Abstract
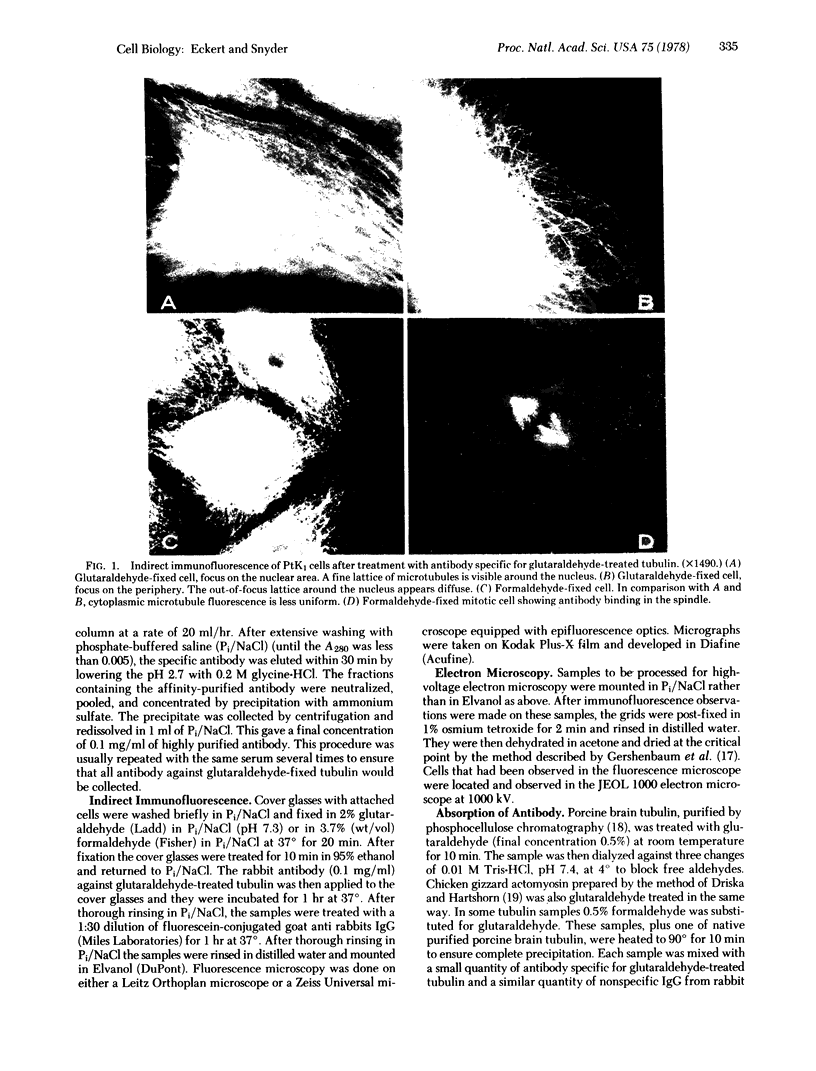
An antibody against tubulin that binds specifically to microtubules in glutaraldehyde-fixed cells has been prepared. Sodium dodecyl sulfate-denatured tubulin treated with glutaraldehyde was used to immunize rabbits. In glutaraldehyde-fixed cells the fluorescent image of this antibody reveals a fine lattice of microtubules around the nucleus of PtK1 (Potorous tridactylis) cells and many uniformly fluorescent microtubules in the peripheral cytoplasm. In cells fixed with formaldehyde the microtubules appear to have a similar distribution, but the fluorescent image is much less uniform. Combined high-voltage electron microscopy and immunofluorescent studies reveal that microtubules are found in the cytoplasm in the same region as the fluorescent antibody stain.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley I. K., Porter K. R. Electron microscopy of critical point dried whole cultured cells. J Microsc. 1975 Jul;104(2):107–120. doi: 10.1111/j.1365-2818.1975.tb04010.x. [DOI] [PubMed] [Google Scholar]
- Cande W. Z., Lazarides E., McIntosh J. R. A comparison of the distribution of actin and tubulin in the mammalian mitotic spindle as seen by indirect immunofluorescence. J Cell Biol. 1977 Mar;72(3):552–567. doi: 10.1083/jcb.72.3.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J., Hoebeke J., De Brabander M., Geuens G., Joniau M. Immunoperoxidase visualisation of microtubules and microtubular proteins. Nature. 1976 Nov 18;264(5583):273–275. doi: 10.1038/264273a0. [DOI] [PubMed] [Google Scholar]
- Driska S., Hartshorne D. J. The contractile proteins of smooth muscle. Properties and components of a Ca2+-sensitive actomyosin from chicken gizzard. Arch Biochem Biophys. 1975 Mar;167(1):203–212. doi: 10.1016/0003-9861(75)90457-9. [DOI] [PubMed] [Google Scholar]
- Fujiwara K., Pollard T. D. Fluorescent antibody localization of myosin in the cytoplasm, cleavage furrow, and mitotic spindle of human cells. J Cell Biol. 1976 Dec;71(3):848–875. doi: 10.1083/jcb.71.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller G. M., Brinkley B. R., Boughter J. M. Immunofluorescence of mitotic spindles by using monospecific antibody against bovine brain tubulin. Science. 1975 Mar 14;187(4180):948–950. doi: 10.1126/science.1096300. [DOI] [PubMed] [Google Scholar]
- Goldman R. D., Lazarides E., Pollack R., Weber K. The distribution of actin in non-muscle cells. The use of actin antibody in the localization of actin within the microfilament bundles of mouse 3T3 cells. Exp Cell Res. 1975 Feb;90(2):333–344. doi: 10.1016/0014-4827(75)90323-7. [DOI] [PubMed] [Google Scholar]
- Lazarides E. Actin, alpha-actinin, and tropomyosin interaction in the structural organization of actin filaments in nonmuscle cells. J Cell Biol. 1976 Feb;68(2):202–219. doi: 10.1083/jcb.68.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E. Two general classes of cytoplasmic actin filaments in tissue culture cells: the role of tropomyosin. J Supramol Struct. 1976;5(4):531(383)–563(415). doi: 10.1002/jss.400050410. [DOI] [PubMed] [Google Scholar]
- Nakane P. K. Recent progress in the peroxidase-labeled antibody method. Ann N Y Acad Sci. 1975 Jun 30;254:203–211. doi: 10.1111/j.1749-6632.1975.tb29170.x. [DOI] [PubMed] [Google Scholar]
- Osborn M., Weber K. Tubulin-specific antibody and the expression of microtubules in 3T3 cells after attachment to a substratum. Further evidence for the polar growth of cytoplasmic microtubules in vivo. Exp Cell Res. 1976 Dec;103(2):331–340. doi: 10.1016/0014-4827(76)90270-6. [DOI] [PubMed] [Google Scholar]
- Snyder J. A., McIntosh J. R. Initiation and growth of microtubules from mitotic centers in lysed mammalian cells. J Cell Biol. 1975 Dec;67(3):744–760. doi: 10.1083/jcb.67.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ternynck T., Avrameas S. Polyacrylamide-protein immunoadsorbents prepared with glutaraldehyde. FEBS Lett. 1972 Jun 1;23(1):24–28. doi: 10.1016/0014-5793(72)80274-6. [DOI] [PubMed] [Google Scholar]
- Weber K., Wehland J., Herzog W. Griseofulvin interacts with microtubules both in vivo and in vitro. J Mol Biol. 1976 Apr 25;102(4):817–829. doi: 10.1016/0022-2836(76)90293-x. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G., Cole R. D. An improved preparation of highly specific tublin antibodies. Exp Cell Res. 1976 Apr;99(1):15–22. doi: 10.1016/0014-4827(76)90674-1. [DOI] [PubMed] [Google Scholar]
- Wolosewick J. J., Porter K. R. Stereo high-voltage electron microscopy of whole cells of the human diploid line, WI-38. Am J Anat. 1976 Nov;147(3):303–323. doi: 10.1002/aja.1001470305. [DOI] [PubMed] [Google Scholar]