Abstract
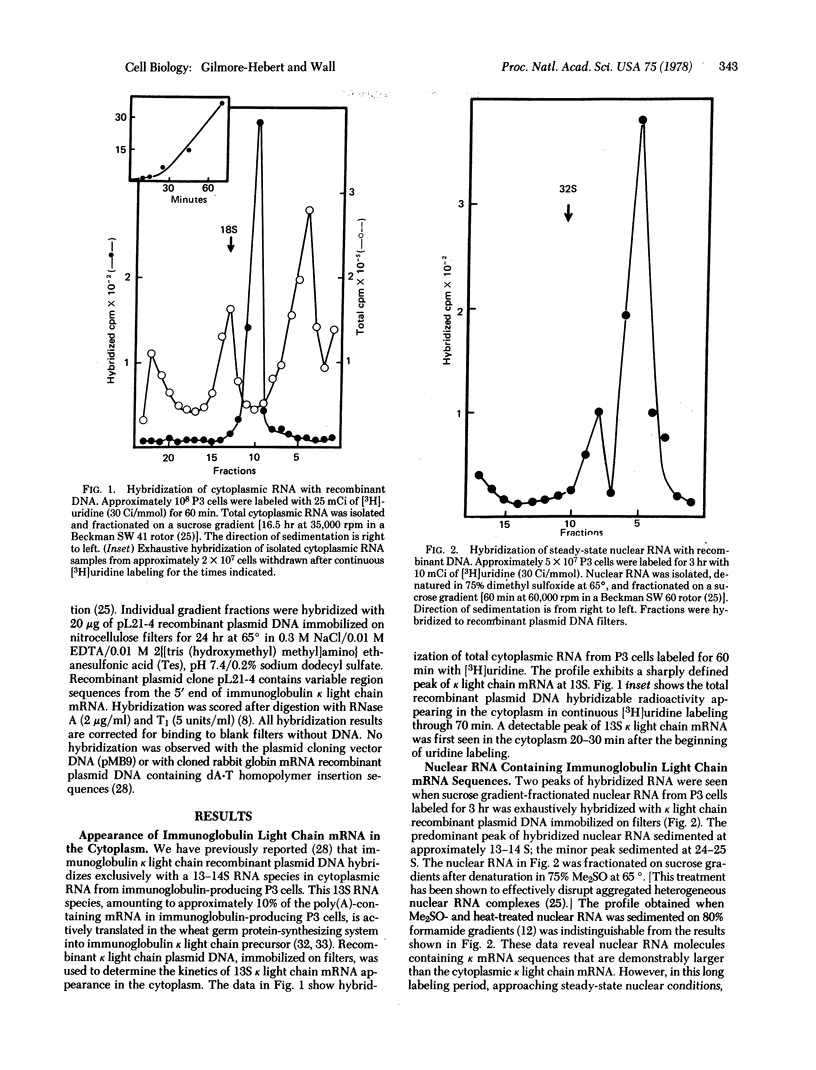
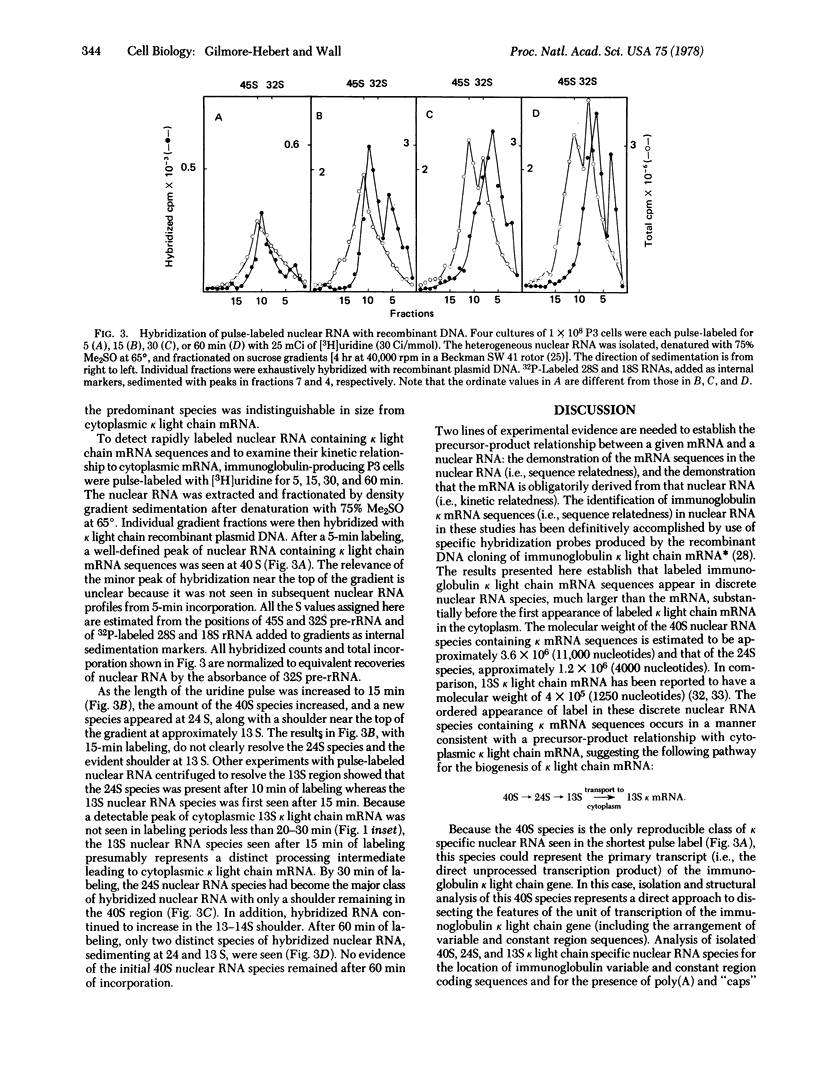
Recombinant DNA probes, produced by the molecular cloning of immunoglobulin kappa light chain mRNA, have been used to analyze heterogenous nuclear RNA for presumptive precursors to cytoplasmic kappa light chain mRNA, Three discrete classes of nuclear RNA containing kappa mRNA sequences were detected after pulse-labeling of immunoglobulin-producing P3 myeloma cells. Two of these were substantially larger than kappa mRNA (approximately 10 and 4 times larger); the third was similar in size to kappa mRNA. Beginning with the largest, the sequential appearance of these three classes of nuclear RNA preceded the first appearance of newly synthesized kappa light chain mRNA in the cytoplasm. The results presented here suggest that immunoglobulin kappa light chain mRNA is generated by the stepwise cleavage and processing of a large nuclear RNA transcript.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aloni Y. Biogenesis and characterization of SV40 and polyoma RNAs in productively infected cells. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):165–178. doi: 10.1101/sqb.1974.039.01.023. [DOI] [PubMed] [Google Scholar]
- Bachenheimer S., Darnell J. E. Adenovirus-2 mRNA is transcribed as part of a high-molecular-weight precursor RNA. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4445–4449. doi: 10.1073/pnas.72.11.4445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastos R. N., Aviv H. Globin RNA precursor molecules: biosynthesis and process in erythroid cells. Cell. 1977 Jul;11(3):641–650. doi: 10.1016/0092-8674(77)90081-2. [DOI] [PubMed] [Google Scholar]
- Baumal R., Potter M., Scharff M. D. Synthesis, assembly, and secretion of gamma globulin by mouse myeloma cells. 3. Assembly of the three subclasses of IgG. J Exp Med. 1971 Nov 1;134(5):1316–1334. doi: 10.1084/jem.134.5.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis P. J., Weissmann C. Purification of globin messenger RNA from dimethylsulfoxide-induced Friend cells and detection of a putative globin messenger RNA precursor. J Mol Biol. 1976 Oct 5;106(4):1067–1075. doi: 10.1016/0022-2836(76)90353-3. [DOI] [PubMed] [Google Scholar]
- Darnell F. E. mRNA structure and function. Prog Nucleic Acid Res Mol Biol. 1976;19:493–511. doi: 10.1016/s0079-6603(08)60941-1. [DOI] [PubMed] [Google Scholar]
- Derman E., Darnell J. E. Relationship of chain transcription to poly(A) addition and processing of hnRNA in HeLa cells. Cell. 1974 Nov;3(3):255–264. doi: 10.1016/0092-8674(74)90140-8. [DOI] [PubMed] [Google Scholar]
- Fan H. RNA metabolism of murine leukemia virus: size analysis of nuclear pulse-labeled virus-specific RNA. Cell. 1977 Jun;11(2):297–305. doi: 10.1016/0092-8674(77)90046-0. [DOI] [PubMed] [Google Scholar]
- Fedoroff N., Wellauer P. K., Wall R. Intermolecular duplexes in heterogeneous nuclear RNA from HeLa cells. Cell. 1977 Apr;10(4):597–610. doi: 10.1016/0092-8674(77)90092-7. [DOI] [PubMed] [Google Scholar]
- Furuichi Y., Morgan M., Shatkin A. J., Jelinek W., Salditt-Georgieff M., Darnell J. E. Methylated, blocked 5 termini in HeLa cell mRNA. Proc Natl Acad Sci U S A. 1975 May;72(5):1904–1908. doi: 10.1073/pnas.72.5.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg S., Weber J., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping by effects of ultraviolet irradiation. Cell. 1977 Apr;10(4):617–621. doi: 10.1016/0092-8674(77)90094-0. [DOI] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi R., Paddock G. V., Wall R., Salser W. A general method for cloning eukaryotic structural gene sequences. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3146–3150. doi: 10.1073/pnas.73.9.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horibata K., Harris A. W. Mouse myelomas and lymphomas in culture. Exp Cell Res. 1970 Apr;60(1):61–77. doi: 10.1016/0014-4827(70)90489-1. [DOI] [PubMed] [Google Scholar]
- Jelinek W., Adesnik M., Salditt M., Sheiness D., Wall R., Molloy G., Philipson L., Darnell J. E. Further evidence on the nuclear origin and transfer to the cytoplasm of polyadenylic acid sequences in mammalian cell RNA. J Mol Biol. 1973 Apr 15;75(3):515–532. doi: 10.1016/0022-2836(73)90458-0. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M. Biogenesis of silk fibroin mRNA: an example of very rapid processing? Prog Nucleic Acid Res Mol Biol. 1976;19:301–312. doi: 10.1016/s0079-6603(08)60927-7. [DOI] [PubMed] [Google Scholar]
- Lizardi P. M., Williamson R., Brown D. D. The size of fibroin messenger RNA and its polyadenylic acid content. Cell. 1975 Mar;4(3):199–205. doi: 10.1016/0092-8674(75)90168-3. [DOI] [PubMed] [Google Scholar]
- Mach B., Faust C., Vassalli P. Purification of 14S messenger RNA of immunoglobulin light chain that codes for a possible light-chain precursor. Proc Natl Acad Sci U S A. 1973 Feb;70(2):451–455. doi: 10.1073/pnas.70.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Kee S. G., Efstratiadis A., Kafatos F. C. Amplification and characterization of a beta-globin gene synthesized in vitro. Cell. 1976 Jun;8(2):163–182. doi: 10.1016/0092-8674(76)90001-5. [DOI] [PubMed] [Google Scholar]
- McKnight G. S., Schimke R. T. Ovalbumin messenger RNA: evidence that the initial product of transcription is the same size as polysomal ovalbumin messenger. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4327–4331. doi: 10.1073/pnas.71.11.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Sullivan N. L., Miller O. L., Jr Visualization of the silk fibroin transcription unit and nascent silk fibroin molecules on polyribosomes of Bombyx mori. Prog Nucleic Acid Res Mol Biol. 1976;19:313–318. doi: 10.1016/s0079-6603(08)60928-9. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Bard E., Hames B. D., Kelly D. E., Schibler U. The relationship between hnRNA and mRNA. Prog Nucleic Acid Res Mol Biol. 1976;19:275–292. doi: 10.1016/s0079-6603(08)60925-3. [DOI] [PubMed] [Google Scholar]
- Perry R. P., Kelley D. E. Kinetics of formation of 5' terminal caps in mRNA. Cell. 1976 Jul;8(3):433–442. doi: 10.1016/0092-8674(76)90156-2. [DOI] [PubMed] [Google Scholar]
- Rabbitts T. H. Bacterial cloning of plasmids carrying copies of rabbit globin messenger RNA. Nature. 1976 Mar 18;260(5548):221–225. doi: 10.1038/260221a0. [DOI] [PubMed] [Google Scholar]
- Ross J. A precursor of globin messenger RNA. J Mol Biol. 1976 Sep 15;106(2):403–420. doi: 10.1016/0022-2836(76)90093-0. [DOI] [PubMed] [Google Scholar]
- Rougeon F., Kourilsky P., Mach B. Insertion of a rabbit beta-globin gene sequence into an E. coli plasmid. Nucleic Acids Res. 1975 Dec;2(12):2365–2378. doi: 10.1093/nar/2.12.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan D., Aviv H., Leder P. Purification and properties of biologically active messenger RNA for a myeloma light chain. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1967–1971. doi: 10.1073/pnas.69.7.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall R., Weber J., Gage Z., Darnell J. E. Production of viral mRNA in adenovirus- transformed cells by the post- transcriptional processing of heterogeneous nuclear RNA containing viral and cell sequences. J Virol. 1973 Jun;11(6):953–960. doi: 10.1128/jvi.11.6.953-960.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J., Jelinek W., Darnell J. E., Jr The definition of a large viral transcription unit late in Ad2 infection of HeLa cells: mapping of nascent RNA molecules labeled in isolated nuclei. Cell. 1977 Apr;10(4):611–616. doi: 10.1016/0092-8674(77)90093-9. [DOI] [PubMed] [Google Scholar]



