Abstract
Aim
To evaluate the efficacy of a standardised combination therapy for clinically significant diabetic macular oedema using bevacizumab injections followed by navigated laser photocoagulation to stabilise retinal thickness.
Methods
In this pilot study we retrospectively reviewed charts and imaging of 23 eyes treated with the standardised combination regimen. Eyes initially received monthly bevacizumab injections, followed by navigated laser photocoagulation when central retinal thickness (CRT) was <440 µm. Patients were then followed monthly for 12 months.
Results
At the time of navigated laser after bevacizumab treatment mean vision gain was +10.4 Early Treatment Diabetic Retinopathy Study letters (p<0.01) and CRT reduction was 146 µm (p<0.001). At 12 months from baseline, the vision gain remained stable at +10.6 Early Treatment Diabetic Retinopathy Study letters (p<0.01), and CRT reduction was stable at 137 µm (p<0.001). At 12 months from laser, the vision gain was 7.8 letters from baseline (p<0.01), with no significant change compared with the gain at 12 months from baseline (p=0.108). At 12 months from laser, CRT reduction was 125 µm from baseline (p<0.001), with no significant change compared with CRT reduction at 12 months from baseline (p=0.601). Total injections needed were 4.4 from baseline to month 12, with 1.3 reinjection needed after laser. 57% of the eyes didn't require injections after laser, while 43% needed two additional injections.
Conclusions
Standardised combination therapy using bevacizumab injections followed by navigated laser treatment for clinically significant diabetic macular oedema demonstrated significant visual gain and CRT reduction after bevacizumab treatment and stabilisation after navigated laser up to 12 months. The number of injections required in 12 months was lower than reported in previous combination studies.
Keywords: Retina, Macula, Treatment Lasers
Introduction
With the introduction of vascular endothelial growth factor inhibitors (anti-VEGF), the management of clinically significant diabetic macular oedema (CSMO) has progressively shifted to the use of intravitreal drug therapies, usually injected every 4–6 weeks.1–8 To limit the treatment burden associated with frequent anti-VEGF injections, combination regimens with macular laser photocoagulation and anti-VEGF drugs have been investigated in multiple studies to determine if they might result in fewer required interventions.3 5–9 However, attempts to limit the number of injections have not produced comparable visual outcomes.3–7 10–12 Two potential limitations of these studies include the variable timing and application of laser therapy. Subgroup analysis of the RESTORE (Ranibizumab Monotherapy or Combined with Laser versus Laser Monotherapy for Diabetic Macular Edema) trial indicated that patients with retinal thickness of 300 µm or less did equally well with laser or anti-VEGF monotherapy, while patients with the thickest retinas benefited the most from anti-VEGF monotherapy.6 From these results, we hypothesised that initial treatment with anti-VEGF monotherapy might be used to reduce retinal thickness in patients with CSMO, and thereby might provide an improved substrate for subsequent focal laser application on thinner retinas.
New laser technologies for retinal treatments have been recently introduced to help physicians in the macular treatment of retinovascular diseases, with the use of computer-guided targeting systems associated with eye-tracking. The Navilas laser (OD-OS GmbH, Teltow, Germany) is an example of image-guided (navigated) photocoagulator system that was designed to improve the accuracy to localise and hit microaneurysms while overcoming the difficulties of slit lamp based laser delivery.13–15 Based on initial results that showed reduced laser retreatment rate in CSMO eyes treated with navigated laser compared with conventional laser,16 we further hypothesised that navigated laser therapy might provide faster stabilisation and closure of leaking retinal microaneurysms, thereby reducing the potential requirement for repeated anti-VEGF injections.
Based on this rationale, we instituted a standardised combination therapy regimen for eyes with clinically significant CSMO at our institutions, using initial bevacizumab injections until central retinal thickness (CRT) was significantly reduced and then applying navigated laser therapy to treat leaking microaneurysms and stabilise retinal thickness. The aim of the present study was to assess the efficacy of this standardised combination regimen with bevacizumab and navigated laser photocoagulation for CSMO eyes over a 12-month period.
Material and methods
Participants
Starting from May 2011, patients with clinically significant CSMO at the Jacobs Retina Center at Shiley Eye Center (University of California San Diego, La Jolla, CA) and at the Instituto de Sub-Especialidades Oftalmologicas (Tijuana, Mexico) were treated with a standardised protocol and followed for at least 12 months from baseline. After approval by the Institutional Review Board of the University of California San Diego, we retrospectively reviewed charts and imaging studies of 23 eyes of 18 subjects seen between May 2011 and June 2012 who met the inclusion/exclusion criteria. We limited our enrolment to patients aged >18 years with diabetes mellitus type 1 or 2, decreased vision from CSMO (≤ 20/40 Snellen equivalent using Early Treatment Diabetic Retinopathy Study (ETDRS) testing) and macular oedema on optical coherence tomography (OCT) with CRT≥400 µm. Key exclusion criteria were retinal thickening due to epiretinal membranes or vitreomacular traction, macular ischaemia on fluorescein angiography (FA), history of laser panphotocoagulation or macular laser treatment within 4 months, history of anti-VEGF injections or steroids for intraocular use received within 3 months of starting the standardised regimen, or major ocular surgery within 4 months of starting the standardised regimen. We also excluded patients with uncontrolled hypertension, and patients who did not complete at least 12 months of follow-up. The study was in adherence to the tenets of the Declaration of Helsinki, and all participants provided signed informed consent form.
Treatment protocol
At baseline, all patients underwent a baseline examination including best-corrected visual acuity (BCVA), slit lamp examination, measurement of intraocular pressure, dilated funduscopic examination, OCT evaluation and FA. This was followed by a standardised treatment protocol. The standardised treatment regimen included an initial series of at least two intravitreal injections of bevacizumab (1.25 mg/0.05 mL) spaced 4 weeks apart. Navigated laser therapy was applied after retinal thinning which was as early as 4 weeks following the second injection in eyes that showed evidence of improvement (defined as greater than 20% decrease in CRT, or more than 5 letters gain in BCVA compared with baseline) and CRT less than 440 µm on Heidelberg Spectralis (Heidelberg Engineering, Carlsbad, CA). This CRT threshold on Heidelberg Spectralis is approximately equivalent to 300 µm on StratusOCT (Carl Zeiss Meditec, Dublin, CA).17 We used this threshold CRT based on results of the RESTORE study, which demonstrated that laser monotherapy was as effective as ranibizumab monotherapy in cases of retinal thickness of 300 µm or below as measured by StratusOCT.6 Eyes that did not reach the threshold CRT for laser therapy by the fifth bevacizumab injection were not considered for this treatment regimen and were not included in the study.
After navigated laser treatment, eyes were followed monthly with BCVA, indirect ophthalmoscopy and OCT. In cases of worsening of CRT greater than 20% from the lowest measurement, or in cases of BCVA loss greater than 5 letters compared with the measurement at the time of laser treatment, eyes received one additional bevacizumab injection and were re-evaluated monthly for additional pro re nata injections. FA was repeated 4–6 months following initial laser therapy.
Navigated laser photocoagulation procedure
Macular laser photocoagulation treatments were performed using the Navilas Laser System. As described previously,13 this computer-based system combines wide-angle imaging and FA to precisely deliver 532 nm laser light. Briefly, treatment plan is made by physicians on a static image from the fluorescein angiogram and then registered and overlaid onto the live retinal image in real time. The Navilas Laser system automatically pre-positions the laser beam to the planned treatment locations using eye-tracking feature, allowing the surgeon to complete the treatment plan with great accuracy.18 A post-treatment report documents the location and parameters for each laser spot.
Treatment plans were based on the modified ETDRS protocol using single spot targeting of microaneurysms with 100 µm laser spot size, 100 ms pulse duration and power to achieve a pale grey burn.19 For macular areas with diffuse leakage, grid pattern spots were added on the extent of the leakage, at least 100 µm apart, using 100 µm laser spot size, 100 ms pulse duration, and power to obtain a barely visible burn. Navigated laser treatments were performed by a single trained physician at the Jacobs Retina Center, and by another trained physician at the Instituto de Sub-Especialidades Oftalmologicas.
Optical Coherence Tomography
OCT was performed at every visit using either the Heidelberg Spectralis or the Ivue SD-OCT (Optovue, Freemont, CA). After checking the correct subfoveal position of the ETDRS map, retinal thickness of the 1-mm central retina was recorded. To compare retinal thickness from the two different SD-OCT devices, the conversion formula provided by Giani et al was applied: Spectralis OCT=Optovue×1+48.4 µm.17
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 V.5.04 (GraphPad Software, La Jolla, California, USA). The outcome measures were: (1) change in BCVA at 12 months from baseline and at 12 months from laser treatment; (2) change in CRT at 12 months from baseline and at 12 months from laser treatment; and (3) number of bevacizumab injections needed at 12 months from baseline and at 12 months from laser treatment. Paired samples t-test was performed to compare the CRT and the BCVA with baseline values. A p<0.05 was considered statistically significant.
Results
A total of 23 eyes from 18 subjects who met the inclusion/exclusion criteria were reviewed, with baseline characteristics presented in table 1. Mean baseline BCVA was 44±20 letters (Snellen equivalent 20/160. Range, count fingers to 20/30) and mean baseline CRT was 484±117 µm (range, 401 µm to 798 µm).
Table 1.
Baseline characteristics of the sample
| Characteristic | Value |
|---|---|
| Number of eyes/patients | 23 eyes/20 patients |
| Sex, n (%) | |
| Male | 13 (65%) |
| Female | 7 (35%) |
| Age (years) | |
| Range | 35–82 |
| Mean±SD | 62±10 |
| Type of diabetes, n (%) | |
| Type 1 | 1 (5%) |
| Type 2 | 19 (95%) |
| Duration of diabetes (years) | |
| Range | 1–27 |
| Mean±SD | 12±8 |
| HbA1c (%) | |
| Range | 6.0–11.0 |
| Mean±SD | 7.2±1.4 |
| Type of CSMO, n (%) | |
| Focal | 5 (22%) |
| Diffuse | 18 (78%) |
| Type of diabetic retinopathy, n (%) | |
| Background | 15 (65%) |
| Proliferative | 8 (35%) |
| CRT (µm) | |
| Mean±SD | 484±117 |
| Range | 401–798 |
| BCVA | |
| Mean±SD in ETDRS letters (Snellen eq.) | 44±20 (20/160) |
| Range in Snellen equivalent | CF—20/30 |
| Previous treatments, n (%) | |
| Anti-VEGF (bevacizumab) | 11 (48%) |
| Focal Laser | 6 (26%) |
| Intravitreal Steroids | 2 (8%) |
| Anti-VEGF (bevacizumab) + focal laser | 4 (17%) |
| PRP | 8 (35%) |
BCVA, best-corrected visual acuity; CF, count fingers; CRT, central retinal thickness; CSME, clinically significant macular oedema; ETDRS, Early Treatment Diabetic Retinopathy Study; PRP, panretinal photocoagulation; VEGF, vascular endothelial grow factor.
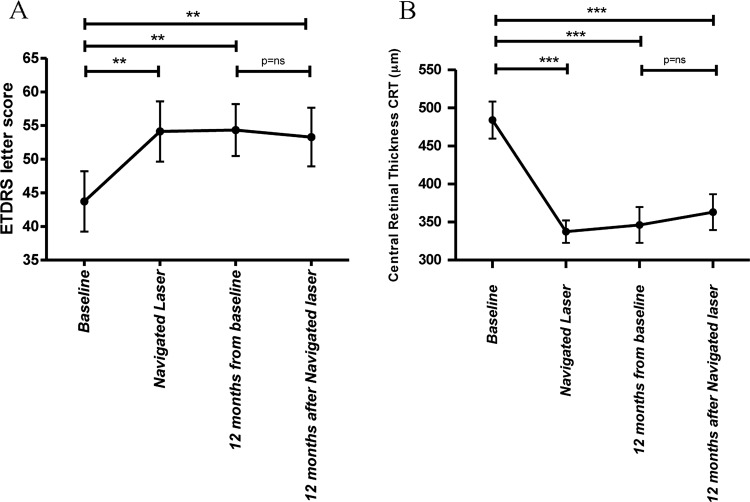
As presented in figure 1A, mean BCVA improved after the initial anti-VEGF therapy by 9.4±14, 10.1±14 and 12.3±16 letters after one, two and three injections, respectively. Navigated laser treatment was performed at 69±24 days from baseline (median=60 days). At the time of laser treatment (table 2), mean BCVA was improved by 10.4±15 letters from baseline (p<0.01, median=7 letters), and remained stable through month 12 from baseline with a mean visual improvement of 10.6±18 letters and a median of 7 letters. At 12 months after laser treatment, BCVA was improved by 7.8±10 letters from baseline (p<0.01, median=7 letters), with no significant changes compared with BCVA scores at 12 months after baseline (p=0.108). Between the time of laser treatment and 12 months from baseline, the change in BCVA was 0.3±10 letters (p=0.670). At 12 months (table 2), 43% (10/23) of the eyes improved by 10 or more letters from baseline, 35% (8/23) improved by more than 15 letters compared with baseline and 8% (2/23) of the eyes had lost 10 or more letters.
Figure 1.
(A) Change in best-corrected visual acuity (BCVA) and (B) change in central retinal thickness (CRT) during the study. At the time of navigated laser (median, 60 days from baseline), mean BCVA showed and improvement of 10.4 letters from baseline; it remained stable at 12 months from baseline with a mean improvement of 10.6 letters, and also at 12 months after navigated laser with a mean improvement of 7.8 letters. A significant difference was found in the change of BCVA from baseline to the time of navigated laser, from baseline to 12 months and from baseline to 12 months after navigated laser (**p<0.01). CRT decreased by a mean of 146 µm at the time of navigated laser; CRT remained stable with a change of 137 µm at 12 months from baseline, and 125 µm at 12 months from navigated laser. A significant difference was found in the change of CRT from baseline to the time of navigated laser, from baseline to 12 months, and from baseline to 12 months after navigated laser (***p<0.001). ETDRS, Early Treatment Diabetic Retinopathy Study.
Table 2.
Mean change in BCVA and CRT from baseline and analysis by subgroup
| Change in BCVA (letters) | Change in CRT (µm) | ||
|---|---|---|---|
| From baseline to laser treatment | Mean: +10.4±15 Median: +7 | Mean: −146±108 Median: −112 | |
| From baseline to month 12 after baseline | Mean: +10.6±18 Median: +7 | Mean: −137±126 Median: −156 | |
| From baseline to month 12 after laser | Mean: +7.8±10 Median: +7 | Mean: −125±104 Median: −134 | |
| Categorised BCVA gain at 12 months after baseline | |||
| Gain≥15 letters | 8/23 (35%) | ||
| Gain≥10 letters | 10/23 (43%) | ||
| Gain≥5 letters | 14/23 (60%) | ||
| No gain | 7/23 (30%) | ||
| Loss≥10 letters | 2/23 (8%) | ||
BCVA, best-corrected visual acuity; CRT, central retinal thickness.
As presented in figure 1B, mean CRT decreased by 119±109 µm (23%), 139±106 µm (27%) and 162±121 µm (32%) after one, two and three injections, respectively. At the time of laser treatment (table 2), mean CRT reduction from baseline was 146±108 µm (28%, p<0.001) and median CRT reduction was 112 µm. At 12 months after baseline, CRT reduction remained stable at 137±126 µm (25%). The most significant change in CRT took place after the first injection (p<0.001) associated with a significant increase in BCVA (p<0.01). At 12 months after laser treatment, mean CRT reduction from baseline was 125±104 µm (p<0.001), with no significant changes compared with CRT at 12 months after baseline (p=0.601). The change in CRT compared with month 12 after baseline was+19±97 µm (p=0.930).
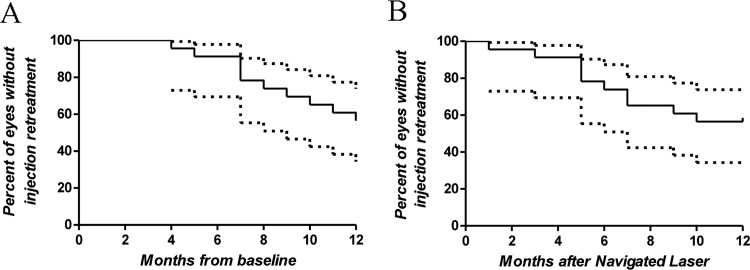
At the time of navigated laser treatment, 78.2% eyes (18/23) had received two bevacizumab injections, 13.0% eyes (3/23) had received three injections, and 8.8% eyes (2/23) had received more than three injections. The mean number of injections before navigated laser was 3.1±0.54. After navigated laser treatment, injection retreatment was performed when eyes met worsening criteria. For all eyes, the mean number of injections needed from navigated laser treatment to month 12 from baseline was 0.8±0.2, and from navigated laser to month 12 from laser was 1.3±1.6. The mean number of injections needed from baseline to month 12 was 4.0±1.2 (range 3–7, table 3), and from baseline to month 12 after laser was 4.4±1.4 (range 3–8). At 12 months from baseline, 13 out of 23 eyes (57%) did not meet worsening criteria and did not require additional intravitreal injections after navigated laser treatment. Ten out of 23 eyes (43%) met worsening criteria and were given a mean of 2±1.1 additional bevacizumab injections after laser treatment (median=2, range 1–4), with mean time to reinjection of 6.5 months (range 2–9 months). Figure 2A,B show the proportion of eyes that did not need additional bevacizumab injections over time.
Table 3.
Analysis of number of injections needed
| Number of injections needed before navigated laser | |
| Mean±SD | 3.1±0.5 |
| Median | 3 |
| Range | 3–5 |
| Number of injections needed after laser to month 12 from baseline | |
| Mean±SD | 0.8±1.2 |
| Median | 0 |
| Range | 0–4 |
| Number of injections needed after laser to month 12 from laser | |
| Mean±SD | 1.32±1.61 |
| Median | 1 |
| Range | 0–5 |
| Total number of injections needed in 12 months from baseline | |
| Mean±SD | 4.0±1.2 |
| Median | 3 |
| Range | 3–7 |
| Total number of injections needed in 12 months from laser | |
| Mean±SD | 4.4±1.2 |
| Median | 4 |
| Range | 3–8 |
| Proportion of eyes receiving reinjections after navigated laser | 43% (10/23) |
| Mean interval to first reinjection | 6½ months |
| Range | 2–9 months |
| Proportion of eyes with no reinjections needed after navigated laser | 57% (13/23) |
Figure 2.
Survival plots showing the percentage of eyes with no need of bevacizumab reinjections after navigated laser. (A) At 12 months from baseline, 57% of the eyes did not require additional injections. (B) At 12 months from navigated laser, 53% of the eyes did not require additional injections.
Discussion
This retrospective review of eyes with CSMO treated with a standardised regimen (monthly bevacizumab injections followed by navigated laser when a threshold CRT was attained) demonstrated a mean improvement of 10 letters in BCVA at 12 months from baseline, and of 8 letters at 12 months after laser. The median improvement of BCVA was constant at 7 letters during all the follow-up. This vision gain was maintained over the 1-year study with only one additional injection needed after stabilisation of retinal thickness using navigated laser. Focal navigated laser therapy applied to an oedematous retina made thin by serial bevacizumab injections appeared to provide excellent visual improvement while limiting the number of reinjections needed over 12 months. While these observations must be verified in a larger, prospective trial, these results may be compared with other clinical studies that have evaluated combination therapy previously using conventional slit lamp based lasers.
In the combination arm of the RESTORE study,6 eyes received three initial consecutive monthly injections of ranibizumab, followed by focal/grid laser treatment at day 1 and at 3-month intervals from the previous treatment, if necessary. Further monthly ranibizumab treatment was needed by protocol if stable visual acuity was not reached at the last two consecutive visits. After suspension, injections were resumed with a pro re nata regimen if there was a decrease in BCVA due to CSMO worsening. Visual outcomes in this combination arm showed a mean improvement of 5.9 letters during the first 12 months, during which they received a mean of 6.8±2.95 injections (median=7) with mean duration of the treatment-free interval of approximately 2½ months. These results were not significantly different from results of the injection monotherapy arm and so no benefit of conventional laser was seen overall. However, laser monotherapy demonstrated to be as effective as ranibizumab monotherapy in cases of retinal thickness of 300 µm or below as measured by StratusOCT (equivalent to 440 µm in the current study using Heidelberg Spectralis17). Therefore, laser alone seemed to have a role in the treatment of CSMO in cases of not too thick retina.
In the DRCR (Diabetic Retinopathy Clinical Research Network) trial evaluating ranibizumab plus laser for CSMO,3 there were multiple combination groups, including ranibizumab plus prompt laser, ranibizumab plus deferred laser and sham plus prompt laser. Ranibizumab/sham injections were given every 4 weeks prior to week 16; afterwards, eyes were treated following a retreatment algorithm based on BCVA and CRT categorised in success, improvement and failure criteria. In the ranibizumab plus prompt laser, the median number of laser treatments was two while in the ranibizumab plus deferred laser, 72% eyes received no focal laser treatment during the 1st year. Both combination arms, either ranibizumab plus prompt or deferred laser showed a mean improvement of 9 letters. The median number of injections at 12 months was eight and nine, respectively suggesting no additional benefit of conventional laser for treatment of CSMO.
While neither of these studies demonstrated a benefit in combining anti-VEGF with conventional laser, there is support in the literature for a regimen with lower treatment burden, for example, requirement of fewer injections. In the READ-2 (Ranibizumab for Edema of the mAcula in Diabetes) trial,7 8 the combination arm received a total number of 4.9 injections compared with 9.3 in the ranibizumab monotherapy group with comparable mean visual improvements of 6.8 and 7.7 letters, respectively. However, the study showed that, when combining ranibizumab with focal or grid laser treatment, the amount of residual oedema was reduced, as well as the frequency of injections needed to control CSMO.
In the present study, following the RESTORE study results,6 we used a quantitative threshold for retinal thickness at which laser should be applied (eg, 440 µm on Heidelberg Spectralis, that are equivalent to 300 µm on StratusOCT17) after retinal thinning using bevacizumab monthly, combined with the use of navigated laser treatment. We know from previous studies that navigated laser allows a great outcome in terms of closure rate of leaking microaneurysms.14 In addition, there is some initial evidence—however not yet confirmed by multicentre randomised clinical trials—that it may increase the accuracy of laser therapy compared with conventional laser.16 The increased accuracy may be related to features of the navigated laser such as retinal eye-tracking and laser stabilisation system.13 A further advantage of the navigated laser system is that it allows standardised and documented laser therapy, potentially providing a method for more uniform results across practitioners. These considerations may explain why our combined treatment protocol led to higher BCVA gain (+10 letters at 1 year) and lower retreatment need (four bevacizumab injections in 1 year, one injection after navigated laser) compared with the previous studies. In other words, navigated focal/laser photocoagulation seemed to be effective in stabilising retinal thickness after thinning with bevacizumab, and therefore in reducing treatment burden for CSMO over 12 months.
Preliminary reports using a similar methodology have also supported this treatment approach. Kernt et al (Kernt M, Ulbig M, Haritoglou C, et al, “Combination of Ranibizumab and Navigated Retinal Photocoagulation vs Ranibizumab Mono-Therapy for Diabetic Macular Oedema: Twelve Month Results”. Paper session presented at the ARVO Annual Meeting, 5–9 May 2013, Seattle, Washington, USA) have compared a consecutive series of eyes treated with monthly ranibizumab injections with or without focal/grid navigated laser therapy when CRT was reduced to 440 µm or less on Heidelberg Spectralis. At 12 months, the laser treated group demonstrated better visual acuity and required approximately half the number of injections as the control group. These results compare favourably with our results, in which a similar mean number of injections (4) was found at 12 months. New anti-VEGF drugs (such as aflibercept for diabetic macular oedema4) and improvement in the navigated laser therapy (such as image stabilisation, or integration with OCT thickness map for treatment plan) may further improve these results.
Our study has some limitations. First, it is retrospective in nature; however, we carefully included in the study only patients who met strict inclusion/exclusion criteria. Second, it lacks of control group of eyes receiving bevacizumab monotherapy, or bevacizumab plus conventional laser. However, the preliminary report by Kernt et al demonstrated superiority of the combination group compared with anti-VEGF monotherapy group in increasing BCVA with low treatment burden. Moreover, the superiority of navigated laser compared with conventional laser in treating CSMO has been already suggested, even if not yet confirmed.16 Third, even if we found a mean improvement of 10 letters from baseline, the final BCVA was low (20/100); this finding was expected since the baseline BCVA was very poor (20/160) and previous studies have shown that the baseline BCVA is a predictor for the final visual outcome after macular treatment.20–22 However, we demonstrated that our combination protocol was helpful in gaining two ETDRS lines in our population with low baseline BCVA. Finally, the small sample size of the study population did not allow performing further analyses, such as evaluation of possible predictor factors for necessity of bevacizumab retreatment after navigated laser.
In conclusion, this pilot study demonstrated that navigated laser photocoagulation seems to have a role in stabilising retinal thickness in CSMO after thinning using bevacizumab therapy, resulting in significantly improved BCVA at 12 months. Using navigated laser to accurately treat leaking microaneurysms or areas of diffuse macular leakage, only 4.0 bevacizumab injections were needed in 12 months after baseline, and 4.4 in 12 months after laser treatment; therefore the treatment burden in patients with CSMO was limited. Further larger randomised clinical trials are necessary to validate our results.
Acknowledgments
The authors thank Lingyun Cheng, MD (Department of Ophthalmology, Jacobs Retina Center at Shiley Eye Center, University of California San Diego, La Jolla, California, USA) for helping with statistical analysis.
Footnotes
Contributors: Design and conduct of study (GB, IK, MC); Collection and management of data (GB, SE-E, JC); Analysis and interpretation of data (GB, IK); Preparation of draft of manuscript (GB, IK); and Review and approval of manuscript (WRF).
Funding: This study was supported by NIH grants R01EY007366 and R01EY018589, and in part by an unrestricted fund from Research to Prevent Blindness to the Department of Ophthalmology, University of California San Diego. The funding organisations had no role in the design or conduct of this research.
Competing interests: WRF is a consultant for OD-OS. The other authors have no proprietary, financial or conflicts of interest to disclose.
Ethics approval: Institutional Review Board of the University of California San Diego.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology 2007;114:743–50 [DOI] [PubMed] [Google Scholar]
- 2.Arevalo JF, Sanchez JG, Fromow-Guerra J, et al. Comparison of two doses of primary intravitreal bevacizumab (Avastin) for diffuse diabetic macular edema: results from the Pan-American Collaborative Retina Study Group (PACORES) at 12-month follow-up. Graefes Arch Clin Exp Ophthalmol 2009;247:735–43 [DOI] [PubMed] [Google Scholar]
- 3.Diabetic Retinopathy Clinical Research N, Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117:1064–77, e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Do DV, Nguyen QD, Boyer D, et al. One-year outcomes of the DA VINCI Study of VEGF Trap-Eye in eyes with diabetic macular edema. Ophthalmology 2012;119:1658–65 [DOI] [PubMed] [Google Scholar]
- 5.Elman MJ, Bressler NM, Qin H, et al. Expanded 2-year follow-up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011;118:609–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118:615–25 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen QD, Shah SM, Heier JS, et al. Primary End Point (Six Months) Results of the Ranibizumab for Edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2009;116:2175–81, e1 [DOI] [PubMed] [Google Scholar]
- 8.Nguyen QD, Shah SM, Khwaja AA, et al. Two-year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ-2) study. Ophthalmology 2010;117:2146–51 [DOI] [PubMed] [Google Scholar]
- 9.Soheilian M, Ramezani A, Obudi A, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009;116:1142–50 [DOI] [PubMed] [Google Scholar]
- 10.Arevalo JF, Garcia-Amaris RA. Intravitreal bevacizumab for diabetic retinopathy. Curr Diabetes Rev 2009;5:39–46 [DOI] [PubMed] [Google Scholar]
- 11.Arevalo JF, Maia M, Garcia-Amaris RA, et al. Intravitreal bevacizumab for refractory pseudophakic cystoid macular edema: the Pan-American Collaborative Retina Study Group results. Ophthalmology 2009;116:1481–7, 7 e1 [DOI] [PubMed] [Google Scholar]
- 12.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119:789–801 [DOI] [PubMed] [Google Scholar]
- 13.Kozak I, Oster SF, Cortes MA, et al. Clinical evaluation and treatment accuracy in diabetic macular edema using navigated laser photocoagulator NAVILAS. Ophthalmology 2011;118:1119–24 [DOI] [PubMed] [Google Scholar]
- 14.Lee SN, Chhablani J, Chan CK, et al. Characterization of microaneurysm closure after focal laser photocoagulation in diabetic macular edema. Am J Ophthalmol 2013;155:905–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chhablani J, Kozak I, Barteselli G, et al. A novel navigated laser system brings new efficacy to the treatment of retinovascular disorders. Oman J Ophthalmol 2013;6:18–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neubauer AS, Langer J, Liegl R, et al. Navigated macular laser decreases retreatment rate for diabetic macular edema: a comparison with conventional macular laser. Clin Ophthalmol 2013;7:121–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giani A, Cigada M, Choudhry N, et al. Reproducibility of retinal thickness measurements on normal and pathologic eyes by different optical coherence tomography instruments. Am J Ophthalmol 2010;150:815–24 [DOI] [PubMed] [Google Scholar]
- 18.Kozak I, Kim JS, Oster SF, et al. Focal navigated laser photocoagulation in retinovascular disease: clinical results in initial case series. Retina 2012;32:930–5 [DOI] [PubMed] [Google Scholar]
- 19.Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Early Treatment Diabetic Retinopathy Study Research Group. Ophthalmology 1987;94:761–74 [DOI] [PubMed] [Google Scholar]
- 20.Bloch SB, la Cour M, Sander B, et al. Predictors of 1-year visual outcome in neovascular age-related macular degeneration following intravitreal ranibizumab treatment. Acta Ophthalmol 2013;91:42–7 [DOI] [PubMed] [Google Scholar]
- 21.Chhablani J, Kim JS, Freeman WR, et al. Predictors of visual outcome in eyes with choroidal neovascularization secondary to age related macular degeneration treated with intravitreal bevacizumab monotherapy. Int J Ophthalmol 2013;6:62–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Emam S, Chhablani J, Barteselli G, et al. Correlation of spectral domain optical coherence tomography characteristics with visual acuity in eyes with subfoveal scarring after treatment for wet age-related macular degeneration. Retina 2013;33:1249–57 [DOI] [PubMed] [Google Scholar]