558. [DOI] [PubMed] [Google Scholar]
- 26.Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382–389. doi: 10.1192/bjp.134.4.382. [DOI] [PubMed] [Google Scholar]
- 27.Rush AJ, Trivedi MH, Ibrahim HM, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C) and Self-Report (QIDS-SR): A Psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003;54:573–583. doi: 10.1016/s0006-3223(02)01866-8. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA. Manual for the Beck Scale for Suicide Ideation. Psychological Corporation; San Antonio, TX: 1991. [Google Scholar]
- 29.Beck A, Steer R, Ranieri W. Scale for Suicide Ideation: psychometric properties of a self-report version. J Clin Psychol. 1988;44:499–505. doi: 10.1002/1097-4679(198807)44:4<499::aid-jclp2270440404>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 30.Nock M, Deliberto T, Dour H, et al. Measuring implicit associations about suicide; Annual Convention of the Association for Behavioral and Cognitive Therapies; Orlando, FL. 2008. [Google Scholar]
- 31.Beck AT, Steer RA. Manual for the Beck Hopelessness Scale. Psychological Corporation; San Antonio, TX: 1988. [Google Scholar]
- 32.Beck AT, Brown G, Berchick RJ, et al. Relationship between hopelessness and ultimate suicide: a replication with psychiatric outpatients. Am J Psychiatry. 1990;147:190–195. doi: 10.1176/ajp.147.2.190. [DOI] [PubMed] [Google Scholar]
- 33.Spielberger CD, Gorsuch RL, Lushene R. State-Trait Anxiety Inventory Test Manual for Form Y. Consulting Psychological Press; Palo Alto: 1983. [Google Scholar]
- 34.Fawcett J, Busch KA, Jacobs D, et al. Suicide: a four-pathway clinical-biochemical model. Ann N Y Acad Sci. 1997;836:288–301. doi: 10.1111/j.1749-6632.1997.tb52366.x. [DOI] [PubMed] [Google Scholar]
- 35.Bremner JD, Krystal JH, Putnam FW, et al. Measurement of dissociative states with the Clinician-Administered Dissociative States Scale (CADSS) J Trauma Stress. 1998;11:125–136. doi: 10.1023/A:1024465317902. [DOI] [PubMed] [Google Scholar]
- 36.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Chapman & Hall; London: 1993. [Google Scholar]
- 37.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 38.Holi MM, Pelkonen M, Karlsson L, et al. Psychometric properties and clinical utility of the Scale for Suicidal Ideation (SSI) in adolescents. BMC Psychiatry. 2005;5:8–15. doi: 10.1186/1471-244X-5-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maeng S, Zarate Ca, Du J, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 40.Li N, Lee B, Liu RJ, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mann JJ. Neurobiology of suicidal behaviour. Nat Rev Neurosci. 2003;4:819–828. doi: 10.1038/nrn1220. [DOI] [PubMed] [Google Scholar]
- 43.Oquendo Ma, Placidi GPa, Malone KM, et al. Positron emission tomography of regional brain metabolic responses to a serotonergic challenge and lethality of suicide attempts in major depression. Arch Gen Psychiatry. 2003;60:14–22. doi: 10.1001/archpsyc.60.1.14. [DOI] [PubMed] [Google Scholar]
- 44.Erhardt S, Lim CK, Linderholm KR, et al. Connecting inflammation with glutamate agonism in suicidality. Neuropsychopharmacol. 2012;38:743–752. doi: 10.1038/npp.2012.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.