Abstract
Background
Transient neonatal hyperglycemia has been reported in up to 80% of extremely preterm human infants. We hypothesize that severe hyperglycemia (HG) is associated with increased morbidity and mortality in preterm baboons.
Methods
Sixty six baboons born at 67% of gestation were studied. Hyperglycemia was defined as serum glucose level ≥ 150mg/dL during the first week of life. Animals were stratified into 2 groups: severe HG (≥8 events) and non-severe HG (<8 events).
Results
Hyperglycemia developed in 65/66 (98%) of the baboons included. A total of 3,386 glucose measurements were obtained. The mean serum glucose level was 159±69 mg/dL for the severe HG group and 130±48 mg/dL for the non-severe HG group during the first week of life. No differences were found in gender, birth weight, sepsis, PDA, or oxygenation/ventilation indices between groups. Severe HG was associated with early death even after controlling for sepsis, postnatal steroid exposure and catecholamine utilization.
Conclusion
Hyperglycemia is common in preterm baboons and is not associated with short term morbidity. Severe hyperglycemia occurring in the first week of life is associated with early death in preterm baboons.
INTRODUCTION
Transient neonatal hyperglycemia has been reported in up to 80% of extremely preterm infants, and has been associated with increased morbidity, including intraventricular hemorrhage, white matter injury, retinopathy of prematurity and necrotizing enterocolitis (1-7). Furthermore, both early and persistent hyperglycemia during the first week of life are associated with higher mortality in extremely preterm infants (2,6,8). The pathogenesis seems to be multi-factorial and is likely secondary to defective islet β-cell processing/secretion of insulin, decreased peripheral insulin sensitivity, and hepatic insulin resistance (5,9,10).
Hyperglycemia is highly prevalent in the preterm baboon and has a reported incidence similar to extremely preterm human infants (3,11). Baboons have 97% phylogenetic proximity with humans and when born preterm, they develop conditions unique to preterm infants, such as respiratory distress syndrome, patent ductus arteriosus and bronchopulmonary dysplasia (12,13).
Baboons have been shown to be a pertinent non-human primate model to examine the underlying cellular/molecular mechanisms responsible for insulin resistance which can develop spontaneously along with diabetes in adult baboons (14,15). Furthermore, preterm fetal baboons have significant down regulation of insulin signaling proteins in skeletal muscle such as AS160, GLUT1, and GLUT4, which may be implicated in the development of hyperglycemia of preterm infants (11).
The preterm baboon is the only animal that survives extreme prematurity (>48 hours) where hyperglycemia develops spontaneously. All of the animals included in this study were under strict protocols for each major organ system and the homogeneity of this group of animals is narrow. In addition, common perinatal events that lead to prematurity are not present (i.e., intrapartum infection and/or maternal disease) and therefore, confounding factors are minimized. The objective of this study is to evaluate if severe hyperglycemia is associated with increased morbidity and mortality in preterm baboons.
MATERIALS AND METHODS
Animal Care
A total of 66 preterm baboons were delivered at 67% of gestation at the Texas Biomedical Research Institute (TBRI) in San Antonio, Texas from 2004 to 2008. Animals were delivered prematurely via caesarean section under general anesthesia from healthy, non-diabetic mothers at 125±2 days (d) gestational age (GA) (full term=185d GA). All studies were approved by the Institutional Animal Care Committee at the TBRI. Animal experiments were conducted in accordance with accepted standards of humane animal care. Mothers were given prenatal steroids initiated 48 hours prior to delivery with betamethasone (6mg intramuscular (IM)) q24h for 2 doses or dexamethasone if pharmaceutical shortage of betamethasone). This dose of prenatal steroids is equivalent to the dose utilized in humans when averaged as mg/kg of body weight.
The animals were intubated immediately after birth and chronically ventilated for a planned survival of 14d, except for one animal where survival was only planned for 6d. None of the animals included on this study received any investigational treatments. Surfactant (Survanta, Abbott Laboratories, Abbott Park, IL) was administered through the endotracheal tube immediately after placement at a dose of 4mL/kg. A standard protocol was followed for sedation and anesthesia allowing animals to move similar to what is tolerated in preterm ventilated infants. This was achieved by titrating their sedation as needed at an approximate interval of every 2-4 hours. A detailed protocol for ventilator management was followed according to blood gas analysis which were measured hourly for the first 48 hours and then every 2 hours for the rest of their stay. Central intravenous lines were placed shortly after birth for fluid management and parenteral nutrition. An echocardiogram was obtained on the first day of life and daily thereafter, details have been published elsewhere (13,16). A 5% dextrose intravenous solution was started after birth at a rate of 150 mL/kg/d. The glucose infusion rate was increased every 2 days as per protocol (by 1-2 mg/kg/min), and total fluid goal was titrated depending on fluid balance. Measurements of glucose levels were obtained shortly after birth and then at a minimum of every six hours using the AU 640 Immuno Analyzer (Olympus Inc., San Diego, CA). Additional glucose measurements were obtained if hyperglycemic. There were 3 professionals in charge of their daily management (2 neonatologist/1 neonatal nurse practitioner) that could request additional measurements but not less. The direct caregivers (technicians) were very familiar with the protocols and followed procedures consistently. Urine glucose was measured at least every twelve hours by Multistix 10 SG reagent strips for urinalysis (Siemens (Bayer) Medical Solutions Inc., Pittsburg, PA). Intravenous insulin was given as a bolus administration dose of 0.5 IU/kg to treat serum glucose levels greater than 200 mg/dL. After insulin administration, glucose measurements were monitored at an minimum interval of four hours until euglycemia was achieved. A parenteral feeding protocol was initiated after 24h of life. Intravenous amino acids were started at 24h of life at a dose of 1.75 g/kg and increased to 3.5 g/kg by 48h of life and kept at that dose for the remainder of the number of days in the protocol. Intravenous lipids were started at 24h of life at a dose of 1g/kg and increased incrementally to a maximum of 3g/kg/day. Enteral feeds were initiated on 3d of age if bowel gas pattern was considered normal on radiograph. Primilac formula (BioServ, Frenchtown, NJ) was initiated as trophic feeds and increased as tolerated by 20 mL/kg/day to a maximum feeding volume of 150 mL/kg/day. Dopamine, dobutamine, epinephrine and hydrocortisone were initiated in that order and advanced to a set maximum amount to maintain mean arterial pressure > 25 mmHg. A weaning protocol was followed as well to avoid hypertension. Sepsis was defined by at least one positive blood, urine or cerebrospinal fluid culture. Prophylactic antibiotics were administered for 48 hours to mimic human care. Thereafter, antibiotics were continued or restarted based on clinical condition.
Data Collection
Demographic data was recorded in the medical chart, including birthweight, gender, length of stay, and death. Clinical data collected included all serum glucose measurement and chemistries, medications (cathecholamines (dopamine, dobutamine, epinephrine), insulin, and steroids), glucose infusion rate (GIR, calculated hourly throughout stay), hourly ventilator settings, urinalysis, sepsis (defined as a positive blood, urine or CSF culture) and any other pertinent clinical diagnosis.
Oxygenation index (OI) was calculated by (MAP × FiO2)/pO2 ×100, where MAP=mean airway pressure, FiO2=fraction of inspired oxygen, and pO2=partial pressure of oxygen. Ventilation index (VI) was calculated by (PIP × respiratory rate × pCO2)/1000, where PIP=peak inspiratory pressure and pCO2=partial pressure of carbon dioxide. The oxygenation and ventilation indices were recorded every 2-4 hours but for statistical purposes, an average was calculated at 12 hour intervals on each animal. Due to the higher mortality in the severe hyperglycemia group, the data sets for the two groups became unbalanced and therefore an ANOVA for repeated measures could not be accomplished. Consequently, a two way ANOVA was done to compare the oxygenation index and ventilation index with glucose level and time as the two parameters.
Hyperglycemia was defined as a serum glucose concentration of ≥150 mg/dL (8.3mmol/L) during the first 7d of life. This level has been previously identified as being clinically relevant in the human population (17,18). Fasting serum glucose has been reported in preterm baboons to be 40.6 ± 4.7 mg/dL, term baboons 59.2±10.6 mg/dL and adult baboons 87.0±16.6 mg/dL (19). Therefore, hyperglycemia defined as above will be highly relevant in the baboon model.
For logistic regression analyses, the animals were defined according to their top quartile distribution for severity of hyperglycemia. The top quartile (i.e. severe HG group) was defined by having ≥ 8 episodes (glucose measurement ≥150 mg/dL) of hyperglycemia during the first 7 days of life. In addition, in order to ensure that this group had in fact a greater exposure to hyperglycemia, and not only a greater number of measurements, a glucose ratio was calculated for all animals. This was defined as the number of hyperglycemic events divided by the number of total measurements. A glucose ratio has been previously found to be a good marker of the time spent in hyperglycemic state and may reflect length of exposure. (6). The glucose ratio utilized by Hay et al was different because in humans the number of measurements per day are not standardized and a time ratio for glucose concentrations of >150 mg/dL, calculated as the number of days with a lowest blood glucose value of >150 mg/dL divided by the total number of days on which blood glucose concentrations were obtained during the first week of life. In this study, since measurements were standardized by a protocol with a minimum of 4 measurements per day, and the incidence of hyperglycemia is so high, we used a glucose ratio to ensure the animals with “severe” hyperglycemia were not classified as such because glucose was checked more frequently but rather they spent more time hyperglycemic.
Serum glucose range was calculated daily by deducting the minimum level from the maximum level in each animal. Similarly, minimum and maximum serum glucose levels were recorded daily.
Statistical Methods
Statistical calculations were performed with SPSS for Windows (Version 16.5, SPSS, Inc., Chicago, IL) and Prism (version 4.0 GraphPad Software, Inc., La Jolla, CA). Distributions and means of demographic and clinical variables were compared across study groups using χ2 tests and t-tests, and Mann-Whitney test when data not normally distributed. Standard deviations are shown for continuous variables when means are calculated and inter-quartile ranges for medians. Binary logistic regression analysis was employed to assess correlates of hyperglycemia, and to assess the association of hyperglycemia with several clinical outcomes, after adjusting for birth weight, catecholamine exposure, postnatal steroid use, presence of sepsis, and/or occurrence of early death when appropriate. Kaplan-Meier curves were utilized to assess probability of survival and Pearson Correlations were utilized with selected continuous variables. Two way ANOVA and repeated measures were utilized to identify within and between subject effects of continuous variables.
RESULTS
Sixty-five (98%) of the 66 preterm baboons included in this study had at least one episode of hyperglycemia within the first 3 days of life and all had at least one episode by 7 days of age. A total of 91% of animals had greater than one episode of hyperglycemia during the first week of life. 19/66 animals were classified as having severe hyperglycemia according to the criteria previously described. No differences in gender or birth weight were found between the severe and non-severe HG groups (Table 1). As anticipated, 100% of all study animals were exposed prenatally to steroids. The demographic details are summarized in Table 1.
Table 1.
Demographic Characteristics
| Study Characteristics | Non-Severe Hyperglycemia (n=47) |
Severe Hyperglycemia (n=19) |
p-value |
|---|---|---|---|
| Birth Weight (grams) | 373±41 | 364 ±45 | 0.458 |
| Male | 30(63%) | 9 (47%) | 0.21 |
| Prenatal Steroids | 100% | 100% | 1.0 |
| PDA | 100% | 100% | 1.0 |
| Sepsis | 2 (4.3%) | 1 (5.6%) | 0.83 |
| Length of Stay (days) | 13±2.5 | 11±5.2 | 0.81 |
| Early Death* | 5 (10%) | 6 (32%) | 0.03 |
| Postnatal Steroids | 13 (28%) | 7 (37%) | 0.46 |
| Hyperglycemic episodes (n) | |||
| First 3 days* | 4.8±3.2 | 8.5±2.9 | <0.001 |
| First 7 days* | 6.6±3.0 | 11.5±3.3 | <0.001 |
| Glucose (mean) first 3 days* | 135±57 | 170±74 | <0.001 |
| Glucose (mean) first 7 days* | 130±48 | 159±69 | <0.001 |
| Glucose (max) first 3 days* | 226±58 | 291±77 | <0.001 |
| Glucose (max) first 7 days* | 243±57 | 295±76 | <0.01 |
| Glucose (range) first 3 days* | 166±69 | 241±83 | <0.001 |
| Glucose (range) first 7 days* | 187±68 | 249±81 | <0.01 |
| GIR first 3 days | 5.3±1.0 | 5.4±1.3 | 0.18 |
| GIR first 7 days | 6.3±1.6 | 6.3±2.0 | 0.98 |
| Insulin doses* | 2.1±2.7 | 4.5±2.8 | 0.002 |
| Catecholamines all days † | 0.13±0.15 | 0.14±0.19 | 0.80 |
| Catecholamines first 3 days † | 0.23±0.22 | 0.18±0.23 | 0.43 |
| Catecholamines first 7 days † | 0.20±0.20 | 0.16±0.21 | 0.47 |
| OI first 3 days | 7.3±3.5 | 6.8±1.6 | 0.46 |
| OI first 7 days | 6.7±2.8 | 6.1±1.5 | 0.41 |
| VI first 3 days | 52.8±20.2 | 51.1±7.9 | 0.64 |
| VI first 7 days | 49.9±17.2 | 47.5±8.2 | 0.46 |
| Total feeding volume (cc) | 95.8±55.9 | 85.5±65.1 | 0.25 |
| Average feeding volume (cc/kg/d) | 18.8±11.2 | 17.0±13.2 | 0.26 |
| Sodium first 3 days (meq/dL) | 146±8 | 147±10 | 0.11 |
| Sodium first 7 days (meq/dL) | 146±8 | 146±10 | 0.21 |
| Potassium first 3 days (meq/dL)* | 3.4±0.6 | 3.3±0.6 | <0.001 |
| Potassium first 7 days (meq/dL) | 3.6±0.6 | 3.6±0.7 | 0.90 |
| Hematocrit first 3 days (meq/dL)* | 45.2±8.1 | 44.0±7.4 | <0.01 |
| Hematocrit first 7 days (meq/dL) | 40.9±8.1 | 40.5±7.2 | 0.16 |
Data is shown as mean ± S.D. unless otherwise specified;
Significance <0.05 by χ2 tests and t-tests for categorical or continuous data respectively. GIR= Glucose Infusion Rate, OI=Oxygenation Index, VI=Ventilation Index,
Catecholamines are calculated from the cumulative mL of dopamine, dobutamine and epinephrine given hourly during either all days alive, first 3 or 7 days. All catecholamines are mixed with same concentration per body weight.
The development of sepsis was similar between the severe and non-severe HG groups (Table 1). The amount of catecholamines utilized during the first 3, 7 or all days alive in order to maintain a normal mean arterial pressure, was similar between the 2 groups (Table 1). In addition, the maximum amount and the mean amount of catecholamines were calculated per day each day alive and were not associated with the development of HG (N.S.). Serum glucose levels did not tightly correlate with the amount of catecholamine infused (r2=0.001, p=0.01). Twenty (30%) of all study animals were exposed to postnatal steroids (hydrocortisone), with no differences between the 2 stratified groups (Table 1).
Glucose levels and Nutritional Data
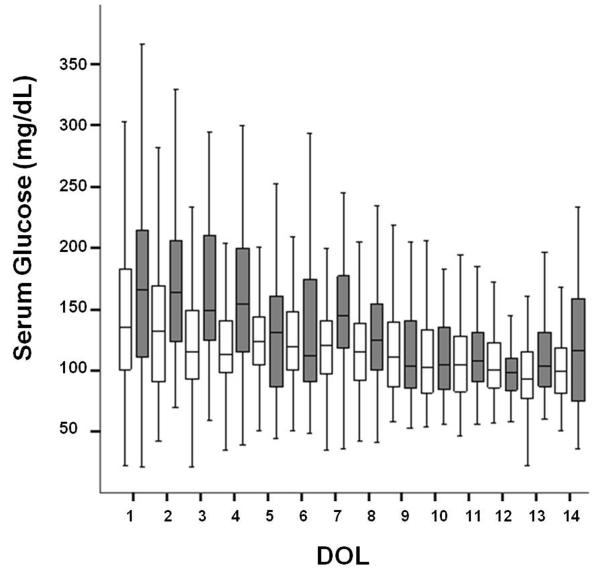
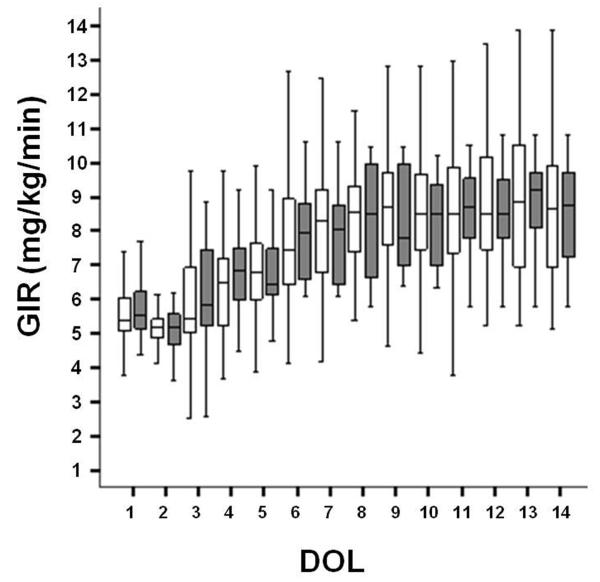
A total of 3,386 glucose measurements were obtained throughout the study period (Figure 1). For the first week of age, the mean serum glucose level for the severe HG group was 159±69 mg/dL and 130±48 mg/dL for the non-severe HG group (p<0.001, Table 1). The severe HG group had significantly higher mean and maximum serum glucose levels along with wide variations of glucose (as expressed by glucose ranges, Table 1) when compared to the non-severe HG group during the first 3 and 7 days of life (p<0.01 for mean, maximum and range differences between groups, Table 1). Interestingly, the mean GIR during these time periods were not different between the two groups (Figure 2). As expected, the numbers of hyperglycemic episodes in the first 3 and 7 days were higher in the severe HG group when compared to the non-severe HG group (Table 1). There was no difference in the number of glucose measurements obtained during the first 3 and 7 days in the severe HG group when compared to the non-severe group (15.2 ± 4.1 vs. 14.7 ± 3.9, p=0.6, and 20.8 ± 6.4 vs. 23.2 ± 4.3, p=0.2, respectively). The glucose ratios were higher at both 3 and 7 days in the severe HG group vs. the non-severe HG group (0.55 ± 0.12 vs. 0.32 ± 0.18, p<0.001 and 0.55 ± 0.07 vs. 0.28 ± 0.11, p<0.001, respectively). Urine glucose was measured a total of 1,011 times for all animals. Urine glucose was negative (<100 mg/dL) in 85% of the samples with a paired median serum glucose of 107 mg/dL (IQR 87-136); 7.3% of the samples had trace glucose in urine (≥ 100 but <250 mg/dL) with paired median serum glucose of 139 mg/dL (IQR 109-186), and 7.7% of the samples had ≥250 mg/dL of glucose in the urine with a paired median serum glucose of 195 mg/dL (IQR 155-265).
Figure 1. Daily Serum Glucose.
Daily serum glucose measurements are represented as medians in the figure with interquartile ranges shown as black lines. The non-severe HG group is shown in white boxes and severe HG group in dark boxes. DOL=day of life.
Figure 2. Glucose Infusion Rates by Group.
Daily median glucose infusion rates (GIR) are expressed as mg/kg/min and compared each day of life with interquartile ranges shown as black lines. The non-severe HG group is shown in white boxes and severe HG group in dark boxes. DOL=day of life.
Insulin was used to treat HG in 70% of the animals (mean 1.7 ± 1.8 d of life). As expected, baboons with severe HG received more insulin doses than those with non-severe HG (Table 1).
Cumulative enteral feed volume and the average amount of feeds per day were similar between the severe and non-severe HG groups (p=0.25). Since serum sodium can affect serum glucose, recorded daily measurements were compared with no differences found between the 2 groups. A statistically significant difference in serum potassium levels was found between the severe and non-severe HG groups at 3 days of life likely due to the large number of measurements (2,652 and 5,337 measurements in the first 3 and 7 days of life respectively)(Table 1); however, this differences are not clinically relevant.
Clinical outcomes
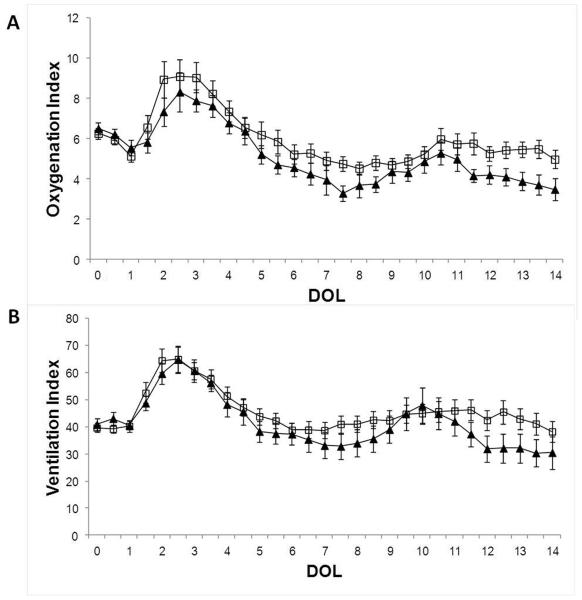
Since animals were planned for euthanasia at day 14, the clinical outcome measures were early death and oxygenation and ventilation indices. There were no differences in the daily oxygenation and ventilation indices between the severe HG group and the non-severe HG animals by independent t-tests group comparisons at day 3 and 7 of life (Table 1), by binary logistic regression (data not shown) nor by two way ANOVA (Figure 3). Serum glucose levels did not tightly correlate with OI or VI (r2=0.001, p=0.1; r2=0.001, p=0.04, respectively). Hemoglobin plays an important role in oxygenation and therefore, anemia was monitored. At day 3 of life, the severe HG group had a statistically lower hematocrit than the non-severe HG group but this level was considered to be clinically irrelevant and was likely due to the large number of measurements performed in each animal (Table 1). By 7 days of life, however, the hematocrit levels were similar between the 2 groups.
Figure 3. Daily Oxygenation and Ventilation Indices.
(A) Daily oxygenation index and (B) ventilation index is shown as mean per day of life (DOL) in each group, S.E are shown. Empty squares represent non-severe HG and black triangles represent severe HG group (N.S.).
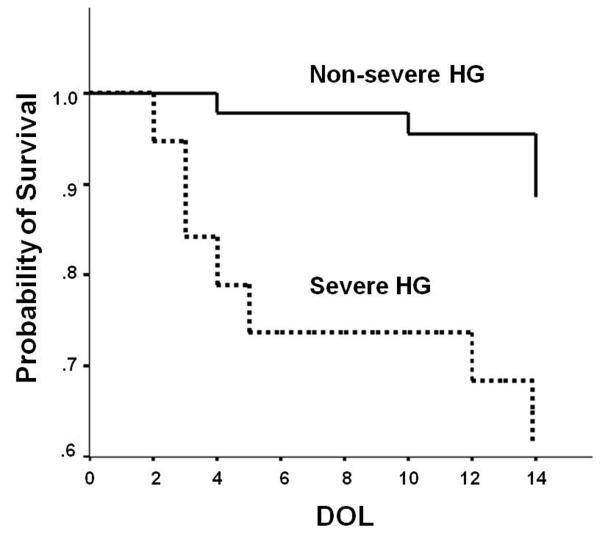
Early death was found in 11/66 animals and occurred at a mean age of 6.7±5 days. One of the non-early death animals was planned for experiment completion at 6 days instead of 14 days; this animal was excluded from the analysis. Clinical characteristics in the early death group (weight, gender, OI or VI) did not differ from the rest of the animals (N.S.). Severe HG was associated with early death, even after controlling for sepsis, postnatal steroid exposure and catecholamine use (OR 3.88, 95% CI 1.02-14.81). As expected, the animals in the severe HG group had a tendency towards a shorter length of stay (secondary to increased number of early deaths) when compared to the non-severe group, but this did not reach statistical significance (Table 1). Early deaths occurred as follows: low quaritile (n=4), second and third quartile (n=1), high quartile (n=6). When severe HG was categorized by tertiles or by highest glucose alone, the association with death did not reach statistical significance (data not shown). The cause of early death for each animal is shown in Table 2. One animal died due to loss of tracheostomy and is unlikely to be associated to hyperglycemia. This animal was not taken out of the analysis since it was in the non-severe HG group and therefore, will be unlikely to make any difference in the outcome measure (death). The probability of survival was lower in the severe HG group but did not reach statistical significance (Figure 4, p=0.05).
Table 2.
Causes of Early Death
| Severe Hyperglycemia |
Cause of Death |
|---|---|
| Y | Cardiac Insufficiency a |
| Y | Cardiac Insufficiency a |
| Y | Sepsis |
| Y | Renal Insufficiency |
| Y | Cardiac Insufficiency a |
| Y | Cardiac Insufficiency a |
| N | Metabolic Acidosis |
| N | Renal Insufficiency |
| N | Unknown |
| N | Loss of Tracheostomy |
| N | Sepsis |
Cardiac Insufficiency defined as severe hypotension unresponsive to vasopressors (dopamine, dobutamine and epinephrine) and fluid resuscitation
Figure 4. Probability of Survival According to Hyperglycemic Status.
This graph shows Kaplan-Meier survival curve comparing non-severe HG and severe HG groups (p=0.05).
DISCUSSION
Hyperglycemia is a known condition that frequently occurs in preterm infants. The development of hyperglycemia has been associated with significant morbidity in these infants, leading to long term consequences and increased hospital stays (1,3,4,6,7,20). Increased mortality has been well documented in diabetic adults, non-diabetic elderly patients and critically ill children with poor glucose control (21-23); there is now data that supports increased mortality in preterm infants who develop hyperglycemia early in life (2,8).
In this study of 66 extremely preterm baboons, hyperglycemia was found in the majority of animals during the first week of life and those with prolonged exposure (29% of these animals) were associated with early death. Recent studies suggest a link between severe hyperglycemia and increased odds of dying within the first week of life in extremely low birth weight preterm infants (8). Although this increased mortality may reflect severity of illness rather than causality, the odds of early death remained high even when controlling for other variables that express severity of illness, such as sepsis, postnatal steroid exposure, and catecholamine utilization. In fact, both groups of hyperglycemic animals (severe and non-severe) had very similar clinical and demographic characteristics. Pulmonary functions, expressed by oxygenation and ventilation indices, were followed very closely and were similar between both groups (Table 1 and Figure 3). Nonetheless, the pulmonary function in the severe HG group (Figure 3) appeared to be slightly better than the non-severe HG group in the last 4 days of life (non statistically significant); this is likely to be due to lack of data from those animals which died early, skewing the data from healthier survivors. Nutritional management was dictated by parenteral nutrition and enteral feeding protocols set a priori and no differences were found (Table 1). Glucose infusion rates were also similar between both groups during the first week of life (Table 1 and Figure 2); therefore, the development of severe hyperglycemia was unlikely to be due to differences in glucose administration. Another strength of this study relies on the frequent glucose measurements obtained per animal (over 3,000 measurements) which contrary to most clinical reports where sick infants get more testing, the number of glucose measurements did not differ between groups since a protocol was tightly followed (as shown in the results). Those animals with hyperglycemia >200 mg/dL received spot doses of insulin and may had more glucose measurements but there are no differences in number of measurements between groups likely due to the high incidence of hyperglycemia. This potential bias is further minimized by utilizing a glucose ratio to assess time spent in hyperglycemia, which is a result of the number of times the animal was hyperglycemic divided by the number of times glucose was measured and not on single hyperglycemic events.
The majority of animals (67%) required vasoactive medications due to their severity of illness from extreme prematurity, but no differences were found in the number of cumulative doses or doses per day of catecholamine administration between the groups at different time points between the severe HG group and non-severe HG group (Table 1). Due to the effect of vasoactive medications in gluconeogenesis (24), the use of these medications may have contributed to the high serum glucose levels and the incidence of hyperglycemia. Most hyperglycemic events occurred within the first 72 hours of life (Table 1) and a large number of glucose levels measured were above 150 mg/dL particularly within the first 2 days of life (Figure 1). Vasoactive medications were initiated at a mean of 37±36 hours of life and may have contributed to prolonging the duration of hyperglycemia. Nevertheless, the amount of vasoactive medications utilized was the same between groups. We may further speculate that the high incidence of hyperglycemia in the first 2 days of life may have led to hyperosmolar dehydration which in turn, may have placed all animals at higher risk of requiring vasoactive support and those with persistent HG at an increased risk of death due to hypovolemia. Furthermore, glucosuria was initially found with median serum glucose of 139 mg/dL and glucosuria increased with worsening hyperglycemia. Lastly, most of the deaths were due to unexplained renal/cardiac insufficiency and metabolic acidosis which may be a consequence of hypovolemia due to hyperglycemic dehydration.
As evidenced in diabetics, it is likely that persistent exposure to hyperglycemia will lead to more consequences whereas variations in glucose time/length of exposure may lead to differences in type of morbidity and mortality. For example, wide variations, the maximum serum glucose level reached and the percent of time spent with hyperglycemia (glucose ratio) may contribute to disease processes due to changes in osmolality, fluid shifts and direct cellular effects in various organs, particularly in the fragile preterm brain (2). Among our study cohort, the severe hyperglycemia group had higher mean glucose levels, wider fluctuations in glucose (expressed as ranges in Table 1) and higher peak levels than the non-severe HG group during the first 3 days of life when compared to the non-severe hyperglycemia group (p<0.01 for mean, maximum and ranges of serum glucose between groups, Table 1). These independent group differences persisted to 7 days of life and may have further contributed to the higher mortality rates but due to sample size, the effect of each type of glucose exposure or contribution from other variables could not be determined.
Previous studies have suggested a relationship between sepsis and the development of hyperglycemia; this was not observed in this study. This lack of association may be secondary to the low number of study animals who had culture-proven sepsis (4.5%). The incidence of sepsis in ELBW infants is much higher (21%, by NICHD data)(25); this difference may be due to low risk of early sepsis secondary to lack of maternal chorioamnionitis in planned preterm delivery by c-section in healthy animals and selection bias since animals did not live long enough to develop the disease (alive for 14 days). A weakness of the study might be the high incidence of hyperglycemia at 98%. Although this incidence is higher than what has been reported in ELBW infants (3,20,26), it is only 10-20% higher. In addition, most human studies are from non-homogenous ELBW populations. This population of animals is homogenous and the gestational age is very narrow (±2 days). Therefore the results of this study may be more representative of an ELBW infant with no confounding perinatal stress factors. This in turn, may inflate the incidence of hyperglycemia as a result of a lack of maturational effects in glucose metabolism from stress (i.e. cortisol, thyroid and/or catecholamine surges). Another potential pitfall of this study is that causes of death may be more aggressively treated in human infants, but due to the great economic demands and involvement of highly skilled professionals in the non-human primate preterm model, a significant attempt to avoid death was sought at all times, which is less common in other animal models. Death alone as an outcome measure may be a limitation of this study since it was planned at 14 days of age and additional spontaneous deaths may have occurred afterwards. Hyperglycemia commonly occurs in the first 2 weeks of life (3,6), therefore, it is likely we have captured the majority of early deaths that will have occurred as a consequence of hyperglycemia, but we may have missed those resulting from late onset hyperglycemia or secondary to long term effects of this condition.
Hyperglycemia is a common side effect of postnatal dexamethasone therapy in preterm infants (27). In our study, only 30% of the hyperglycemic animals were exposed to postnatal steroids (hydrocortisone) and exposures to steroids were similar between groups (Table 1).
The pathogenesis of hyperglycemia in critically ill preterm infants seems to be multifactorial including abnormalities likely related to prematurity (persistent gluconeogenesis, inadequate pancreatic insulin release and underdeveloped insulin signaling pathways in the muscle (5,9-11) in addition to superimposed postnatal factors (sepsis, medications). Although it is well-known that stress and severity of illness can contribute to development of hyperglycemia, this was not evident in our study population. This is a homogenous population of extremely ill, preterm animals who all received prenatal steroids. This population is very difficult to obtain since there are multiple genetic and environmental differences in human populations. Therefore, this is a very suitable model to study hyperglycemia where the effects of this condition alone can be studied.
Among critically ill adults, better glucose control improves clinical outcomes (28) but intensive insulin therapy leads to higher incidence of death (29). For critically ill children with cardiac surgery, trauma, sepsis or burns, hyperglycemia has been shown to correlate with worse outcomes (30-33,33). Studies in newborns are limited, and so far, insulin therapy does not seem to decrease the incidence of hyperglycemia, but tight glucose control has not been evaluated (34). Others have attempted early insulin therapy in very low birth weight preterm infants (without hyperglycemia) but this strategy was linked to higher mortality at 28 days of life; it is important to note that in some of these studies, the majority of infants were normoglycemic when insulin infusions were initiated (35). As expected, baboons in the present study with severe hyperglycemia received more insulin doses than those with non-severe hyperglycemia. Insulin is more commonly given as a continuous infusion in human preterm infants (35) and insulin boluses may have contributed to wider range of serum glucose seen in the severe HG group. One may argue that this may have contributed to the higher mortality rate seen in the severe hyperglycemic group, but it is unlikely that a difference of 2 extra doses of insulin in the severe HG group was clinically relevant as a cause of mortality. On the other hand, the majority of deaths occurred in the low (4 deaths) and high (6 deaths) quartiles. These findings may reflect what is seen in diabetic adults, where patients with low glucose have an increased risk of death (29). Additional studies need to be performed to determine the highest level of blood glucose that safely provides the maximum amount of nutrients without increasing the risk of morbidity/death. Moreover, it is well documented that preterm and small for gestational age infants are at increased risk of cardiovascular disease and diabetes early in life; therefore, the long term consequences of exposure to high glucose levels at critical periods of development need to be evaluated (36-38). It has been demonstrated that maternal hyperglycemia leads to altered embryogenesis secondary to apoptotic effects, altered mitochondrial physiology and generation of oxygen radicals resulting in neural tube, musculoskeletal and cardiac defects (39,40); in addition exposure to gestational diabetes is associated with an increased incidence of adolescent obesity and glucose intolerance in the off-spring, and therefore exposures to high glucose during the extra-uterine life at these critical periods of development may have life-long consequences (37).
The present study demonstrates the preterm baboon is a novel and clinically relevant model for the study of neonatal hyperglycemia. Furthermore, preterm baboons develop conditions in the neonatal period that are similar to human infants (11-13). Previously, it has been demonstrated that the adult baboon is a pertinent non-human primate model to examine mechanisms behind aberrant glucose control; baboons have close (97%) phylogenetic proximity with humans and can develop insulin resistance and diabetes spontaneously (14). Therefore, the results of this study are highly translational to humans.
In conclusion, extremely preterm baboons have a high incidence of hyperglycemia and severe hyperglycemia occurring during the first week of life is associated with early death. No other morbidities were associated with severe hyperglycemia. Future studies of this model will enhance our understanding of an optimal glycemic management to improve morbidity and mortality in premature infants.
ACKNOWLEDGEMENTS
We thank Dr. Steve Seidner and Dr. Ralph DeFronzo for their advice and continuous support as well as the personnel at the Texas Biomedical Research Institute for their dedication to this project.
Statement of Financial Support: This study was supported by grants from the Robert Wood Johnson Foundation (67067, C.B.), CTSA (UL1RR025767 to C.B.), the National Institutes of Health (HL52636 to BPD resource Center and NCRR P51 RR013986 to the SNPRC, now TBRI).
REFERENCES
- 1.Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol. 2003;23:186–194. doi: 10.1038/sj.jp.7210879. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrou G, Skiold B, Karlen J, et al. Early Hyperglycemia Is a Risk Factor for Death and White Matter Reduction in Preterm Infants. Pediatrics. 2010;125:e584–e591. doi: 10.1542/peds.2009-0449. [DOI] [PubMed] [Google Scholar]
- 3.Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006;26:737–741. doi: 10.1038/sj.jp.7211594. [DOI] [PubMed] [Google Scholar]
- 4.Hall NJ, Peters M, Eaton S, Pierro A. Hyperglycemia is associated with increased morbidity and mortality rates in neonates with necrotizing enterocolitis. J Pediatric Surg. 2004;39:898–901. doi: 10.1016/j.jpedsurg.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 5.Liechty EA. The Resistant Premie: Documenting the Prevalence of Hyperglycemia in the Extremely Low Birth Weight Infant. J Pediatr. 2010;157:699–700. doi: 10.1016/j.jpeds.2010.05.028. [DOI] [PubMed] [Google Scholar]
- 6.Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006;118:1811–1818. doi: 10.1542/peds.2006-0628. [DOI] [PubMed] [Google Scholar]
- 7.Kaempf JW, Kaempf AJ, Wu Y, Stawarz M, Niemeyer J, Grunkemeier G. Hyperglycemia, insulin and slower growth velocity may increase the risk of retinopathy of prematurity. J Perinatol. 2011;31:251–257. doi: 10.1038/jp.2010.152. [DOI] [PubMed] [Google Scholar]
- 8.Kao LS, Morris BH, Lally KP, Stewart CD, Huseby V, Kennedy KA. Hyperglycemia and morbidity and mortality in extremely low birth weight infants. J Perinatol. 2006;26:730–736. doi: 10.1038/sj.jp.7211593. [DOI] [PubMed] [Google Scholar]
- 9.Mitanchez-Mokhtari D, Lahlou N, Kieffer F, Magny JF, Roger M, Voyer M. Both relative insulin resistance and defective islet β-cell processing of proinsulin are responsible for transient hyperglycemia in extremely preterm infants. Pediatrics. 2004;113:537–541. doi: 10.1542/peds.113.3.537. [DOI] [PubMed] [Google Scholar]
- 10.Chacko SK, Ordonez J, Sauer PJJ, Sunehag AL. Gluconeogenesis is not regulated by either glucose or insulin in extremely low birth weight infants receiving total parenteral nutrition. J Pediatr. 2011;158:891–896. doi: 10.1016/j.jpeds.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blanco CL, Liang H, Joya-Galeana J, DeFronzo RA, McCurnin D, Musi N. The ontogeny of insulin signaling in the preterm baboon model. Endocrinology. 2010;151:1990–1997. doi: 10.1210/en.2009-0777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escobedo MB, Leonard Hilliard J, Smith F, et al. A baboon model of bronchopulmonary dysplasia: I. Clinical features. Exp Mol Pathol. 1982;37:323–34. doi: 10.1016/0014-4800(82)90045-4. [DOI] [PubMed] [Google Scholar]
- 13.McCurnin D, Clyman RI. Effects of a patent ductus arteriosus on postprandial mesenteric perfusion in premature baboons. Pediatrics. 2008;122:e1262–e1267. doi: 10.1542/peds.2008-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chavez AO, Lopez-Alvarenga JC, Tejero ME, et al. Physiological and molecular determinants of insulin action in the baboon. Diabetes. 2008;57:899–908. doi: 10.2337/db07-0790. [DOI] [PubMed] [Google Scholar]
- 15.Guardado-Mendoza R, Dick EJ, Jr., Jimenez-Ceja LM, Davalli A, Chavez AO. Spontaneous pathology of the baboon endocrine system. J Med Primatol. 2009;38:383–389. doi: 10.1111/j.1600-0684.2009.00384.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCurnin DC, Yoder BA, Coalson J, et al. Effect of ductus ligation on cardiopulmonary function in premature baboons. Am J Respir Crit Care Med. 2005;172:1569–1574. doi: 10.1164/rccm.200502-230OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cowett RM, Farrag HM, Cowett RM, Farrag HM. Selected principles of perinatal-neonatal glucose metabolism. Semin Neonatol. 2004;9:37–47. doi: 10.1016/S1084-2756(03)00113-1. [DOI] [PubMed] [Google Scholar]
- 18.Denne SC, Poindexter BB, Leitch CA, Ernst JA, Lemons PK, Lemons JA. Nutrition and metabolism in the high risk neonate. In: Fanaroff AA, Martin RJ, editors. Neonatal-Perinatal Medicine. Diseases of the Fetus and Infant. Mosby-Elseiver; Philadephia, Pa: 2006. pp. 661–691. [Google Scholar]
- 19.Quinn AR, Blanco C, Perego C, et al. The ontogeny of the endocrine pancreas in the fetal/newborn Baboon. J Endocrinol. 2012 Jun; doi: 10.1530/JOE-12-0070. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van der Lugt NM, Smits-Wintjens V, van Zwieten P, Walther F. Short and long term outcome of neonatal hyperglycemia in very preterm infants: a retrospective follow-up study. BMC Pediatrics. 2010;10(1):52. doi: 10.1186/1471-2431-10-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muggeo M, Verlato G, Bonora E, Zoppini G, Corbellini M, de marco R. Long-term instability of fasting plasma glucose, a novel predictor of cardiovascular mortality in elderly patients with non-insulin-dependent diabetes mellitus : The Verona Diabetes Study. Circulation. 1997;96:1750–1754. doi: 10.1161/01.cir.96.6.1750. [DOI] [PubMed] [Google Scholar]
- 22.Muggeo M, Verlato G, Bonora E, et al. Long-term instability of fasting plasma glucose predicts mortality in elderly NIDDM patients: the Verona Diabetes Study. Diabetologia. 1995;38:672–679. doi: 10.1007/BF00401838. [DOI] [PubMed] [Google Scholar]
- 23.Rake AJ, Srinivasan V, Nadkarni V, Kaptan R, Newth CJL. Glucose variability and survival in critically ill children: Allostasis or harm? Pediatr Crit Care Med. 2010;11:707–712. doi: 10.1097/PCC.0b013e3181e88b1f. [DOI] [PubMed] [Google Scholar]
- 24.Chu CA, Sindelar DK, Neal DW, Allen EJ, Donahue EP, Cherrington AD. Comparison of the direct and indirect effects of epinephrine on hepatic glucose production. J Clin Invest. 1997;99:1044–1056. doi: 10.1172/JCI119232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stoll BJ, Hansen N, Fanaroff AA, et al. Late-onset sepsis in very low birth weight neonates: The experience of the NICHD Neonatal Research Network. Pediatrics. 2002;110:285–291. doi: 10.1542/peds.110.2.285. [DOI] [PubMed] [Google Scholar]
- 26.Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, et al. Prevalence and determinants of hyperglycemia in very low birth weight infants: cohort analyses of the NIRTURE Study. J Pediatr. 2010;157:715–719. doi: 10.1016/j.jpeds.2010.04.032. [DOI] [PubMed] [Google Scholar]
- 27.Halliday HL, Ehrenkranz RA, Doyle LW. Early (< 8 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane database Syst Rev. 2010;1:CD001146. doi: 10.1002/14651858.CD001146.pub3. [DOI] [PubMed] [Google Scholar]
- 28.Van den Berghe G, Wilmer A, Hermans G, et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449–461. doi: 10.1056/NEJMoa052521. [DOI] [PubMed] [Google Scholar]
- 29.Finfer S, Chittock DR, Su SY, et al. Intensive versus conventional glucose control in critically ill patients. N Engl J Med. 2009;360:1283–1297. doi: 10.1056/NEJMoa0810625. [DOI] [PubMed] [Google Scholar]
- 30.Preissig C, Rigby M, Maher K. Glycemic control for postoperative pediatric cardiac patients. Pediatr Cardiol. 2009;30:1098–1104. doi: 10.1007/s00246-009-9512-4. [DOI] [PubMed] [Google Scholar]
- 31.Garcia BR, Tasker RC, Ramos Garcia PC, Piva JP, Dias XL. Glycemic control and insulin therapy in sepsis and critical illness. J Pediatr. 2007;83:S128–S136. doi: 10.2223/JPED.1710. [DOI] [PubMed] [Google Scholar]
- 32.Bochicchio GV, Sung J, Joshi M, et al. Persistent hyperglycemia is predictive of outcome in critically ill trauma patients. J Trauma. 2005;58:921–924. doi: 10.1097/01.ta.0000162141.26392.07. [DOI] [PubMed] [Google Scholar]
- 33.Gore DC, Chinkes D, Heggers J, Herndon DN, Wolf SE, Desai M. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 34.Ng SM, May JE, Emmerson AJ. Continuous insulin infusion in hyperglycaemic extremely-low-birth-weight neonates. Biol Neonate. 2005;87:269–272. doi: 10.1159/000083863. [DOI] [PubMed] [Google Scholar]
- 35.Beardsall K, Dunger D. Insulin therapy in preterm newborns. Early Hum Dev. 2008;84:839–842. doi: 10.1016/j.earlhumdev.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Whincup PH, Kaye SJ, Owen CG, et al. Birth weight and risk of type 2 diabetes. JAMA. 2008;300:2886–2897. doi: 10.1001/jama.2008.886. [DOI] [PubMed] [Google Scholar]
- 37.Catalano PM, Kirwan JP, Haugel-de Mouzon S, King J. Gestational diabetes and insulin resistance: role in short- and long-term implications for mother and fetus. J Nutr. 2003;133:1674S–1683S. doi: 10.1093/jn/133.5.1674S. [DOI] [PubMed] [Google Scholar]
- 38.Hofman PL, Regan F, Jackson WE, et al. Premature birth and later insulin resistance. N Engl J Med. 2004;351:2179–2186. doi: 10.1056/NEJMoa042275. [DOI] [PubMed] [Google Scholar]
- 39.Chi MMY, Hoehn A, Moley KH. Metabolic changes in the glucose-induced apoptotic blastocyst suggest alterations in mitochondrial physiology. AM J Physiol Endocrinol Metab. 2002;283:E226–E232. doi: 10.1152/ajpendo.00046.2002. [DOI] [PubMed] [Google Scholar]
- 40.Wang R, Martinez-Frias ML, Graham JM., Jr Infants of diabetic mothers are at increased risk for the oculo-auriculo-vertebral sequence: A case-based and case-control approach. J Pediatr. 2002;141:611–617. doi: 10.1067/mpd.2002.128891. [DOI] [PubMed] [Google Scholar]