Abstract
The slit diaphragm (SD), the specialized intercellular junction between renal glomerular epithelial cells (podocytes), provides a selective-filtration barrier in renal glomeruli. Dysfunction of the SD results in glomerular diseases that are characterized by disappearance of SD components, such as nephrin, from the cell surface. Although the importance of endocytosis and degradation of SD components for the maintenance of SD integrity has been suggested, the dynamic nature of the turnover of intact cell-surface SD components remained unclear. Using isolated rat glomeruli we show that the turnover rates of cell-surface SD components are relatively high; they almost completely disappear from the cell surface within minutes. The exocytosis, but not endocytosis, of heterologously expressed nephrin requires the kinase activity of the cell polarity regulator atypical protein kinase C (aPKC). Consistently, we demonstrate that podocyte-specific deletion of aPKCλ resulted in a decrease of cell-surface localization of SD components, causing massive proteinuria. In conclusion, the regulation of SD turnover by aPKC is crucial for the maintenance of SD integrity and defects in aPKC signalling can lead to proteinuria. These findings not only reveal the pivotal importance of the dynamic turnover of cell-surface SD components but also suggest a novel pathophysiological basis in glomerular disease.
Keywords: aPKC, cell-surface localization, glomerular disease, nephrin, slit diaphragm
The glomerular filtration barrier is composed of fenestrated capillary endothelial cells, the glomerular basement membrane (GBM) and glomerular visceral epithelial cells (podocytes). These components work together to form a size- and charge-selective filtration barrier that prevents the leakage of cells or macromolecules into the urine. Podocytes extend primary processes with branched protrusions called foot processes to cover the outer surface of glomerular capillaries. These foot processes from neighbouring podocytes are interdigitated to form a specialized intercellular junction, called the slit diaphragm (SD), that plays a critical role in glomerular filtration (1). Most glomerular disease states are characterized by dramatic morphological alteration of podocyte foot processes, called foot process effacement, which involves loss of typical SD structure, resulting in proteinuria, progressive renal damage and eventually the loss of renal function (1).
The framework of the SD is composed largely of nephrin, a transmembrane protein of the immunoglobulin superfamily. Nephrin molecules interact with one another in a homophilic manner, or with other SD components, such as neph1 and podocin, to form the zipper-like structure of the SD (2, 3). Nephrin is encoded by the NPHS1 gene, and patients who harbour mutations in the NPHS1 gene develop heavy proteinuria before birth and rapidly progress to end stage renal failure, this is known as congenital nephrotic syndrome of the Finnish type (4, 5). Mutated forms of nephrin or podocin found in congenital nephrotic syndrome show defects in cell-surface localization when expressed in HEK293 cells; most localized to the endoplasmic reticulum (ER), whereas wild-type proteins localized to the cell surface (6, 7). In addition, staining of nephrin in patients with various nephrotic syndromes shows defects in the continuous linear pattern, a decrease in the staining at foot processes and an increase in intracellular compartments (8–12). Furthermore, administration of a monoclonal antibody against the extracellular domain of nephrin into rats causes proteinuria, which is also associated with defects in the cell-surface localization of nephrin (13, 14). Change of the staining pattern of nephrin from a linear pattern to a granular pattern is also seen in an animal model, puromycin aminonucleoside nephrosis (15–18). Previously, nephrin has been shown to be rapidly endocytosed through clathrin and raft-dependent pathways in cultured COS-7 cells (19). Furthermore, the endocytosis of nephrin is facilitated by disease-causing conditions through the molecular interaction with β-arrestin2, PKCα or CIN85 (20–23). Recent reports also revealed the importance of protein trafficking, autophagy and protein degradation in the maintenance of the SD (24–27). These observations all support the importance of membrane trafficking of SD components, especially the cell-surface localization of nephrin and other SD components, in the physiology and pathology of the SD. However, the molecular mechanisms leading to cell-surface localization of SD components, as well as those regulating the turnover of SD, are largely unknown.
The atypical protein kinase C-partitioning defective (aPKC-Par) complex is an evolutionally conserved ternary complex composed of the serine-threonine kinase aPKC and two scaffold proteins, Par3 and Par6 (28). This complex plays a critical role in the formation and maintenance of the cell–cell junction in epithelial cells (28). The importance of the aPKC-Par complex is highlighted by the finding that it associates with nephrin, neph1 and podocin through the direct interaction between nephrin and Par3 (29, 30). Furthermore, prevention of the formation of the aPKC-Par complex by podocyte-specific depletion of the aPKC isoform aPKCλ in mice (aPKCλ conditional knockout (cKO)) leads to massive proteinuria, the disassembly of the SD with effacement of podocyte foot processes, and finally develops into severe glomerulosclerosis (30, 31). However, the precise role of the aPKC-Par complex in the maintenance of the SD is largely unknown. Several recent studies have shown that aPKC regulates the turnover of adherence junction proteins and cell-surface receptors through the suppression of their endocytosis (32–34). aPKC is also suggested to regulate polarized exocytosis in epithelial cells (35, 36). These observations raise the possibility that aPKC regulates the cell-surface localization of SD components to maintain SD integrity.
In this study, we employed isolated intact glomeruli in a cell-surface biotinylation assay system to evaluate the turnover of cell-surface SD components. We found that SD components were constantly and rapidly exocytosed, endocytosed and degraded in steady-state glomeruli. We also revealed that aPKC is crucial for the turnover of cell-surface SD components to maintain SD integrity through the regulation of exocytosis in cellular models, isolated glomeruli and in aPKCλ cKO mice. These findings not only reveal a novel aspect of the mechanism regulating the integrity of the SD but also imply that the impairment of SD turnover might contribute to the pathogenesis of proteinuria.
Materials and Methods
Antibodies and reagents
The antibodies used in this study were: rabbit anti-nephrin and rabbit anti-podocin (IBL; Immuno-Biological Laboratories, Fujioka, Gunma, Japan), rabbit anti-aPKC and mouse anti-GFP (B-2; Santa Cruz Biotechnology, Dallas, TX, USA), HRP-conjugated rat anti-HA (Roche Diagnostics, Basel, Switzerland), rabbit anti-Par3 (Merck Millipore, Billerica, MA, USA), mouse anti-E-cadherin (36/E-Cadherin) and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (BD Biosciences, San Jose, CA, USA), mouse anti-transferrin receptor (TfR) (H68.4), mouse anti-zonula occludens-1 (ZO1-1A12), Alexa488 and Alexa555-conjugated secondary antibodies (Life Technologies, Carlsbad, CA, USA), HRP-conjugated secondary antibodies (GE Healthcare Bio-Sciences, Piscataway, NJ, USA) and rabbit anti-neph1 (as previously described (37)). The reagents used in this study were chlorpromazine (LKT Laboratories Inc., St Paul, MN, USA), methyl-β-cyclodextrin (MβCD) (Sigma, St Louis, MO, USA) and doxycycline-HCl (ICN Biochemicals Inc., Irvine, CA, USA). Myristoylated pseudosubstrate inhibitor specific for atypical PKC subtypes (myr-SIYRRGARRWRKL) or scramble peptide (SC) (myr-RLYRKRIWRSAGR) was purchased from Peptide Institute Inc. (Osaka, Japan).
Animals
Podocyte-specific aPKCλ cKO mice (aPkcλΔE5/floxE5; Nphs1-CreTg mice) were described previously (30). Male Wistar rats (200–400 g, 5–9 weeks old) were purchased from Oriental Yeast Co. (Tokyo, Japan). Animal care and handling in experiments were carried out in accordance with protocols approved by the Institutional Animal Care and Use Committee at Yokohama City University.
Cell lines and transfections
We generated HCT116-nephrin cells using the Flp-In System (Life Technologies). First, we made the lentivirus-based Flp-In target site vector, pLenti6/FRT/LacZeo2. pLenti6/FRT/LacZeo2 was constructed by replacing the segment containing pCMV to ZeoR of pLenti6/V5-DEST with the segment of SV40Δ to FRT/LacZ-Zeocin of pFRTZeo2. Then, we constructed the pLenti6/FRT/enhanced green fluorescent protein (eGFP)-blasticidin-S-deaminase (BSD), which replaced the LacZeo2 coding region of pLenti6/FRT/LacZeo2 to enhanced green fluorescent protein (eGFP)–blasticidin-S-deaminase (BSD) fusion protein coding sequence for efficient selection of HCT116_FRT cells. The detailed sequence will be provided upon request. Lentivirus was produced according to the instructions for the pLenti6 system (Life Technologies). HCT116 cells were infected with pLent6/FRT/eGFP-BSD lentivirus, selected with 400 µg/ml of blasticidin, single clones isolated and a single integration of Flp recombination target (FRT) site were confirmed by Southern blotting. HCT116-nephrin cells were prepared using clone #19, by Flp-In System (Life Technologies) and maintained in McCOY’s 5 A medium (Life Technologies) containing 10% foetal bovine serum. HeLa Tet-On Advanced cells (Clontech Laboratories Inc., Mountain View, CA, USA) were maintained in Dulbecco’s modified Eagle medium containing 10% foetal bovine serum. Plasmid transfections were performed using lipofectamine LTX reagent (Invitrogen) or linear polyethyleneimine (MW 25,000 Da; Polyscience Inc., Warrington, PA, USA), and siRNA transfections were performed using lipofectamine RNAiMAX (Life Technologies), following the manufacturer’s instructions.
Expression constructs and siRNA
Human nephrin and podocin cDNA was described previously (30). cDNA fragments of human nephrin were amplified by PCR, and subcloned into pcDNA5-FRT-TO (Life Technologies) or pTRET-FRT-Hyg-TetOn vectors. HA-tagged wild-type mouse aPKCλ and its kinase-deficient mutant (kinase negative form of aPKC (aPKC KN): K273E) were described previously (38).
pTRE-tight(TRET)/FRT vector was constructed by linking the fragment of SV40pA to AmpR of pcDNA5/FRT/TO (Life Technologies) and the PCR amplified tetracycline response element (TRE)-tight promoter, containing six TRE, from pTRE-tight (Clontech). The fragment of CMV promoter to SV40pA from the pTet-On Advanced vector (Clontech Laboratories Inc.) was then inserted at the blunted PciI site of pTRET/FRT vector to generate pTERT/FRT/TetOn vector. Detailed sequence information is available upon request.
siRNA duplexes were purchased from Sigma-Genosys, and nucleotide sequences were as follows: aPKCλ knockdown, sense, 5′-CAAGUGUUCUGAAGAGUUUdTdT-3′ and antisense, 5′-AAACUCUUCAGAACACUUGdTdT-3′; aPKCζ knockdown, sense, 5′-GGAAGCAUAUGGAUUCUGUdTdT-3′ and antisense, 5′-ACAGAAUCCAUAUGCUUCCdTdG-3′; Par3 knockdown #3, sense, 5′-UAGCUGUUUAGAAUACUAUUU-3′ and antisense, 5′-AAAUAGUAUUCUAAACAGCUA-3′; #6, sense, 5′-AUCCAUAUCGACUGCUCUGAU-3′ and antisense, 5′-AUCAGAGCAGUCGAUAUGGAU-3′; #8, sense, 5′-GUCACUUAACCUAAAGCAAUU-3′ and antisense, 5′-AAUUGCUUUAGGUUAACUGAC-3′. For a negative control, we used the All-Stars negative control siRNA (Qiagen, Hilden, Germany, catalogue number 1027281).
Isolation of intact glomeruli from rat kidneys
Glomerular isolation was performed as described previously (30, 39). Deeply anaesthetized rats (50 mg/kg nembutal and 5 mg/kg ketoprofen, i.p.) were transcardially perfused with ice-cold phosphate-buffered saline (PBS) containing protease/phosphatase inhibitors and the sliced cortices from the dissected kidneys were minced into small pieces at 4°C. The minced tissues suspended in the same buffer were passed through successive stainless steel sieves (pore size: 250, 125 and 63 µm, respectively; AS ONE, Osaka, Japan) at 4°C. The glomeruli were collected with ice-cold PBS containing 1 mM MgCl2 and 0.1 mM CaCl2 by centrifugation at 2,000 × g for 10 min at 4°C, and then processed for the surface biotinylation assay.
Surface biotinylation assay
The details of the surface biotinylation assay have been described previously (40). Briefly, the isolated glomeruli, HCT116-nephrin cells or HeLa Tet-On Advanced cells were washed with PBS containing 0.1 mM CaCl2 and 1 mM MgCl2 (PBSCM), and cell-surface proteins biotinylated with 0.5 mg/ml sulfo-NHS-SS-biotin (Thermo Scientific, Waltham, MA, USA) in PBSCM at 4°C for 30 min. Then, the glomeruli or cells were washed with 20 mM glycine in PBSCM at 4°C for 15 min to quench free sulfo-NSH-SS-biotin. In this protocol, we can specifically biotinylate the cell-surface proteins. Cell proteins were extracted with lysis buffer (20 mM Hepes–NaOH (pH7.5), 150 mM NaCl, 5 mM EGTA, 15 mM MgCl2, 0.1% SDS, 0.2% sodium deoxycholate, 1% TritonX-100, protease inhibitor cocktail (Sigma–Aldrich) and PhosSTOP (Roche Diagnostics)). The biotinylated proteins were isolated with streptavidin sepharose (GE Healthcare Bio-Sciences). The samples were then prepared for immunoblot analysis. The total protein level and the cell-surface localization of SD components were normalized to those at the start of labelling.
Endocytosis assay
The details of the endocytosis assay were described previously (40). The isolated glomeruli or HCT116-nephrin cells were cell-surface biotinylated as described above, and incubate at 37°C in HBSS (Life Technologies) for the indicated times to allow endocytosis. The remaining sulfo-NHS-SS-biotin on the cell surface was stripped for 1 h at 4°C with 200 mM (isolated glomeruli) or 50 mM β-mercaptoethane sulfonate (MESNA) (Sigma–Aldrich) in 100 mM Tris–HCl (pH 8.6) containing 100 mM NaCl and 2.5 mM CaCl2 and washed with 5 mg/ml iodoacetamide in PBSCM for 15 min at 4°C to quench remaining MESNA. Cell proteins were extracted with cell lysis buffer and the biotinylated proteins were isolated and affinity purified with streptavidin sepharose (GE Healthcare Bio-Sciences). The samples were then prepared for immunoblot analysis. The percentages of internalized proteins were calculated using the following formula: internalized proteins = [(biotinylated protein after incubation at 37°C) − (biotinylated protein at 0 min)]/(biotinylated protein at the start of labelling) × 100 (40).
Biotinylation degradation assay
The details of the biotinylation degradation assay have been described previously (41). The isolated glomeruli were cell-surface biotinylated as described above, and incubated at 37°C in HBSS to allow endocytosis and degradation. At the indicated time points, glomeruli were lysed and analysed as per the endocytosis assay. The total protein level and the cell-surface localization of SD components were normalized to those at the start of labelling. The percentages of degraded proteins were calculated using the following formula: degraded proteins = [(biotinylated protein at the start of labelling) − (biotinylated protein after incubation at 37°C)]/(biotinylated protein at the start of labelling) × 100 (41).
Doxycycline-inducible expression of nephrin
HeLa Tet-On Advanced cells were transiently transfected with the pTRET-FRT-Hyg-Teton-nphs1 vector. After incubation for 48 h, cells were incubated in growth medium with 100 ng/ml doxycycline (ICN Biomedicals Inc.) for the indicate times. After induction, cells were subjected to surface biotinylation and affinity purification with streptavidin sepharose. The samples were then prepared for immunoblot analysis.
Immunofluorescence
Isolated rat glomeruli or HCT116-nephrin cells were fixed with 2% paraformaldehyde in PBS for 10 min at room temperature and permeabilized with 0.1% TritonX-100 for 10 min. The glomeruli or the cells were then incubated with the indicated primary antibodies in TBST-containing 0.1% BSA for 1 h, followed by incubation with Alexa Fluor-conjugated secondary antibodies for 1 h. The glomeruli or the cells were mounted on glass slides using Prolong Gold anti-fade reagent (Invitrogen). The samples were examined and photographed with a fluorescent microscope (Axioimager Z1, Carl Zeiss, Oberkochen, Germany), equipped with a confocal unit (CSU10, Yokogawa Electric Corporation, Tokyo, Japan) and a cooled CCD camera (ORCA-R2, Hamamatsu Photonics K.K., Hamamatsu, Shizuoka, Japan), and objective lens (Plan-Apochromat NA 1.4 63×, Carl Zeiss). Images were processed using ImageJ (NIH, Bethesda, MD, USA) and Photoshop CS4 (Adobe, San Jose, CA, USA).
For stimulation emission depletion (STED) microscopic analysis, 5 -µm thick cryosections were cut using a Jung Frigocut 2800E (Leica, Wetzlar, Germany), mounted on silane-coated glass slides and processed for staining with several antibodies. Sections were incubated for 4 h at room temperature with primary antibodies. Next, the sections were incubated with Alexa 488-labelled anti-rabbit IgG (Life Technologies) and V500-labelled streptavidin (BD Biosciences) for 1 h at room temperature. In control experiments, incubation with the primary antibody was omitted. All sections were examined with a TCS STED CW (Stimulated Emission Depletion) super-resolution microscope from Leica Microsystems GmbH (Mannheim, Germany). Samples were imaged using with a 1.4 NA 100× objective lens (HCX PL APO, Leica). Confocal images were acquired using the 488 nm argon laser line for the excitation of Alexa 488 and 458 nm to excite V500. Alexa 488 was detected using PMT2 of the spectral detection unit with the detection range set to 519–602 nm and V500 was detected with the detection range of 463–502 nm. Imaging speed was at 400 Hz using 4× line averaging and the pinhole was set to 1.0 Airy units. For STED microscopy, all conditions were identical. For the stimulated emission depletion the pulsed Ti:Sapphire laser (MaiTai laser, Spectra-Physics, Santa Clara, CA, USA) was tuned to 592 nm and the power set to 100%. Signal was detected by use of a GaAsP hybrid detection system (Leica). We performed deconvolution by applying Huygens STED deconvolution option software (Leica). Colocalization analysis of double-stained samples was performed with the LAS-AF software provided by Leica.
In vivo biotinylation of mouse kidney
aPKC cKO mice at P10 or P11, or control mice were transcardially perfused sequentially for 2 min with 1 ml/g body weight of PBSCM, 2 mg/ml sulfo-NHS-SS-biotin/PBSCM and 20 mM glycine/PBSCM. All solutions were prepared at room temperature. After perfusion, the kidneys were homogenized with a Potter homogenizer in 1 ml of lysis buffer on ice. The lysates were diluted to a protein concentration of 0.3 mg/ml and subjected to affinity purification using streptavidin sepharose. The samples were then prepared for immunoblot analysis.
Statistical analysis
Two-tailed Student’s t-test (Microsoft Excel 2004) and two-tailed Mann–Whitney U-test (VassarStats, http://faculty.vassar.edu/lowry/VassarStats.html) were used to analyse the differences between the pairs of groups. Values were regarded statistically significant at P < 0.05.
Results
SD components are persistently and rapidly exocytosed and endocytosed in isolated glomeruli
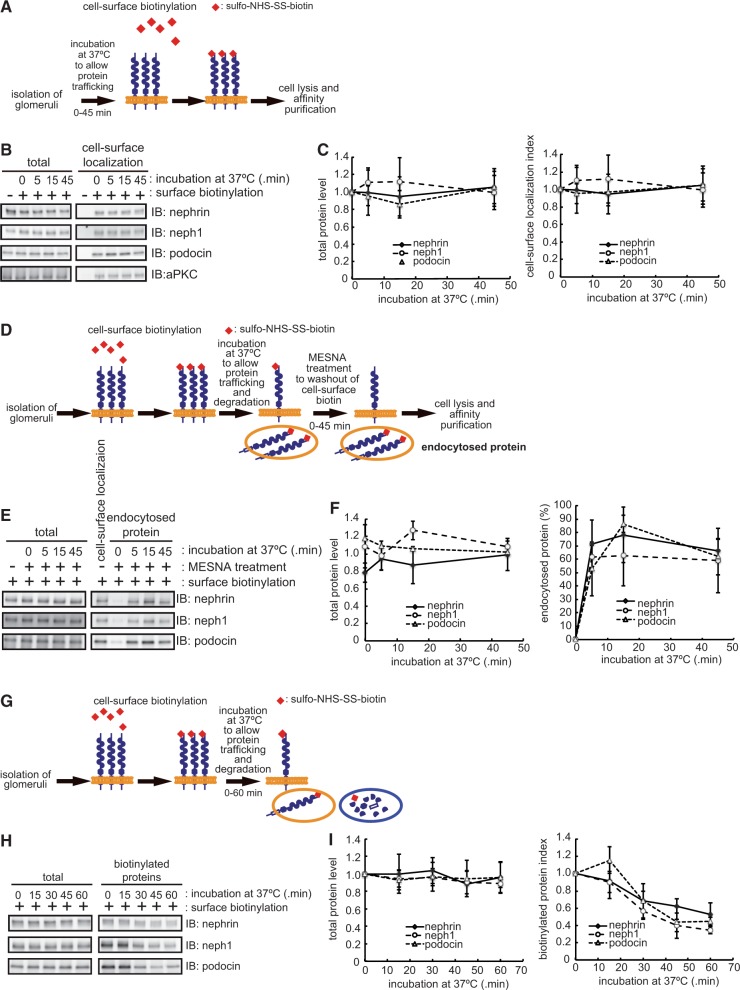
To evaluate the turnover rate of cell-surface SD components in intact glomeruli, we employed rat glomeruli isolated by sieving techniques (39) and examined whether total expression and cell-surface localization of SD components were maintained during culture conditions. In isolated glomeruli, the immunofluorescence signals for nephrin and podocin were detected as a linear pattern along the glomerular capillary wall. This staining pattern was preserved in the 60 min incubation at 37°C, suggesting that SD integrity is maintained during this period (Supplementary Fig. S1). Then, we measured the total protein and cell-surface localization level of SD components using a cell-surface biotinylation system (Fig. 1A). Not only the total protein level of nephrin, neph1 and podocin, but also their cell-surface localization levels remained stable for 45 min during incubation (Fig. 1B and C). It has been reported that SD components are localized both in detergent soluble and detergent-resistant membrane domains called lipid-rafts (42). In our experiments, the majority of the nephrin was detected in the detergent soluble fraction and only part of the nephrin was detected in the detergent-resistant fraction. However, we found that both fractions of nephrin were biotinylated with a similar efficacy (Supplementary Fig. S2). This result eliminates the possibility that only a subset of SD components is preferentially biotinylated. These results also indicate that SD integrity is maintained in isolated glomeruli ex vivo and suggests that they faithfully recapitulate steady-state glomeruli in vivo.
Fig. 1.
SD integrity is maintained by rapid turnover of cell-surface SD components. (A) Schematic representation of the cell-surface biotinylation assay. (B) Isolated glomeruli were subjected to the cell-surface biotinylation assay as in (A). Biotinylated SD components were isolated with streptavidin sepharose and isolated proteins detected by immunoblot. (C) Quantification of the results in (B). The total protein level and cell-surface localization of SD components were normalized to those at the start of labelling. (D) Schematic representation of the endocytosis assay. (E) Isolated glomeruli were subjected to the endocytosis assay and analysed by immunoblot. (F) Quantification of the results in (E). The endocytosed proteins were expressed as the percentage of to those at the start of labelling (see ‘Materials and Methods’ section). (G) Schematic representation of the biotinylation degradation assay. (H) Isolated glomeruli were subjected to biotinylation degradation and analysed by immunoblot. (I) Quantification of the results in (H). The biotinylated SD components were normalized to those at the start of labelling. The data shown in C, F and I are the mean ± SD of three independent experiments.
Next, we evaluated the amount of protein internalized from the cell surface by an endocytosis assay (40) (Fig. 1D). Cell surface proteins were biotinylated and incubated at 37°C for the indicated times to allow endocytosis. By stripping of the remaining biotin from the cell surface with MESNA, we can specifically maintain the biotinylation of the endocytosed proteins and can quantitatively evaluate the endocytosis rate of SD components. As shown in Fig. 1E and F, all three SD components examined were rapidly internalized within 5 min after the induction of protein trafficking (nephrin, 72.1 ± 8.9%; neph1, 61.0 ± 4.4%; podocin, 52.7 ± 10.0%; mean ± SD (n = 3)). This suggests that the remaining fraction consists of SD components that were either degraded after the induction of protein trafficking or retained at the cell surface that was not endocytosed or both. Based on the data taken at 45 min after the induction of protein trafficking, we assume that ∼30–40% of SD components were either degraded and/or retained at the cell surface. To evaluate these possibilities, we chased the amount of SD components after biotinylation (41) (Fig. 1G). To do this, cell-surface proteins were first biotinylated and then incubated at 37°C for various durations to allow endocytosis and degradation. By chasing the remaining amount of biotinylated proteins, we can determine the degradation rate of SD components that localized to the cell surface from the start of labelling. Our quantitative analysis demonstrates a 31.4 ± 11.0% decrease in the amount of biotinylated nephrin and 43.6 ± 9.6% decrease in neph1 (mean ± SD (n = 3)) 30 min after the induction of protein trafficking (Fig. 1H and I), suggesting that most of the internalized SD components were rapidly degraded rather than recycled back to the cell surface. In addition, the amount of podocin, the affinity-purified intracellular binding partner of nephrin and neph1, was also decreased in a time-dependent manner (Fig. 1H and I), suggesting that podocin is also rapidly degraded with these proteins or dissociates from nephrin and neph1. Therefore, although cell-surface localized SD components are rapidly endocytosed and degraded, total expression and cell-surface levels of SD components were constantly maintained, probably by compensating de novo synthesis and exocytosis.
aPKC plays a critical role in the surface localization of SD components
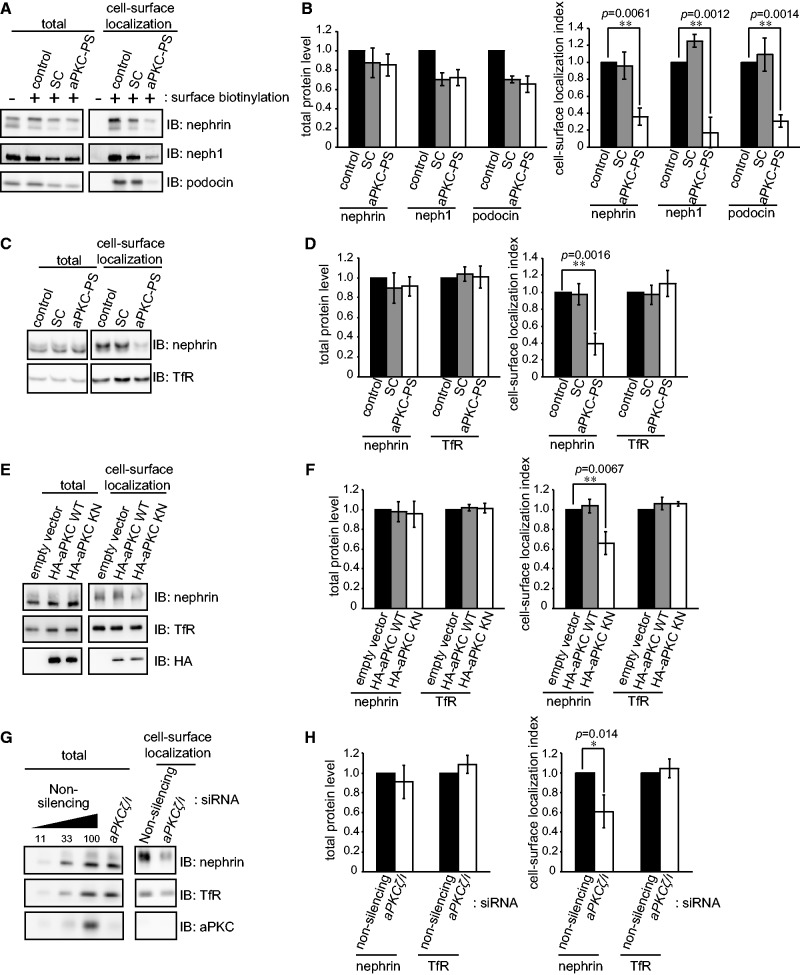
As described above, aPKC has an essential role in SD integrity (30, 31) and is also involved in both endocytosis and exocytosis in a variety of other systems (32–36, 43–45). These observations prompted us to investigate the involvement of aPKC in the turnover of cell-surface SD components. First, we evaluated the cell-surface localization of SD components in isolated glomeruli with or without treatment with an aPKC pseudosubstrate inhibitor (aPKC-PS). The amount of SD components at the cell surface was dramatically decreased by aPKC-PS treatment for 30 min compared with control or SC-treated glomeruli, whereas the total glomerular expression level of these proteins was unaffected (Fig. 2A and B). The result implies that aPKC is involved in the turnover of cell-surface SD components in glomeruli. To further examine the involvement of aPKC, we established epithelial cells stably expressing human nephrin using the colon cancer cell-line HCT116 (HCT116-nephrin cells). In these cells, nephrin localized to both apical and intercellular junction regions, and co-localized with the adherens junction protein, E-cadherin (Supplementary Fig. S3 and data not shown). Not only aPKC-PS treatment (Fig. 2C and D), but also the overexpression of a aPKC KN (Fig. 2E and F), as well as the siRNA knockdown of aPKC (Fig. 2G and H) all resulted in a decrease of the cell-surface localization of nephrin, without affecting the total expression level. The cell-surface localization, as well as the total amount, of the TfR was not affected by the suppression of aPKC. These data indicate that aPKC plays a crucial role in the cell-surface localization of SD components, especially nephrin. We also examined the role of Par3, a component of aPKC-Par complex, in the maintenance of cell-surface localization of nephrin. Knockdown of Par3 in HCT116-nephrin cells also resulted in a significant decrease of the cell-surface localization of nephrin (Supplementary Fig. S4), suggesting that the aPKC-Par3 complex regulates the cell-surface localization of nephrin.
Fig. 2.
aPKC is required for the cell-surface localization of SD components, including nephrin. (A) Isolated rat glomeruli were treated with 10 µM aPKC pseudosubstrate (PS) or SC for 30 min at 37°C in HBSS(+), then subjected to the cell-surface biotinylation assay. (B) Quantification of the results in (A). (C) HCT116-nephrin cells were treated with 20 µM of aPKC-PS or SC for 2 h at 37°C and subjected to the cell-surface biotinylation assay. (D) Quantification of the results in (C). (E) HCT116-nephrin cells were transiently transfected with aPKC WT or KN cDNA and incubated for 48 h and then subjected to the cell-surface biotinylation assay. (F) Quantification of the results in (E). (G) HCT116-nephrin cells were transiently transfected with aPKCλ/ζ siRNA and incubated for 70 h. Both isotypes of aPKC are expressed in HCT116 cells (data not shown). After incubation, the cells were subjected to the cell-surface biotinylation assay. (H) Quantification of the results in (G). The values shown in B, D, F and H were normalized to the appropriate control and are the mean ± SD of three independent experiments. The P values were determined by two-tailed Student’s t-test.
The kinase activity of aPKC is required for exocytosis of newly synthesized nephrin, but not for the suppression of endocytosis
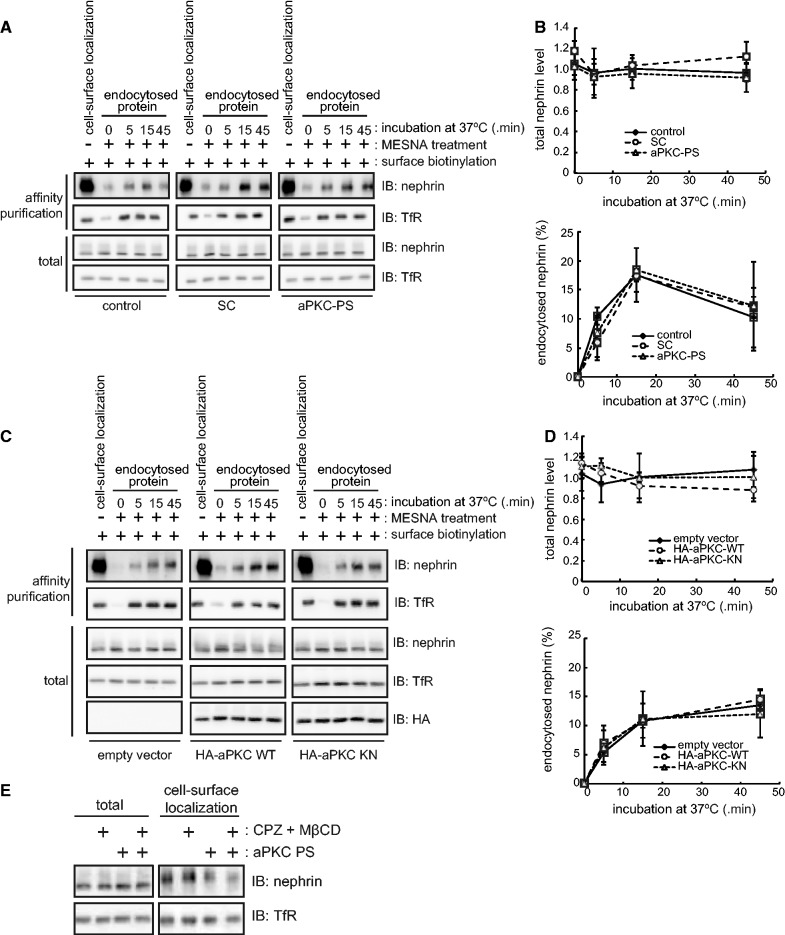
The decrease of cell-surface localization of nephrin by aPKC inhibition is caused by either the facilitation of endocytosis or the suppression of exocytosis, or both, because the total amount of nephrin was not altered by aPKC inhibition (Fig. 2). To evaluate these possibilities, we first examined whether the inhibition of aPKC activity affects the rate of endocytosis of nephrin in HCT116-nephrin cells using the endocytosis assay (Fig. 1D). aPKC-PS treatment (Fig. 3A and B) or overexpression of aPKC KN (Fig. 3C and D) did not alter the rate of nephrin endocytosis. The total expression level of nephrin was also unaffected by these manipulations. We further investigated whether endocytosis inhibitors can compensate for the decrease in cell-surface localization of nephrin by aPKC-PS treatment. Neither chlorpromazine (clathrin-dependent endocytosis inhibitor (46)) nor MβCD (raft-mediated endocytosis inhibitor (47)) could compensate for the decreased cell-surface localization of nephrin induced by aPKC-PS treatment (Fig. 3E). These data suggest that aPKC does not play a significant role in the endocytosis of nephrin in our system.
Fig. 3.
aPKC does not suppress the endocytosis of nephrin. (A) HCT116-nephrin cells were treated with 20 µM aPKC-PS or SC for 2 h at 37°C and then subjected to the endocytosis assay as in Fig. 1D. (B) Quantification of the results in (A). (C) HCT116-nephrin cells were transiently transfected with aPKC WT or KN cDNA and incubate for 48 h and then subjected to the endocytosis assay. (D) Quantification of the results in (C). The amount of endocytosed nephrin shown in B and D was expressed as the percentage of those at the start of labelling and are the mean ± SD of three independent experiments. (E) HCT116-nephrin cells were treated with 20 µM aPKC PS with or without 10 µM chlorpromazine or 10 mM MβCD for 30 min at 37°C and then subjected to the cell-surface biotinylation assay.
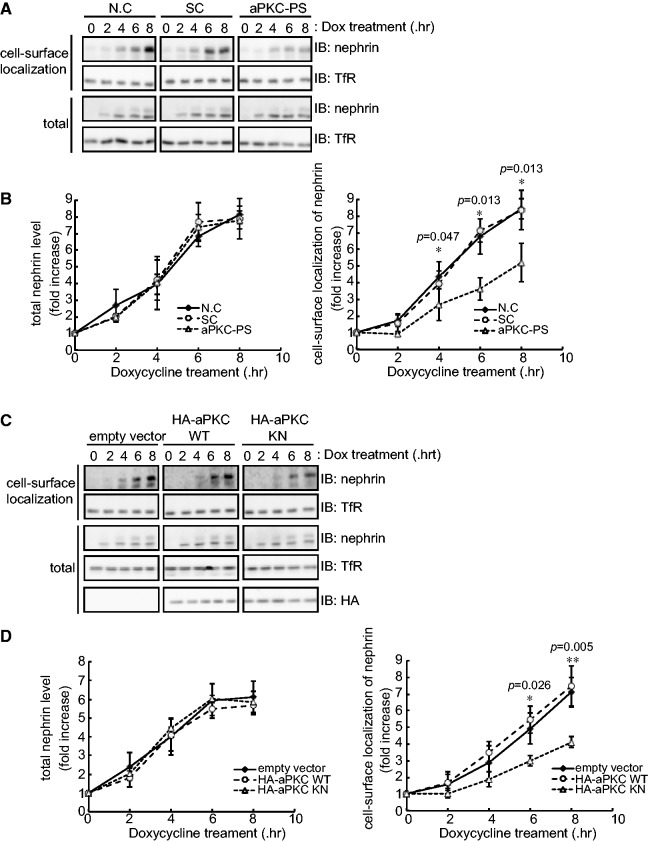
To directly evaluate exocytosis of nephrin, we employed a tetracycline-inducible (Tet-On) expression system in the HeLa Tet-On Advanced cell line. In this cell line, the total expression level of nephrin accumulated and reached a constant at 6 h after induction (Fig. 4A–D). The amount of nephrin targeted to the cell surface continued to increase during the 8 h following induction of nephrin expression (Fig. 4A–D). Suppression of aPKC by aPKC-PS treatment (Fig. 4A and B) or overexpression of aPKC KN (Fig. 4C and D) significantly decreased cell-surface localization of newly synthesized nephrin, while the total amount of nephrin was not affected (Fig. 4A–D). The cell-surface localization of TfR was not affected by the inhibition of aPKC in this cell line (Fig. 4A and C). Taken together, these results indicate that aPKC is required for exocytosis of newly synthesized nephrin, but not for the suppression of endocytosis.
Fig. 4.
aPKC is required for exocytosis of newly synthesized nephrin. (A) HeLa Tet-On Advanced cells were transiently transfected with nephrin cDNA and incubated for 48 h. After incubation, cells were treated with 20 µM of aPKC-PS or SC for 2 h, and then incubated with 100 ng/ml doxycycline for the indicated times to induce the expression of nephrin. After doxycycline treatment, the cells were subjected to the cell-surface biotinylation assay. (B) Quantification of the results in (A). (C) HeLa Tet-On Advanced cells were transiently transfected with nephrin and aPKC WT or KN cDNA, and incubated for 48 h. After incubation, the cells were incubated with 100 ng/ml doxycycline for the indicated times to induce the expression of nephrin. After doxycycline treatment, the cells were subjected to the cell-surface biotinylation assay. (D) Quantification of the results in (C). The values shown in B and D were normalized to those at the start of doxycycline treatment and are the mean ± SD of three independent experiments. The P values were determined by two-tailed Student’s t-test.
aPKC is required for the surface localization of SD components in vivo
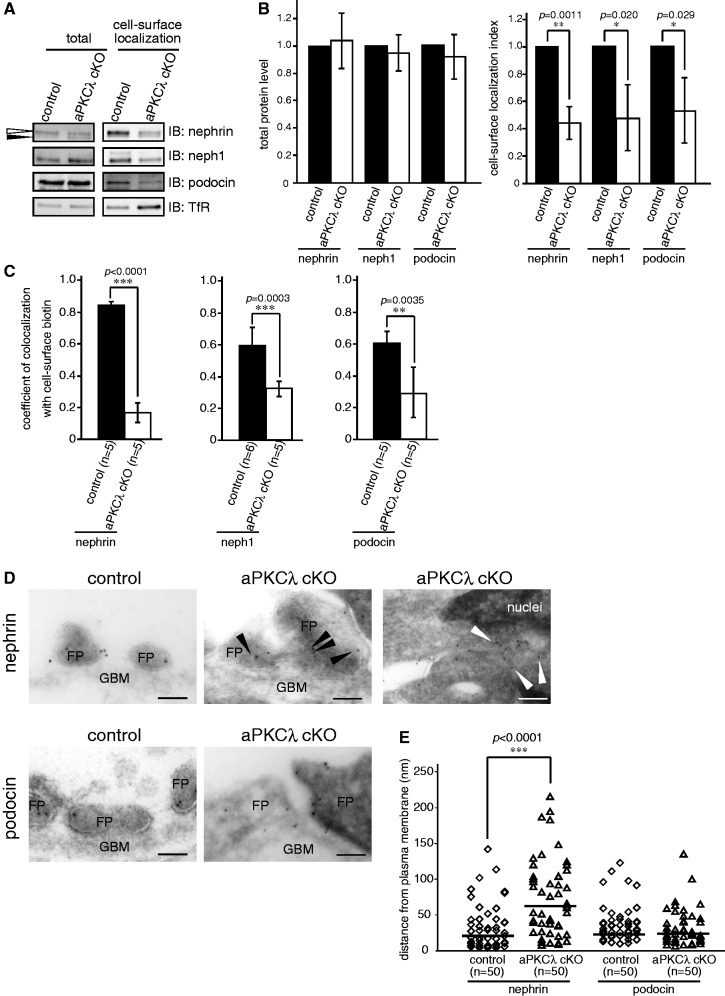
The above experiments using isolated glomeruli and nephrin-expressing epithelial cells strongly support the role of aPKC in maintaining the cell-surface localization of nephrin by stimulating the exocytosis of newly synthesized nephrin. To evaluate this possibility in vivo, we next analysed the cell-surface localization of SD components in the aPKC-deficient kidney by cell-surface biotinylation in vivo. aPKCλ cKO mice at P10 or P11, which developed heavy proteinuria (30), and control mice were transcardially perfused with sulfo-NHS-SS-biotin and the biotinylated cell-surface proteins were isolated with streptavidin sepharose from the kidney lysate. The total expression level of SD components was not different between control and mutant mice. However, the ratio between total and cell-surface localization of SD components significantly differed between control and aPKCλ cKO mice (nephrin, 0.447 ± 0.116; neph1, 0.480 ± 0.238; podocin, 0.533 ± 0.234; mean ± SD (n = 3), Fig. 5A and B). Exocytosis of nephrin is associated with specific glycosylation of its extracellular domain. Thus by western blot analysis, the upper band of nephrin (Fig. 5A, white arrowhead) represents the mature-glycosylated cell-surface form, and the lower band (Fig. 5A, black arrowhead) represents the N-glycosylated ER-form (48). Notably, the proportion of the ER-form of nephrin was higher in aPKCλ cKO mice compared with that of control mice; however, these mice also had a reduction in the cell-surface fraction (Fig. 5A). To our knowledge, there is no evidence that aPKC regulates the glycosylation of cell-surface proteins. Therefore, this suggests that the exocytic process of nephrin is disturbed by podocyte-specific deletion of aPKCλ.
Fig. 5.
aPKC is required for the cell-surface localization of SD components in vivo. (A) aPKC cKO and control mice at P10 or P11 were transcardially perfused with 2 mg/ml sulfo-NHS-SS-biotin/PBSCM for 5 min. Then, the kidneys were lysed and biotinylated proteins isolated with streptavidin sepharose and detected by immunoblot. The white arrowhead represents the mature-glycosylated, cell-surface form, and the black arrowhead represents the N-glycosylated, ER-form of nephrin. (B) Quantification of the results in (A). The values were normalized to control mice and are the mean ± SD of three independent experiments. The P values were determined by two-tailed Student’s t-test. (C) The cell-surface biotinylated aPKC cKO and control kidney were immunostained with nephrin, neph1 or podocin and biotin (Supplementary Fig. S5), and the colocalization coefficient was calculated with LAS-AF software provided by Leica. The P values were determined by two-tailed Student’s t-test. (D) The ultrathin cryosections of aPKC cKO and control kidney were labelled with anti-nephrin or anti-podocin antibodies followed by 10 nm gold particle-conjugated secondary antibody. Black arrowheads represent nephrin localized to the intracellular region, and white arrowheads represent nephrin localized to the rough ER. FP, foot process; GBM, glomerular basement membrane. (E) The distance of gold particle-labelled nephrin or podocin from the plasma membrane in aPKC cKO podocytes was compared with that of the control kidney. The P values were determined by two-tailed Mann–Whitney U-test.
To confirm the disturbance of cell-surface localization of nephrin in aPKCλ-deficient glomeruli, we assessed the localization of SD components using super-resolution confocal microscopy based on the STED system (49). In control glomeruli, the signals of nephrin, neph1 and podocin were detected as a linear pattern along the glomerular capillary and colocalized with cell-surface biotin labels. However, in glomeruli from aPKCλ cKO mice, the colocalization with cell-surface biotin was disrupted and the signals of nephrin, neph1 and podocin were mainly seen at the cell body of podocytes (Fig. 5C and Supplementary Fig. S5). Finally, we examined the localization of nephrin and podocin using immunoelectron microscopy. In control podocytes, most of the immunolabelled gold particles for nephrin and podocin were detected at the plasma membrane of foot processes. However, in aPKCλ-deficient podocytes, the gold particles for nephrin were dissociated from the plasma membrane and predominantly detected in the intracellular region (Fig. 5D and E). Conversely, the particles for podocin were detected near the plasma membrane, similar to control podocytes. Taken together, these findings demonstrate that aPKC is required for the cell-surface localization of SD components in vivo through the regulation of their exocytosis.
Discussion
Our previous observations in aPKCλ cKO mice revealed that aPKCλ depletion gradually causes severe glomerular dysfunction with a drastic change in the ultrastructure of the SD resulting in apically dislocated SDs and foot process effacement, suggesting the involvement of aPKCλ in the turnover of cell-surface SD components (30). In this study, we provide direct evidence demonstrating the rapid turnover of SD components at the cell surface using both isolated glomeruli and aPKCλ cKO mice. Furthermore, we provide evidence for the involvement of aPKCλ in the trafficking of SD components to the cell surface.
The physiological and pathological importance of the rapid turnover of SD components at the cell surface
Previous studies have revealed the critical importance of the specialized intercellular junctions formed between podocyte foot processes, the SD, in the maintenance of the glomerular filtration barrier and that the disassembly of the SD leads directly to the pathogenesis of proteinuria (1, 50). These studies also revealed the importance of SD components and associated proteins in the development and maintenance of SD structures and function. Among the mechanisms implicated in SD integrity, endocytosis of SD components and regulation of actin cytoskeleton have been extensively investigated using cultured podocytes or epithelial cell lines (50, 51). However, these in vitro culture systems do not recapitulate the dynamic nature of the SD, thus they are insufficient to directly evaluate the turnover of cell-surface SD components.
By using cell-surface biotinylation and endocytosis assays on isolated glomeruli, we revealed an unexpectedly high turnover rate of cell-surface-localized SD components such as nephrin; they almost completely undergo exocytosis within minutes. We further show that suppression of aPKC kinase activity greatly affected cell-surface localization of SD components, while it did not affect endocytosis, implying the critical role of aPKC on exocytosis of SD components and the critical importance of exocytosis of SD components in maintaining SD integrity. This notion is supported by the parallel experiments on nephrin-expressing cultured epithelial cells with direct evaluation of exocytosis of newly synthesized nephrin. Finally, the involvement of aPKCλ in the exocytosis of nephrin was confirmed in vivo using aPKCλ cKO mice.
Reduced expression and abnormal distribution of SD components are observed in several nephrotic syndromes in humans, including minimal change nephrotic syndrome, focal and segmental glomerulosclerosis, lupus nephritis, diabetic nephropathy and membranous nephropathy (8–12) and also in various disease models (15–18). However, little is known about the trafficking of SD components in the intact SD (52, 53). Our present findings indicating the importance of the rapid turnover of SD components to the cell surface are consistent with these previous observations, highlighting the importance of endocytosis of SD components in the maintenance of SD integrity. Taken together with previous observations, our present results suggest that the balance between exocytosis and endocytosis is tightly associated not only with the maintenance of SD integrity, but also with the pathogenesis of glomerular diseases. To elucidate the alteration of the high turnover rate of cell-surface SD components under disease conditions would provide a new pathophysiologic basis of proteinuria in glomerular diseases.
What is the physiological reason for rapid turnover of cell-surface SD components? It is suggested that this model can explain how podocytes replace clogged SD components with new ones to prevent overall SD clogging and minimize the unnecessary breakdown of SD structure (50). In addition, the high turnover of cell-surface SD components further enables the remodelling of SD architecture in response to various physiological parameters such as blood flow-dependent change of glomerular capillary diameter (22).
The molecular mechanisms regulating the turnover of SD components at the cell surface: the role of aPKC
Although we and other groups have shown that aPKC plays an essential role in the maintenance of SD integrity (30, 31), the precise role of aPKC has been largely unknown. In this study, we demonstrated that aPKC plays a critical role in the cell-surface localization of SD components, including nephrin, possibly through the exocytosis of newly synthesized ones. Furthermore, our results suggest that Par3, a component of the aPKC-Par complex, is also required for the cell-surface localization of proteins including nephrin. As previously demonstrated in columnar epithelial cells, aPKC forms a complex with Par3 in podocytes (30). Therefore, these observations suggest that aPKC and Par3 might jointly regulate the cell-surface localization of SD components.
Previous studies suggested that podocin is necessary for the cell-surface localization of nephrin in vitro (6, 7). However, we show that nephrin can be targeted to the plasma membrane without podocin since HCT116-nephrin cells do not express it. Moreover, aPKC is required for the cell-surface localization of both nephrin and neph1 in kidneys and isolated glomeruli. These observations suggest that aPKC can regulate the cell-surface localization of nephrin independently of podocin. The molecular mechanisms regulating the exocytosis of nephrin through aPKC are currently uncertain. One possible mechanism is that aPKC regulates the exocytosis of nephrin through the modulation of the exocyst complex, the octameric complex that tethers transport vesicle to the plasma membrane (54). Since several studies have suggested that aPKC associates with the exocyst complex (35, 55), the kinase activity of aPKC might regulate the formation or appropriate localization of the exocyst complex for the exocytosis of nephrin. In podocytes, it is suggest that aPKC form a complex with Par3 and nephrin at the plasma membrane (30). Furthermore, it has been reported that combined deletion of aPKCλ and aPKCζ isoforms in podocytes associates with incorrectly positioned Golgi apparatus (56). Based on these observations and our results, aPKC might regulate multiple steps in the exocytosis of nephrin; transport from ER to Golgi apparatus and from Golgi apparatus to plasma membrane.
The activation of aPKC is regulated by multiple mechanisms including the phosphatidylinositol 3-kinase (PI3K)-dependent pathway (57), and aPKC under control of PI3K has been implicated in insulin-stimulated surface mobilization of GLUT4 (44). Because nephrin interacts with PI3K in a tyrosine phosphorylation-dependent manner and activates PI3K signalling (58), it is plausible that aPKC is activated downstream of nephrin through PI3K. In addition, it has been demonstrated that the tyrosine phosphorylation of nephrin by Src family kinases triggers endocytosis of nephrin (19). Based on these previous observations and our current findings, we propose a model of negative feedback mechanisms to maintain cell-surface localization of nephrin. First, cell-surface nephrin that is tyrosine-phosphorylated by Src family kinases leads to the binding and activation of PI3K and endocytosis. In turn, activated PI3K leads to the activation of downstream effector aPKC, which leads to the exocytosis of newly synthesized nephrin to cell surface.
The correlation between the alteration of the kinase activity of aPKC and the pathological conditions present in glomerular diseases is still unclear. It has been suggested that the kinase activity of aPKC is regulated by both insulin and epidermal growth factor through PI3K (57, 59), and that these observations imply that the change in the composition of serum circulating factors may alter the kinase activity of aPKC in pathological conditions. To investigate the expression, localization or kinase activity of aPKC and the upstream factors in the pathogenesis or recovery phase of proteinuria will provide novel insights into the pathophysiological basis, and a potential therapeutic target, for glomerular diseases.
Taken together, we present a novel model for the maintenance of SD integrity, where the cell-surface localization of SD components is dynamically regulated by persistent and rapid turnover of these proteins at the cell surface. Furthermore, the cell polarity regulator aPKC plays a critical role in the cell-surface localization of SD components through the regulation of exocytosis. As mentioned above, the disturbance in the cell-surface localization of SD components is tightly associated with the pathogenesis and progression of proteinuria. Therefore, the signalling leading to the inhibition of aPKC activity would be one of the therapeutic targets for proteinuria. These results provide a new insight into the pathophysiological basis for glomerular diseases and shed light on the exocytosis pathway as a potential therapeutic target for proteinuria.
Supplementary Data
Supplementary Data are available at JB Online.
Acknowledgements
The authors thank Hiroyasu Tsukaguchi (Kansai Medical University) for providing human nephrin cDNA, Taiji Matsusaka for providing Nphs1-Cre transgenic mice, Chiho Kusaka and Hiromi Kazama for their animal care and technical assistance, and all the members of Ohno laboratory for helpful discussion.
Glossary
Abbreviations
- aPKC
atypical protein kinase C
- aPKC KN
kinase negative form of aPKC
- aPKC-Par
aPKC (partitioning defective)
- aPKC-PS
aPKC pseudosubstrate inhibitor
- BSD
blastcidin-S-deaminase
- cKO
conditional knockout
- EGF
epidermal growth factor
- eGFP
enhanced green fluorescent protein
- ER
endoplasmic reticulum
- FRT
Flp recombination target
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GBM
glomerular basement membrane
- MβCD
methyl-β-cyclodextrin
- MESNA
β-mercaptoethane sulfonate
- PI3K
phosphatidylinositol 3-kinase
- SC
scramble peptide
- SD
slit diaphragm
- STED
stimulation emission depletion
- TfR
transferrin receptor
- TRE
tetracycline response element
- ZO-1
zonula occludens-1
Funding
This work was partially supported by the fund for Creation of Innovation Centers for Advanced Interdisciplinary Research Areas Program in the Project for Developing Innovation Systems from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan (to S.O.); Grant-in-Aid for Scientific Research on Innovative Areas ‘Tubulology’ of MEXT (to S.O., 22247030); Grant-in-Aid for Scientific Research (to H.K. and S.O., 20590964 and 23659174, respectively) and Young Scientists (to T.H., Y.H. and A.Y., 20790261, 22790991, 21790289, respectively) from the Japan Society for the Promotion of Science; and the Strategic Research Project of Yokohama City University (to T.H., K2103).
Conflict of Interest
None declared.
References
- 1.Tryggvason K, Patrakka J, Wartiovaara J. Hereditary proteinuria syndromes and mechanisms of proteinuria. N. Engl. J. Med. 2006;354:1387–1401. doi: 10.1056/NEJMra052131. [DOI] [PubMed] [Google Scholar]
- 2.Ruotsalainen V, Ljungberg P, Wartiovaara J, Lenkkeri U, Kestila M, Jalanko H, Holmberg C, Tryggvason K. Nephrin is specifically located at the slit diaphragm of glomerular podocytes. Proc. Natl Acad. Sci. U. S. A. 1999;96:7962–7967. doi: 10.1073/pnas.96.14.7962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Welsh GI, Saleem MA. Nephrin-signature molecule of the glomerular podocyte? J. Pathol. 2010;220:328–337. doi: 10.1002/path.2661. [DOI] [PubMed] [Google Scholar]
- 4.Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K. Positionally cloned gene for a novel glomerular protein—nephrin—is mutated in congenital nephrotic syndrome. Mol. Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- 5.Putaala H, Soininen R, Kilpelainen P, Wartiovaara J, Tryggvason K. The murine nephrin gene is specifically expressed in kidney, brain and pancreas: inactivation of the gene leads to massive proteinuria and neonatal death. Hum. Mol. Genet. 2001;10:1–8. doi: 10.1093/hmg/10.1.1. [DOI] [PubMed] [Google Scholar]
- 6.Liu L, Done SC, Khoshnoodi J, Bertorello A, Wartiovaara J, Berggren PO, Tryggvason K. Defective nephrin trafficking caused by missense mutations in the NPHS1 gene: insight into the mechanisms of congenital nephrotic syndrome. Hum. Mol. Genet. 2001;10:2637–2644. doi: 10.1093/hmg/10.23.2637. [DOI] [PubMed] [Google Scholar]
- 7.Roselli S, Moutkine I, Gribouval O, Benmerah A, Antignac C. Plasma membrane targeting of podocin through the classical exocytic pathway: effect of NPHS2 mutations. Traffic. 2004;5:37–44. doi: 10.1046/j.1600-0854.2003.00148.x. [DOI] [PubMed] [Google Scholar]
- 8.Doublier S, Ruotsalainen V, Salvidio G, Lupia E, Biancone L, Conaldi PG, Reponen P, Tryggvason K, Camussi G. Nephrin redistribution on podocytes is a potential mechanism for proteinuria in patients with primary acquired nephrotic syndrome. Am. J. Pathol. 2001;158:1723–1731. doi: 10.1016/S0002-9440(10)64128-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan N, Ding J, Zhang J, Yang J. Expression of nephrin, podocin, alpha-actinin, and WT1 in children with nephrotic syndrome. Pediatr. Nephrol. 2003;18:1122–1127. doi: 10.1007/s00467-003-1240-z. [DOI] [PubMed] [Google Scholar]
- 10.Huh W, Kim DJ, Kim MK, Kim YG, Oh HY, Ruotsalainen V, Tryggvason K. Expression of nephrin in acquired human glomerular disease. Nephrol. Dial. Transplant. 2002;17:478–484. doi: 10.1093/ndt/17.3.478. [DOI] [PubMed] [Google Scholar]
- 11.Koop K, Eikmans M, Baelde HJ, Kawachi H, De Heer E, Paul LC, Bruijn JA. Expression of podocyte-associated molecules in acquired human kidney diseases. J. Am. Soc. Nephrol. 2003;14:2063–2071. doi: 10.1097/01.asn.0000078803.53165.c9. [DOI] [PubMed] [Google Scholar]
- 12.Wernerson A, Duner F, Pettersson E, Widholm SM, Berg U, Ruotsalainen V, Tryggvason K, Hultenby K, Soderberg M. Altered ultrastructural distribution of nephrin in minimal change nephrotic syndrome. Nephrol. Dial. Transplant. 2003;18:70–76. doi: 10.1093/ndt/18.1.70. [DOI] [PubMed] [Google Scholar]
- 13.Orikasa M, Matsui K, Oite T, Shimizu F. Massive proteinuria induced in rats by a single intravenous injection of a monoclonal antibody. J. Immunol. 1988;141:807–814. [PubMed] [Google Scholar]
- 14.Topham PS, Kawachi H, Haydar SA, Chugh S, Addona TA, Charron KB, Holzman LB, Shia M, Shimizu F, Salant DJ. Nephritogenic mAb 5-1-6 is directed at the extracellular domain of rat nephrin. J. Clin. Invest. 1999;104:1559–1566. doi: 10.1172/JCI7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan N, Ding J, Deng J, Zhang J, Yang J. Key molecular events in puromycin aminonucleoside nephrosis rats. Pathol. Int. 2004;54:703–711. doi: 10.1111/j.1440-1827.2004.01683.x. [DOI] [PubMed] [Google Scholar]
- 16.Hosoyamada M, Yan K, Nishibori Y, Takiue Y, Kudo A, Kawakami H, Shibasaki T, Endou H. Nephrin and podocin expression around the onset of puromycin aminonucleoside nephrosis. J. Pharmacol. Sci. 2005;97:234–241. doi: 10.1254/jphs.fp0040802. [DOI] [PubMed] [Google Scholar]
- 17.Kawachi H, Koike H, Kurihara H, Yaoita E, Orikasa M, Shia MA, Sakai T, Yamamoto T, Salant DJ, Shimizu F. Cloning of rat nephrin: expression in developing glomeruli and in proteinuric states. Kidney Int. 2000;57:1949–1961. doi: 10.1046/j.1523-1755.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 18.Luimula P, Ahola H, Wang SX, Solin ML, Aaltonen P, Tikkanen I, Kerjaschki D, Holthofer H. Nephrin in experimental glomerular disease. Kidney Int. 2000;58:1461–1468. doi: 10.1046/j.1523-1755.2000.00308.x. [DOI] [PubMed] [Google Scholar]
- 19.Qin XS, Tsukaguchi H, Shono A, Yamamoto A, Kurihara H, Doi T. Phosphorylation of nephrin triggers its internalization by raft-mediated endocytosis. J. Am. Soc. Nephrol. 2009;20:2534–2545. doi: 10.1681/ASN.2009010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L. beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc. Natl Acad. Sci. U. S. A. 2006;103:14110–14115. doi: 10.1073/pnas.0602587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quack I, Woznowski M, Potthoff SA, Palmer R, Konigshausen E, Sivritas S, Schiffer M, Stegbauer J, Vonend O, Rump LC, Sellin L. PKC alpha mediates beta-arrestin2-dependent nephrin endocytosis in hyperglycemia. J. Biol. Chem. 2011;286:12959–12970. doi: 10.1074/jbc.M110.204024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tossidou I, Teng B, Menne J, Shushakova N, Park JK, Becker JU, Modde F, Leitges M, Haller H, Schiffer M. Podocytic PKC-alpha is regulated in murine and human diabetes and mediates nephrin endocytosis. PLoS One. 2010;5:e10185. doi: 10.1371/journal.pone.0010185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tossidou I, Teng B, Drobot L, Meyer-Schwesinger C, Worthmann K, Haller H, Schiffer M. CIN85/RukL is a novel binding partner of nephrin and podocin and mediates slit diaphragm turnover in podocytes. J. Biol. Chem. 2010;285:25285–25295. doi: 10.1074/jbc.M109.087239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bechtel W, Helmstadter M, Balica J, Hartleben B, Kiefer B, Hrnjic F, Schell C, Kretz O, Liu S, Geist F, Kerjaschki D, Walz G, Huber TB. Vps34 deficiency reveals the importance of endocytosis for podocyte homeostasis. J. Am. Soc. Nephrol. 2013;24:727–743. doi: 10.1681/ASN.2012070700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen J, Chen MX, Fogo AB, Harris RC, Chen JK. mVps34 deletion in podocytes causes glomerulosclerosis by disrupting intracellular vesicle trafficking. J. Am. Soc. Nephrol. 2013;24:198–207. doi: 10.1681/ASN.2012010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartleben B, Godel M, Meyer-Schwesinger C, Liu S, Ulrich T, Kobler S, Wiech T, Grahammer F, Arnold SJ, Lindenmeyer MT, Cohen CD, Pavenstadt H, Kerjaschki D, Mizushima N, Shaw AS, Walz G, Huber TB. Autophagy influences glomerular disease susceptibility and maintains podocyte homeostasis in aging mice. J. Clin. Invest. 2010;120:1084–1096. doi: 10.1172/JCI39492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yaddanapudi S, Altintas MM, Kistler AD, Fernandez I, Moller CC, Wei C, Peev V, Flesche JB, Forst AL, Li J, Patrakka J, Xiao Z, Grahammer F, Schiffer M, Lohmuller T, Reinheckel T, Gu C, Huber TB, Ju W, Bitzer M, Rastaldi MP, Ruiz P, Tryggvason K, Shaw AS, Faul C, Sever S, Reiser J. CD2AP in mouse and human podocytes controls a proteolytic program that regulates cytoskeletal structure and cellular survival. J. Clin. Invest. 2011;121:3965–3980. doi: 10.1172/JCI58552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J. Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- 29.Hartleben B, Schweizer H, Lubben P, Bartram MP, Moller CC, Herr R, Wei C, Neumann-Haefelin E, Schermer B, Zentgraf H, Kerjaschki D, Reiser J, Walz G, Benzing T, Huber TB. Neph-Nephrin proteins bind the Par3-Par6-atypical protein kinase C (aPKC) complex to regulate podocyte cell polarity. J. Biol. Chem. 2008;283:23033–23038. doi: 10.1074/jbc.M803143200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirose T, Satoh D, Kurihara H, Kusaka C, Hirose H, Akimoto K, Matsusaka T, Ichikawa I, Noda T, Ohno S. An essential role of the universal polarity protein, aPKClambda, on the maintenance of podocyte slit diaphragms. PLoS One. 2009;4:e4194. doi: 10.1371/journal.pone.0004194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huber TB, Hartleben B, Winkelmann K, Schneider L, Becker JU, Leitges M, Walz G, Haller H, Schiffer M. Loss of podocyte aPKClambda/iota causes polarity defects and nephrotic syndrome. J. Am. Soc. Nephrol. 2009;20:798–806. doi: 10.1681/ASN.2008080871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harris KP, Tepass U. Cdc42 and Par proteins stabilize dynamic adherens junctions in the Drosophila neuroectoderm through regulation of apical endocytosis. J. Cell Biol. 2008;183:1129–1143. doi: 10.1083/jcb.200807020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sato K, Watanabe T, Wang S, Kakeno M, Matsuzawa K, Matsui T, Yokoi K, Murase K, Sugiyama I, Ozawa M, Kaibuchi K. Numb controls E-cadherin endocytosis through p120 catenin with aPKC. Mol. Biol. Cell. 2011;22:3103–3119. doi: 10.1091/mbc.E11-03-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nakayama M, Nakayama A, van Lessen M, Yamamoto H, Hoffmann S, Drexler HC, Itoh N, Hirose T, Breier G, Vestweber D, Cooper JA, Ohno S, Kaibuchi K, Adams RH. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat. Cell Biol. 2013;15:249–260. doi: 10.1038/ncb2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rosse C, Formstecher E, Boeckeler K, Zhao Y, Kremerskothen J, White MD, Camonis JH, Parker PJ. An aPKC-exocyst complex controls paxillin phosphorylation and migration through localised JNK1 activation. PLoS Biol. 2009;7:e1000235. doi: 10.1371/journal.pbio.1000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoshihama Y, Sasaki K, Horikoshi Y, Suzuki A, Ohtsuka T, Hakuno F, Takahashi S, Ohno S, Chida K. KIBRA suppresses apical exocytosis through inhibition of aPKC kinase activity in epithelial cells. Curr. Biol. 2011;21:705–711. doi: 10.1016/j.cub.2011.03.029. [DOI] [PubMed] [Google Scholar]
- 37.Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, Hattori S. Neph1, a component of the kidney slit diaphragm, is tyrosine-phosphorylated by the Src family tyrosine kinase and modulates intracellular signaling by binding to Grb2. J. Biol. Chem. 2008;283:9177–9186. doi: 10.1074/jbc.M707247200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horikoshi Y, Suzuki A, Yamanaka T, Sasaki K, Mizuno K, Sawada H, Yonemura S, Ohno S. Interaction between PAR-3 and the aPKC-PAR-6 complex is indispensable for apical domain development of epithelial cells. J. Cell Sci. 2009;122:1595–1606. doi: 10.1242/jcs.043174. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T. Isolation and enrichment of glomeruli using sieving techniques in Renal and Urinary Proteomics. In: Thongboonkerd V, editor. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2009. pp. 1–7. [Google Scholar]
- 40.Morimoto S, Nishimura N, Terai T, Manabe S, Yamamoto Y, Shinahara W, Miyake H, Tashiro S, Shimada M, Sasaki T. Rab13 mediates the continuous endocytic recycling of occludin to the cell surface. J. Biol. Chem. 2005;280:2220–2228. doi: 10.1074/jbc.M406906200. [DOI] [PubMed] [Google Scholar]
- 41.Joffre C, Barrow R, Menard L, Calleja V, Hart IR, Kermorgant S. A direct role for Met endocytosis in tumorigenesis. Nat. Cell Biol. 2011;13:827–837. doi: 10.1038/ncb2257. [DOI] [PubMed] [Google Scholar]
- 42.Huber TB, Simons M, Hartleben B, Sernetz L, Schmidts M, Gundlach E, Saleem MA, Walz G, Benzing T. Molecular basis of the functional podocin–nephrin complex: mutations in the NPHS2 gene disrupt nephrin targeting to lipid raft microdomains. Hum. Mol. Genet. 2003;12:3397–3405. doi: 10.1093/hmg/ddg360. [DOI] [PubMed] [Google Scholar]
- 43.Bandyopadhyay G, Sajan MP, Kanoh Y, Standaert ML, Quon MJ, Lea-Currie R, Sen A, Farese RV. PKC-zeta mediates insulin effects on glucose transport in cultured preadipocyte-derived human adipocytes. J. Clin. Endocrinol. Metab. 2002;87:716–723. doi: 10.1210/jcem.87.2.8252. [DOI] [PubMed] [Google Scholar]
- 44.Dugani CB, Klip A. Glucose transporter 4: cycling, compartments and controversies. EMBO Rep. 2005;6:1137–1142. doi: 10.1038/sj.embor.7400584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Imamura T, Huang J, Usui I, Satoh H, Bever J, Olefsky JM. Insulin-induced GLUT4 translocation involves protein kinase C-lambda-mediated functional coupling between Rab4 and the motor protein kinesin. Mol. Cell. Biol. 2003;23:4892–4900. doi: 10.1128/MCB.23.14.4892-4900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang LH, Rothberg KG, Anderson RG. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J. Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parpal S, Karlsson M, Thorn H, Stralfors P. Cholesterol depletion disrupts caveolae and insulin receptor signaling for metabolic control via insulin receptor substrate-1, but not for mitogen-activated protein kinase control. J. Biol. Chem. 2001;276:9670–9678. doi: 10.1074/jbc.M007454200. [DOI] [PubMed] [Google Scholar]
- 48.Simons M, Schwarz K, Kriz W, Miettinen A, Reiser J, Mundel P, Holthofer H. Involvement of lipid rafts in nephrin phosphorylation and organization of the glomerular slit diaphragm. Am. J. Pathol. 2001;159:1069–1077. doi: 10.1016/S0002-9440(10)61782-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Willig KI, Kellner RR, Medda R, Hein B, Jakobs S, Hell SW. Nanoscale resolution in GFP-based microscopy. Nat. Methods. 2006;3:721–723. doi: 10.1038/nmeth922. [DOI] [PubMed] [Google Scholar]
- 50.Johnstone DB, Holzman LB. Clinical impact of research on the podocyte slit diaphragm. Nat. Clin. Pract. Nephrol. 2006;2:271–282. doi: 10.1038/ncpneph0180. [DOI] [PubMed] [Google Scholar]
- 51.Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- 52.Brinkkoetter PT, Ising C, Benzing T. The role of the podocyte in albumin filtration. Nat. Rev. Nephrol. 2013;9:328–336. doi: 10.1038/nrneph.2013.78. [DOI] [PubMed] [Google Scholar]
- 53.Swiatecka-Urban A. Membrane trafficking in podocyte health and disease. Pediatr. Nephrol. 2013;28:1723–1737. doi: 10.1007/s00467-012-2281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He B, Guo W. The exocyst complex in polarized exocytosis. Curr. Opin. Cell Biol. 2009;21:537–542. doi: 10.1016/j.ceb.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lalli G. RalA and the exocyst complex influence neuronal polarity through PAR-3 and aPKC. J. Cell Sci. 2009;122:1499–1506. doi: 10.1242/jcs.044339. [DOI] [PubMed] [Google Scholar]
- 56.Hartleben B, Widmeier E, Suhm M, Worthmann K, Schell C, Helmstadter M, Wiech T, Walz G, Leitges M, Schiffer M, Huber TB. aPKClambda/iota and aPKCzeta contribute to podocyte differentiation and glomerular maturation. J. Am. Soc. Nephrol. 2013;24:253–267. doi: 10.1681/ASN.2012060582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Akimoto K, Takahashi R, Moriya S, Nishioka N, Takayanagi J, Kimura K, Fukui Y, Osada S, Mizuno K, Hirai S, Kazlauskas A, Ohno S. EGF or PDGF receptors activate atypical PKClambda through phosphatidylinositol 3-kinase. EMBO J. 1996;15:788–798. [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73:556–566. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 59.Herr HJ, Bernard JR, Reeder DW, Rivas DA, Limon JJ, Yaspelkis BB., 3rd Insulin-stimulated plasma membrane association and activation of Akt2, aPKC zeta and aPKC lambda in high fat fed rodent skeletal muscle. J. Physiol. 2005;565:627–636. doi: 10.1113/jphysiol.2005.086694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.