Abstract
Background
Germline mutations in the tumour suppressor gene CDKN2A occur in 5–20% of familial melanoma cases. A single founder mutation, p.Arg112dup, accounts for the majority of CDKN2A mutations in Swedish carriers. In a national program, carriers of p.Arg112dup mutation have been identified. The aim of this study was to assess cancer risks in p.Arg112dup carriers and their first degree relatives (FDRs) and second degree relatives (SDRs).
Methods
In this prospective cohort study, cancer diagnoses in carriers (n=120), non-carriers (n=111), carriers’ FDRs (n=275) and SDRs (n=321) and controls (n=3976) were obtained from the Swedish Cancer Registry. Relative risks (RRs) for cancers were calculated (number of cancers/person years). Two-sided 95% CIs were calculated for all RRs.
Results
In carriers prospective RR for non-melanoma cancers was 5.0 (95% CI 3.7 to 7.3), for pancreatic cancer 43.8 (95% CI 13.8 to 139.0), for cancers in upper digestive tissues 17.1 (95% CI 6.3 to 46.5), and in respiratory tissues 15.6 (5.4 to 46.0). In FDRs and SDRs RRs were significantly elevated for cancers in pancreas, respiratory and upper digestive tissues. In ever-smoking carriers compared with never-smoking carriers, the odds ratio (OR) of cancers in pancreas, respiratory or upper digestive tissues was 9.3 (95% CI 1.9 to 44.7).
Conclusions
CDKN2A p.Arg112dup mutation carriers from melanoma-prone families and their FDRs and SDRs have elevated risk for pancreatic, lung, head and neck and gastro-oesophageal carcinomas. These cancers were mainly seen in ever-smoking carriers. Germline CDKN2A mutations may confer an increased sensitivity to carcinogens in tobacco smoke. CDKN2A mutation carriers should be counselled to abstain from smoking.
Keywords: Cancer: dermatological, Cancer: lung, Cancer: head and neck, Genetic epidemiology, Genetic screening/counselling
Introduction
It is estimated that approximately 10% of all cases of cutaneous malignant melanoma occur in kindreds with hereditary predisposition for melanoma.1 2 Among melanoma families 5–20% carry a germline mutation of the CDKN2A gene on chromosome 9p21 coding for the cell cycle inhibitors and tumour suppressors p16-INK4A and p14-ARF.3 In Swedish melanoma families, occurrence of CDKN2A mutations has been analysed in studies from Southern Sweden and from Stockholm and were found in 19% and 8% of the families, respectively.4 5 In Sweden a single CDKN2A mutation, NM_000077.4: c.335_337dup, p.Arg112dup is the predominant mutation in melanoma families. The mutation inserts (duplicates) an arginine at codon 112 in one of the ankyrin repeats of p16-INK4A, disrupting its binding to CDK4/6. The mutation is located in CDKN2A exon 2 in a region that is also part of a second transcript with alternative reading frame, giving rise to a duplication of Ser-127 in p14-ARF, still of unknown functional consequence.4 5 This mutation, which has only been detected in Sweden is a founder mutation estimated to have arisen in Sweden approximately 2000 years ago, and it is possible (but not confirmed) that the mutation may have spread with Swedish emigration to European and North American countries.6 Individuals with p.Arg112dup and several other CDKN2A mutations also have an increased risk of developing pancreatic carcinoma.4 7–10 Several studies have reported an excess risk of other cancer types in CDKN2A mutated families, including gastrointestinal, breast, lung, central nervous system (CNS), gynaecological, childhood, head and neck, non-melanoma skin cancers and uveal melanomas,4 7 11–19 but these cancer risks are not as well established, nor as consistently observed as the increased risks of melanoma and pancreatic cancer. In CDKN2A carriers, melanoma risk has been positively associated with sun exposure,20 but apart from this there have been no studies so far investigating the association of exposures to carcinogens, such as those in tobacco smoke, on cancer risk in CDKN2A carriers from melanoma-prone families.
In 1987 the Swedish Melanoma Study Group initiated a national program to identify kindreds with familial cutaneous malignant melanoma and to provide the members of these families with the possibility to participate in a preventive program.21 The original criteria for participation were two or more blood relatives (including first degree relatives (FDRs), second degree relatives (SDRs) and third degree relatives) with histopathologically confirmed melanoma. Later these criteria have been narrowed according to more recent guidelines from the International Melanoma Genetics Consortium to include only kindreds with two FDRs with melanoma or three or more melanomas in at least two blood related individuals. Since 1995, CDKN2A mutation analysis has been available for family members; to date at least one member in 455 families has undergone testing. Protein altering mutations have been found in 35 families, corresponding to 8% of the analysed families (unpublished data). Of these, 29 families are carriers of the p.Arg112dup founder mutation. The first aim of the study was to prospectively determine the risks in p.Arg112dup carriers for non-melanoma cancers (all combined) and also risk for individual cancer types. Second, we tested independently if the risks seen in confirmed carriers were also seen in the carrier's non-genotyped FDRs and SDRs.
Materials and methods
Participants, register linkages and questionnaire
The cohorts of carriers (n=120) and non-carriers (n=111) of the Swedish CDKN2A p.Arg112dup founder mutation were identified in 28 melanoma-prone families, of which 12 have previously been described.4 5 13 22 All known p.Arg112dup families were included in the study, except one. This family was excluded because several family members are also carriers of a BRCA1 mutation and a high occurrence of breast cancer had been observed in family members. Informed consent was obtained before family members underwent germline CDKN2A analysis and the study was approved by Lund University Ethical Committee.
To extend the families the unique 10-digit personal identity numbers of the carriers were linked with the Swedish Multi-Generation registry which contains connections between all individuals born after 1931 and their biological parents that have been registered in Sweden after 1960.23 This allowed identification of carriers’ FDRs (parents, siblings and children) and SDRs (grandparents, uncles/aunts, half-siblings, nieces/nephews and grandchildren). Since the individuals within a family are related, an individual that is a known carrier also could be identified as a FDR or SDR to another known carrier. In this study each individual was assigned only to one group; known carriers were only accounted for as carriers even when they had been identified as FDRs or SDRs to other known carriers; FDRs were only accounted for as FDRs even if they had also been identified as SDRs. In this manner 275 FDRs and 321 SDRs of mutation carriers were identified. This extension of the families showed that two of the p.Arg112dup families are closely related, but in none of the other families there were shared FDRs or SDRs.
An age-matched and sex-matched control population for the carriers was obtained from the Swedish Civil Registry (n=360). FDRs (n=1586) and SDRs (n=2030) of controls were obtained from the Multi-Generation Registry. In the age-matched and sex-matched control group for the carriers (n=360) there were so few cancer occurrences that relative risks (RRs) for several cancer types became non-calculable and interpretations of risks uncertain. To increase power of the analysis, the control groups were combined (n=3976). An analysis was performed to calculate the power to detect significant (p<0.05; two-sided test) RRs in carriers compared with the prespecified control group and the combined control group. When assessing risks for all cancers combined (set at 10% in the control population), both control groups had 80% power to significantly detect twofold RRs. When assessing risks for rare cancer types (set at 0.2% in the control population), using the prespecified control group, there was 80% power to detect 20-fold RRs, whereas using the combined control groups there was 80% power to detect 10-fold RRs. This analysis demonstrated that the combination of the control groups was helpful in increasing the power in the analysis of RRs of rare cancer types. In the prespecified control groups the age and sex distributions were similar (see online supplementary table S1). The analysis based on the combined control groups did not alter the trends observed using specific control groups.
Cancer diagnoses in carriers, non-carriers, FDRs, SDRs and controls were obtained from the Swedish Cancer registry. Reporting to this registry, established in 1958, is compulsory for clinicians and pathologists/cytologists diagnosing a cancer. Completeness of the register in 2011 was estimated to 97%.24 All cancers registered from start of registry in 1958 to 31 December 2011 were obtained. ICD7 codes and histology codes (WHO/HS/CANC/24.1) were used to identify cancer types.
Information on tobacco smoking habits among carriers was obtained from questionnaires that were distributed to members of melanoma families with germline CDKN2A mutations. In the questionnaire it was asked how many years they had smoked and how many cigarettes they smoked per day on average. Ever-smoker was defined as in lifetime having smoked 100 cigarettes or more. Never-smoker was defined as having smoked less than 100 cigarettes in lifetime. For the deceased carriers, smoking history was retrieved from medical records.
DNA isolation, PCR and direct sequencing of PCR products
See online supplementary materials and methods.
Follow-up
In carriers and non-carriers follow-up started the date the first blood sample was taken for CDKN2A analysis in each family. When the controls were compared with the carriers, follow-up started the date the blood sample was taken for CDKN2A analysis in the carriers they were controls for. For FDRs and SDRs follow-up started at their date of birth. When the controls were compared with FDRs and SDRs, follow-up started at the controls’ date of birth. The rationale behind having different definitions of start of follow-up for carriers and non-carriers versus FDRs and SDRs was twofold. First, using the start of follow-up date determined in the carriers for the FDRs and SDRs meant that there were too few events for a risk analysis, many cancer occurrences that had occurred in the older generations were then left out. Second, there was no apparent sign of ascertainment bias for other cancers than melanoma, and since the risk of carriers and their relatives for non-melanoma cancers was the main aim of the study we found this approach acceptable. For all individuals follow-up ended at date of death, emigration or census date of 31 December 2011.
Statistics
For the genotyped p.Arg112dup carriers and non-carriers median age was calculated for age at study inclusion and age at first melanoma diagnosis. RR for all non-melanoma cancers and for specific cancer types was calculated from incidence rates (number of cancers/person years). In individuals with multiple occurrences of the same cancer diagnosis, each diagnosis was only counted once in every individual. To estimate age-specific cumulative cancer incidence in carriers, the incidence of cancer was analysed in 10 year intervals from 0 year to 80 years of age (number of cancers/persons alive in each interval). Odds ratio (OR) was calculated for smoking status (ever/never) and having been diagnosed with cancer in pancreas, respiratory and upper digestive tissues (yes/no). Two-sided 95% CIs were calculated for all RRs. Database handling and statistical calculations were done in Microsoft Office Excel 2007 and StatSoft Statistica V.10.
Results
Table 1 shows baseline characteristics of CDKN2A p.Arg112dup mutation carriers and non-carriers. At study inclusion, 39 carriers had been diagnosed with melanoma, and of those 20 had been diagnosed with multiple primary melanomas. Among non-carriers, five had been diagnosed with melanoma, whereof none had multiple primary melanomas. The median age at diagnosis of first melanoma was 39 years for carriers and 45 years for non-carriers. At baseline 13 carriers and 3 non-carriers had been diagnosed with non-melanoma tumours.
Table 1.
Baseline characteristics of carriers and non-carriers from CDKN2A p.Arg112dup mutated families*
| Carriers (n=120) | Non-carriers (n=111) | |
|---|---|---|
| Sex (men/women) | 56/64 | 54/57 |
| Age at study inclusion (years; median, range) | 35 (7–80) | 33 (3–69) |
| No. diagnosed with melanoma | 39 | 5 |
| No. diagnosed with multiple primary melanomas | 20 | 0 |
| Age at first melanoma diagnosis (years; median, range) | 39 (16–64) | 45(24–68) |
| No. diagnosed with non-melanoma tumours | 13 | 3 |
| Gynaecological | 4 | 2 |
| Digestive—upper | 2 | 0 |
| Haematopoietic or lymphatic | 2 | 0 |
| Breast | 1 | 1 |
| Respiratory | 1 | 0 |
| Skin (non-melanoma) | 1 | 0 |
| Digestive—lower | 1 | 0 |
| Endocrine | 1 | 0 |
| Pancreas | 0 | 0 |
*Baseline is defined as the date when each family’s index case was tested for mutation.
Prospective RRs for all cancers in p.Arg112dup carriers compared with non-carriers and controls are shown in table 3. Carriers contributed 1657, non-carriers 1837 and controls 51 952 person years. Observed cases of each cancer type for carriers are shown. In non-carriers and controls, numbers of expected cases are calculated to correspond to equal person years as for carriers. Prospective RRs for all non-melanoma cancers in mutation carriers compared with controls was 5.0 (95% CI 3.7 to 7.3). The RR for melanoma was 64.8 (95% CI 36.9 to 117.9), for pancreatic cancer 43.8 (95% CI 13.8 to 139.0), for primary cancers in upper digestive tissues (oral cavity, tongue, pharynx, oesophagus, stomach, liver, gall bladder) 17.1 (95% CI 6.3 to 46.5), for primary cancers in respiratory tissues (lung, bronchi, larynx) 15.6 (95% CI 5.4 to 46.0), for gynaecological cancers 8.8 (95% CI 3.8 to 20.4) and for non-melanoma skin cancer 3.3 (95% CI 1.0 to 10.7). Compared with non-carriers there was a significantly higher risk in carriers for all non-melanoma cancers and for melanoma. In non-carriers there were no cases of pancreatic cancers or cancers in upper digestive tissues and one case of lung cancer, indicating a marked excess risk for these tumours in carriers compared with non-carriers. In tables 1–3 cancers in upper digestive tissues are shown separately from pancreatic cancer, since this tumour is of special interest being previously known to be more common in CDKN2A mutation carriers.
Table 3.
Lifetime risk of all cancers in CDKN2A p.Arg112dup carriers FDRs (n=275) and SDRs (n=321)
| FDRs | Expected* | RR (95% CI) | SDRs | Expected* | RR (95% CI) | |
|---|---|---|---|---|---|---|
| Melanoma | 28 | 1.36 | 20.6 (11.6 to 36.7) | 8 | 1.66 | 4.8 (2.1 to 10.9) |
| Pancreas | 13 | 0.6 | 21.6 (9.1 to 49.9) | 3 | 0.8 | 3.8 (1.1 to 14.9) |
| Respiratory | 9 | 1.5 | 6.0 (2.8 to 13.1) | 5 | 1.8 | 2.8 (1.0 to 7.2) |
| Digestive—upper | 7 | 2.1 | 3.3 (1.5 to 7.6) | 6 | 2.6 | 2.3 (1.0 to 5.6) |
| Haematopoietic or lymphatic | 2 | 1.8 | 1.1 (0.3 to 4.6) | 2 | 2.2 | 0.9 (0.2 to 3.8) |
| Gynaecological | 7 | 6.7 | 1.0 (0.5 to 2.2) | 8 | 8.2 | 1.0 (0.5 to 2.0) |
| Endocrine | 4 | 1.4 | 2.9 (1.0 to 8.6) | 0 | 1.7 | 0.0 |
| Skin (non-melanoma) | 4 | 2.9 | 1.4 (0.5 to 3.9) | 0 | 4.3 | 0.0 |
| Breast | 8 | 4.2 | 1.9 (0.9 to 4.0) | 7 | 5.1 | 1.4 (0.6 to 3.0) |
| CNS | 3 | 1.4 | 2.1 (0.6 to 7.1) | 0 | 1.7 | 0.0 |
| Digestive—lower | 5 | 4.3 | 1.2 (0.5 to 2.9) | 2 | 5.3 | 0.4 (0.1 to 1.5) |
| Urinary | 8 | 7.3 | 1.1 (0.5 to 2.9) | 12 | 8.9 | 1.3 (0.7 to 2.5) |
| Connective tissue | 1 | 0.3 | 3.3 (0.3 to 25.3) | 1 | 0.4 | 2.5 (0.3 to 20.6) |
| Unknown primary tumour | 3 | 1.2 | 2.5 (0.8 to 8.9) | 0 | 1.4 | 0.0 |
| All non-melanoma cancers | 74 | 35.7 | 2.1 (1.6 to 2.7) | 46 | 44.4 | 1.0 (0.8 to 1.4) |
*In controls, numbers of expected cases are calculated to correspond to equal person years as for carriers.
FDR, first degree relative; RR, relative risk; SDR, second degree relative.
Table 2.
Prospective risk of cancers in CDKN2A p.Arg112dup carriers (n=120) compared with non-carriers (n=111) and controls (n=3976)
| Tumour type | p.Arg112dup | Non-carriers | Controls | ||
|---|---|---|---|---|---|
| observed | expected* | RR (95% CI) | expected* | RR (95% CI) | |
| Melanoma | 35 | 3.61 | 9.7 (3.5 to 27.2) | 0.54 | 64.8 (36.9 to 117.9) |
| Pancreas | 7 | 0 | NA† | 0.16 | 43.8 (13.8 to 139.0) |
| Digestive—upper | 6 | 0 | NA† | 0.35 | 17.1 (6.3 to 46.5) |
| Respiratory | 5 | 0.90 | 5.6 (0.7 to 47.4) | 0.32 | 15.6 (5.4 to 46.0) |
| Gynaecological | 7 | 3.61 | 1.9 (0.6 to 6.6) | 0.80 | 8.8 (3.8 to 20.4) |
| Haematopoietic or lymphatic | 2 | 0 | NA† | 0.51 | 3.9 (0.6 to 10.6) |
| CNS | 1 | 0 | NA† | 0.29 | 3.4 (0.4 to 27.5) |
| Skin (non-melanoma) | 3 | 0.90 | 3.3 (0.3 to 31.9) | 0.92 | 3.3 (1.0 to 10.7) |
| Breast | 3 | 0.90 | 3.3 (0.3 to 31.9) | 0.99 | 3.0 (0.9 to 9.9) |
| Endocrine | 1 | 0 | NA† | 0.35 | 2.9 (0.4 to 22.1) |
| Urinary | 3 | 0.90 | 3.3 (0.3 to 31.9) | 1.75 | 1.7 (0.5 to 5.5) |
| Digestive—lower | 1 | 0 | NA† | 0.96 | 1.0 (0.1 to 7.7) |
| Connective tissue | 0 | 0 | NA† | 0.03 | 0.0 |
| Unknown primary tumour | 0 | 0 | NA† | 0.29 | 0.0 |
| All non-melanoma cancers | 39 | 8.12 | 4.8 (2.4 to 10.1) | 7.78 | 5.0 (3.7 to 7.3) |
*In non-carriers and controls, numbers of expected cases are calculated to correspond to equal person years as for carriers.
†NA=RR not calculable since 0 cases of cancer type in non-carriers.
RR, Relative risk.
Relative cancer risks for FDRs and SDRs are shown in table 3, FDRs contributed 12 683, SDRs 15.525 and controls 187.179 person years. RRs for all non-melanoma cancers was significantly elevated in FDRs, 2.1 (95% CI 1.6 to 2.7) but not in SDRs, 1.0 (95% CI 0.8 to 1.4). RRs for pancreatic cancer was significantly elevated in FDRs, 21.6 (95% CI 9.1 to 49.9) and in SDRs, 3.8 (95% CI 1.1 to 14.9). RRs for primary cancers in respiratory tissues were significantly elevated in FDRs, 6.0 (95% CI 2.8 to 13.1) and in SDRs, 2.8 (95% CI 1.0 to 7.2). RRs for primary cancers in upper digestive tissues were significantly elevated in FDRs, 3.3 (95% CI 1.5 to 7.6) and in SDRs, 2.3 (95% CI 1.0 to 5.6). Specification of all cancer occurrences and RRs in carriers, FDRs and SDRs compared with controls are shown in online supplementary table S2. The following cancers were significantly more frequent in the p.Arg112dup families compared with controls; cutaneous melanoma, cancer of the tongue, oral cavity, oesophagus, stomach, pancreas, larynx, lung, breast and cervix.
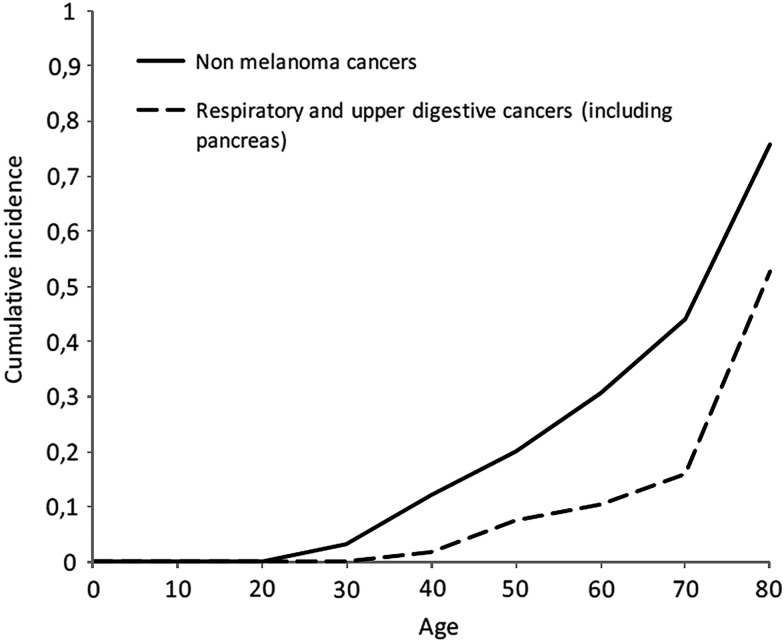
Age-specific cumulative incidence in p.Arg112dup carriers of all non-melanoma cancers and combined for cancers in pancreas, upper digestive and respiratory tissues is shown in figure 1. The numerical values of the cumulative incidences used for figure 1 are shown in online supplementary table S3. Among carriers at age 50 years, 20% had been diagnosed with non-melanoma cancers and 7% with cancers in pancreas, upper digestive and respiratory tissues. At age 80 years, 76% had been diagnosed with non-melanoma cancers and 53% with tumours in pancreas, upper digestive and respiratory tissues.
Figure 1.
Age-specific cumulative incidence of non-melanoma cancers and for cancers in respiratory and upper digestive tissues (including pancreas) among the cohort of CDKN2A p.Arg112dup carriers (n=120).
In table 4 the numbers of members in each family (p.Arg112dup carriers, FDRs and SDRs) with cancers in respiratory, upper digestive tissues and pancreas are shown. Pancreatic cancer, cancer in respiratory and upper digestive tissues was seen in 16, 12 and 12 families, respectively. In all families with more than 16 family members at least one subject was diagnosed with these cancers.
Table 4.
Numbers of subjects per CDKN2A p.Arg112dup positive family diagnosed with cancers in pancreas, respiratory and upper digestive tissues
| Family ID | Family size no.* |
Pancreas no. w/diagnosis |
Respiratory tissues no. w/diagnosis |
Upper digestive tissues no. w/diagnosis |
|---|---|---|---|---|
| 3998 | 10 | 1 | ||
| 8508 | 14 | 1 | 1 | 1 |
| 8512 | 31 | 1 | ||
| 8523 | 48 | 2 | 1 | |
| 8528 | 13 | |||
| 8551 | 9 | 1 | 1 | 1 |
| 8581 | 21 | 3 | ||
| 8601 | 35 | 1 | 1 | |
| 8611 | 17 | 1 | ||
| 8621 | 40 | 1 | 3 | |
| 8793 | 10 | 1 | 1 | |
| 8795 | 34 | 2 | ||
| 8839 | 18 | 1 | ||
| 8866 | 11 | 1 | 1 | |
| 12 519 | 13 | |||
| 12 546 | 15 | |||
| 12 551 | 18 | 1 | ||
| 13 502 | 143 | 2 | 5 | 6 |
| 13 509 | 34 | 2 | 2 | |
| 13 512 | 12 | 2 | ||
| 13 531 | 49 | 1 | 2 | |
| 13 545† | 9 | 3 | 1 | |
| 13 549† | 23 | 2 | ||
| 13 562 | 4 | |||
| 13 569 | 5 | 1 | ||
| 13 581 | 28 | 1 | 1 | |
| 13 592 | 16 | |||
| 13 616 | 38 | 2 | 1 | 1 |
*Total numbers of carriers, FDRs and SDRs per family.
†Families 13 545 and 13 549 were found to have shared SDRs.
FDR, First degree relative; SDR, second degree relative.
In the carriers, FDRs and SDRs we observed significantly elevated risks for pancreatic, lung, head and neck and gastro-oesophageal malignancies. In a normal population these cancer types are highly associated with tobacco smoke and to some extent other agents such as alcohol, chewing tobacco, certain foods, pollution and human papillomavirus (HPV). Although not a predefined aim in this study we found it important to address if the elevated risks of the observed cancers among carriers and their relatives was dependent primarily on the genotype or if environmental factors contributed to the phenotype observed. For this reason data on cigarette smoking was collected among all carriers that had reached the age of 29 years, which was the youngest age when any of the cancers described above had been diagnosed. Of the 116 carriers that had reached that age, information on smoking history was retrieved from 60% of the individuals (67% in carriers with any of these cancers and 57% in carriers without), in 61 individuals information was obtained from questionnaires and in 11 from medical records. In ever-smoking carriers compared with never-smoking carriers, the OR of cancers in pancreas, respiratory or upper digestive tissues was 9.3 (1.9 to 44.7) (table 5). Among ever-smokers that had the diagnoses, the following cancers were observed: pancreatic cancer (three cases), tongue cancer (three cases), lung cancer (three cases), cancer of oral cavity (one case), larynx cancer (one case), oesophageal cancer (one case). The median age at end of follow-up of smokers that had been diagnosed with cancers in pancreas, upper digestive or respiratory tissues was 70 years while in smokers that had none of these diagnoses, the median age at end of follow-up was 50 years.
Table 5.
Smoking status among CDKN2A p.Arg112dup carriers* diagnosed with cancers in respiratory or upper digestive tissues (including pancreas)
| Total | Diagnosed with cancers in respiratory or upper digestive tissues | |||
|---|---|---|---|---|
| no. | Yes | No | OR (95% CI) | |
| Never-smoker | 37 | 2 | 35 | 1.0 (reference) |
| Ever-smoker | 35 | 12 | 23 | 9.3 (1.9 to 44.7) |
*Only carriers that had reached the age of 29 years were included in this analysis.
OR, odds ratio.
Discussion
In this study we studied melanoma kindreds and investigated the cancer risks in carriers of the Swedish p.Arg112dup CDKN2A founder mutation and their FDRs and SDRs. We found a significantly elevated risk in carriers and FDRs for all non-melanoma cancers combined. More specifically, in carriers, FDRs and SDRs, there were significantly elevated risks for cancers in pancreas, upper digestive and respiratory tissues. At age 80 years, 53% of the carriers had one of these diagnoses. Cancers in pancreas, upper digestive and respiratory tissues were found in the majority of families and not limited to a small subset of kindreds, indicating that it is unlikely that the association between the mutation and these cancers is confounded by other genetic causes. Interestingly among carriers that had ever smoked cigarettes, the risk for pancreatic, respiratory and upper digestive cancers was significantly elevated compared with non-smokers. The difference in median age at the end of observation between smokers that had these cancers compared with those that did not was 20 years, indicating that younger smokers are at increased risk to develop these cancer types later in life.
In a study by de Snoo et al,7 specific cancer risks in melanoma families with the Dutch founder mutation (p16-Leiden) in CDKN2A were analysed. In this study, the 221 mutation carriers from 22 melanoma families had a significantly increased risk for cancers of the pancreas, respiratory tissues, lip, mouth and pharynx, digestive tissues (not specified if upper or lower), female genital tissues and eye/brain (only two cases). Interestingly, the specific cancers with increased risks were largely the same among carriers of the p16-Leiden and the p.Arg112dup mutations. These mutations have the following in common: they are founder mutations identified in a number of families and have originated in North European countries (Netherlands and Sweden). The mutations are both located in exon 2 of the gene, and both are set in ankyrin repeats 3–4, where significantly higher risks of pancreatic cancers have been reported compared with mutations in ankyrin repeats 1–2 of the gene.25 In the report by de Snoo et al, there was no data or statement on a possible association with tobacco smoking, but since there were increased risks for mainly the same specific cancers as were observed in our study, it is likely that carriers of the p16-Leiden mutation, and possibly also of other CDKN2A mutations perturbing ankyrin repeats 3–4, have increased risk for smoking induced cancers compared with the wild type population.
The CDKN2A encoded proteins p16-INK4A and p14-ARF are cell cycle inhibitors and tumour suppressors, where p16-INK4A is an inhibitor of CDK4/CDK6 in the retinoblastoma pathway and p14-ARF through its inhibition of HDM2, is a regulator of p53. Notably, germline mutations in TP53 and RB1 genes are associated with high risks for lung cancer (and other specific cancers, in particular retinoblastomas in RB1 mutated and sarcomas in TP53 mutated).26 27 In RB1 and TP53 mutated the elevated risks for lung cancers are mainly observed in smokers.27 28 Thus, it seems that carriers of CDKN2A mutations, and of mutations in tumour suppressors linked to p16-INK4A and p14-ARF, are at elevated risks for smoking induced cancers.
Upper digestive tissues (including pancreas) and respiratory tissues are derived from foregut endoderm29 and are known to be sensitive to exposures from certain carcinogens, in particular a strong association with cancers in these tissues and tobacco smoke and/or alcohol has been established.30 31 In three separate case reports of CDKN2A mutation carriers that were smokers and/or alcohol consumers, cancers of the tongue, oral cavity, pharynx and lung have been reported.12 17 19 Loss of the wild type CDKN2A allele was observed in tumours from these individuals, supporting the role of inactivation of this tumour suppressor gene in these tumours.17 19 Another study showed that among subjects that ever smoked, the risk for pancreatic cancer was higher for CDKN2A mutation carriers compared with non-carriers, but among non-smokers the risk for pancreatic cancer was not significantly different in carriers and non-carriers. Although interesting, this study was limited by a low number of confirmed carriers (n=9), which were all carriers of different CDKN2A mutations.32 Somatic CDKN2A alterations are frequently observed in pancreatic, lung, head and neck, oesophageal and gastric cancers, where they are believed to be driver mutations.33–36 In lung and head and neck cancers it has been shown that somatic alterations in the CDKN2A gene are associated with tobacco smoke and/or alcohol exposure.37–39 In a rodent model it has been demonstrated that exposure to a tobacco smoke derived carcinogen (4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone) induces tumours with hypermethylation of the p16 gene promoter leading to loss of p16 expression.40 In a recent study of mutational processes in multiple human cancers, it was reported that of the 30 different tumour types analysed, melanoma, lung, oesophageal, head and neck and gastric cancers were all among the tumour types that had most acquired mutations.41 Digestive and respiratory organs are highly exposed to multiple carcinogens in our environment in analogy to the skin being exposed to ultraviolet radiation, and it has been shown that melanoma penetrance in CDKN2A mutation carriers is associated with the environmental ultraviolet (UV) exposure in the country of residence.20 In melanoma tumours, UV-B signature DNA changes are commonly observed in the CDKN2A gene.42 In carriers we also found an increased risk of gynaecological malignancies, largely due to cervical carcinomas (see online supplementary table S2). It will be of interest to follow whether this increase is abolished by the introduction of prophylactic HPV vaccination. Our findings support the concept that germline CDKN2A mutations may confer an increased sensitivity to carcinogens, resulting in the observed pattern of increased cancer risks.
In p.Arg112dup carriers, three adolescents were diagnosed with melanoma, but no melanomas or other tumours were seen in young children, in FDRs there was one case of CNS tumour at age 9 years and in SDRs one case of leukaemia at age 2 years (data not shown). Although these are more cases than would be expected in the general population, they are too few for statistical analysis and the association previously reported for childhood cancer15 in p.Arg112dup families cannot be confirmed nor refuted in this study. For breast cancer, the RR was moderately elevated in carriers, FDRs and SDRs but not significantly increased. Higher risk for breast cancer has previously been reported in p.Arg112dup carriers.4 In the study by Borg et al, nine p.Arg112dup families were analysed, but it was later discovered that in one family (excluded from the current study) with a high burden of breast cancer, family members had a germline mutation in the BRCA1 gene in addition to the CDKN2A mutation, which may have contributed to the conclusion that there was a marked increase in breast cancer risk. Also carriers might be more likely to participate in population screening programs for breast and cervical cancer, possibly increasing the incidence of these cancer types in this group.
A few limitations of our study deserve attention. The CDKN2A p.Arg112dup carriers are identified solely from melanoma-prone families and we have limited knowledge on the mutation frequencies in other populations. Therefore we cannot automatically draw conclusions that all carriers of this mutation have the risks found in this study. But in Stockholm we have previously tested sporadic melanoma cases and controls for CDKN2A p.Arg112dup mutations and found mutations in 0.2% (n=526) and 0% (n=663), respectively, indicating that the mutation frequency in the population is low and likely mainly observed in melanoma-prone families.43 In studies as this one there is always a risk for ascertainment bias, and for melanoma we cannot exclude that there is some ascertainment bias since there are more melanoma cases than would be expected in the non-carriers from the mutation carrying families. This is to some degree expected when individuals are selected solely for belonging to families with multiple melanoma cases. In this study the main aim was the risk for non-melanoma cancers, and by observing the frequencies of non-melanoma cancers in non-carriers, we cannot see an obvious tendency of ascertainment bias for non-melanoma tumours (tables 1 and 2). In addition to assessing the combined risk for all non-melanoma cancers we also intended to assess the risks for tissue-specific cancers. This is a highly relevant issue, since for the families it is important to recognise the total risk and the risk for certain cancer types. Although we have included all the known families carrying the p.Arg112dup mutation in our study, the size of the cohorts is too small for an adjustment of statistical significance for multiple outcomes. To still address this issue we have done comparisons in multiple groups, first comparing carriers with non-carriers and with controls and also independently comparing FDRs and SDRs with controls. In online supplementary table S2 we also compare all individuals in p.Arg112dup mutation families (except the non-carriers) with the total control population. We only draw conclusions on an association between certain cancer forms and the mutation when we have seen significantly elevated risks in all comparisons. When smoking history was collected in mutation carriers, data was missing from 33% of carriers with cancers in pancreas, upper digestive and respiratory tissues and 43% of those not diagnosed with these cancer types. This difference was likely mostly explained by that in those that had died being diagnosed with lung, head and neck, pancreatic or gastro-oesophageal cancers there was more often information on smoking history in medical records, than in deceased carriers that had not been diagnosed with these cancer types. We cannot rule out that there might have been an issue of misclassification with respect to smoking status from data collected from medical records or from the questionnaires, but it is very unlikely that the marked association seen between smoking and these cancers, would only have been due to information bias.
The strength of this study is that it involved the hitherto largest number of families with the same germline CDKN2A mutation, with long time follow-up for occurrence of cancer. We have investigated independently the cancer risks in carriers and their FDRs and SDRs by linkage to national registries with near-complete coverage. We found high risks among carriers for cancers in pancreas, respiratory and upper gastrointestinal tissues. In a gene-dose manner we confirmed elevated risks for the same cancer types in FDRs and SDRs as were observed in carriers. By collecting information on smoking history, we show in mutation carriers, a previously not recognised association between tobacco smoking and cancers in pancreas, respiratory and upper gastrointestinal tissues.
We conclude that the risk for non-melanoma cancers in CDKN2A p.Arg112dup mutation carriers is not limited to pancreatic cancer and the phenotype should be extended to include tumours in respiratory and upper digestive tissues. As has been reported for pancreatic cancer, it is not unlikely that the risk for cancers in respiratory and upper gastrointestinal tissues may vary significantly between carriers of specific germline CDKN2A mutations.25 This emphasises the need for further collaborative analyses of cancer occurrence among carriers of specified CDKN2A germline mutations. Further molecular and epidemiological studies should be done to examine the relation between carcinogens and cancers in CDKN2A mutation carriers. CDKN2A p.Arg112dup mutation carriers should be strongly advised to abstain from tobacco smoking. Moreover, families with multiple cases of melanoma as well as cancers in respiratory and upper digestive tissues should be regarded as candidates for CDKN2A mutation screening. We propose that, particularly in mutation carriers who smoke, screening for lung, head and neck, and gastro-oesophageal malignancies should be considered.
Supplementary Material
Acknowledgments
This work was supported by grants from The Swedish Cancer Society, The Radiumhemmet Research Funds, Stockholm County Council, European Research Council Advanced Grant (ERC-2011–294576), Gunnar Nilsson Foundation and Regional and Hospital Funds. The authors thank Diana Lindén, Lena Westerberg and Anita Schmidt Casslén for their help with this study.
Footnotes
Contributors: All authors of this paper have participated/contributed to this work as follows: Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; Drafting the work or revising it critically for important intellectual content; Final approval of the version to be published; Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Competing interests: None.
Ethics approval: Lund University (Sweden) Ethical Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Novelty and Impact Statements: In this study we describe in members of melanoma-prone families with germline CDKN2A mutation, a significantly increased risk of pancreatic, lung, head and neck and gastro-oesophageal cancers. We show a positive association between tobacco smoking and these non-melanoma cancers among mutation carriers. This is the first study that shows association between smoking history and cancer diagnoses in CDKN2A mutation carriers. Our study has important implications for counselling and monitoring of members of melanoma-prone families with germline CDKN2A mutations.
References
- 1.Greene MH, Fraumeni JF. The hereditary variant of malignant melanoma. New York: Grune & Stratton, 1979 [Google Scholar]
- 2.Skolnick MH, Cannon-Albright LA, Kamb A. Genetic predisposition to melanoma. Eur J Cancer 1994;30A:1991–5 [DOI] [PubMed] [Google Scholar]
- 3.Goldstein AM, Struewing JP, Chidambaram A, Fraser MC, Tucker MA. Genotype-phenotype relationships in u.S. Melanoma-prone families with cdkn2a and cdk4 mutations . J Natl Cancer Inst 2000;92:1006–10 [DOI] [PubMed] [Google Scholar]
- 4.Borg A, Sandberg T, Nilsson K, Johannsson O, Klinker M, Masback A, Westerdahl J, Olsson H, Ingvar C. High frequency of multiple melanomas and breast and pancreas carcinomas in cdkn2a mutation-positive melanoma families. J Natl Cancer Inst 2000;92:1260–6 [DOI] [PubMed] [Google Scholar]
- 5.Platz A, Hansson J, Mansson-Brahme E, Lagerlof B, Linder S, Lundqvist E, Sevigny P, Inganas M, Ringborg U. Screening of germline mutations in the cdkn2a and cdkn2b genes in swedish families with hereditary cutaneous melanoma. J Natl Cancer Inst 1997;89:697–702 [DOI] [PubMed] [Google Scholar]
- 6.Hashemi J, Bendahl PO, Sandberg T, Platz A, Linder S, Stierner U, Olsson H, Ingvar C, Hansson J, Borg A. Haplotype analysis and age estimation of the 113insr cdkn2a founder mutation in swedish melanoma families. Genes Chromosomes Cancer 2001;31:107–16 [DOI] [PubMed] [Google Scholar]
- 7.de Snoo FA, Bishop DT, Bergman W, van Leeuwen I, van der Drift C, van Nieuwpoort FA, Out-Luiting CJ, Vasen HF, ter Huurne JA, Frants RR, Willemze R, Breuning MH, Gruis NA. Increased risk of cancer other than melanoma in cdkn2a founder mutation (p16-leiden)-positive melanoma families. Clin Cancer Res 2008;14:7151–7 [DOI] [PubMed] [Google Scholar]
- 8.Ghiorzo P, Ciotti P, Mantelli M, Heouaine A, Queirolo P, Rainero ML, Ferrari C, Santi PL, De Marchi R, Farris A, Ajmar F, Bruzzi P, Bianchi-Scarra G. Characterization of ligurian melanoma families and risk of occurrence of other neoplasia. Int J Cancer 1999;83:441–8 [DOI] [PubMed] [Google Scholar]
- 9.Goldstein AM, Fraser MC, Struewing JP, Hussussian CJ, Ranade K, Zametkin DP, Fontaine LS, Organic SM, Dracopoli NC, Clark WH, Jr, Tucker MA. Increased risk of pancreatic cancer in melanoma-prone kindreds with p16ink4 mutations. N Engl J Med 1995;333:970–4 [DOI] [PubMed] [Google Scholar]
- 10.Goldstein AM, Struewing JP, Fraser MC, Smith MW, Tucker MA. Prospective risk of cancer in cdkn2a germline mutation carriers. J Med Genet 2004;41:421–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bahuau M, Vidaud D, Jenkins RB, Bieche I, Kimmel DW, Assouline B, Smith JS, Alderete B, Cayuela JM, Harpey JP, Caille B, Vidaud M. Germ-line deletion involving the ink4 locus in familial proneness to melanoma and nervous system tumors. Cancer Res 1998;58:2298–303 [PubMed] [Google Scholar]
- 12.Cabanillas R, Astudillo A, Valle M, de la Rosa J, Alvarez R, Duran NS, Cadinanos J. Novel germline cdkn2a mutation associated with head and neck squamous cell carcinomas and melanomas. Head Neck 2013;35:E80–84 [DOI] [PubMed] [Google Scholar]
- 13.Goldstein AM, Chan M, Harland M, Gillanders EM, Hayward NK, Avril MF, Azizi E, Bianchi-Scarra G, Bishop DT, Bressac-de Paillerets B, Bruno W, Calista D, Cannon Albright LA, Demenais F, Elder DE, Ghiorzo P, Gruis NA, Hansson J, Hogg D, Holland EA, Kanetsky PA, Kefford RF, Landi MT, Lang J, Leachman SA, Mackie RM, Magnusson V, Mann GJ, Niendorf K, Newton Bishop J, Palmer JM, Puig S, Puig-Butille JA, de Snoo FA, Stark M, Tsao H, Tucker MA, Whitaker L, Yakobson E, Melanoma Genetics C. High-risk melanoma susceptibility genes and pancreatic cancer, neural system tumors, and uveal melanoma across genomel. Cancer Res 2006;66:9818–28 [DOI] [PubMed] [Google Scholar]
- 14.Kannengiesser C, Avril MF, Spatz A, Laud K, Lenoir GM, Bressac-de-Paillerets B. Cdkn2a as a uveal and cutaneous melanoma susceptibility gene. Genes Chromosomes Cancer 2003;38:265–8 [DOI] [PubMed] [Google Scholar]
- 15.Magnusson S, Borg A, Kristoffersson U, Nilbert M, Wiebe T, Olsson H. Higher occurrence of childhood cancer in families with germline mutations in brca2, mmr and cdkn2a genes. Fam Cancer 2008;7:331–7 [DOI] [PubMed] [Google Scholar]
- 16.Mukherjee B, Delancey JO, Raskin L, Everett J, Jeter J, Begg CB, Orlow I, Berwick M, Armstrong BK, Kricker A, Marrett LD, Millikan RC, Culver HA, Rosso S, Zanetti R, Kanetsky PA, From L, Gruber SB, Investigators GEMS. Risk of non-melanoma cancers in first-degree relatives of cdkn2a mutation carriers. J Natl Cancer Inst 2012;104:953–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oldenburg RA, de Vos tot Nederveen Cappel WH, van Puijenbroek M, van den Ouweland A, Bakker E, Griffioen G, Devilee P, Cornelisse CJ, Meijers-Heijboer H, Vasen HF, Morreau H. Extending the p16-leiden tumour spectrum by respiratory tract tumours. J Med Genet 2004;41:e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasen HF, Gruis NA, Frants RR, van Der Velden PA, Hille ET, Bergman W. Risk of developing pancreatic cancer in families with familial atypical multiple mole melanoma associated with a specific 19 deletion of p16 (p16-leiden). Int J Cancer 2000;87:809–11 [PubMed] [Google Scholar]
- 19.Vinarsky V, Fine RL, Assaad A, Qian Y, Chabot JA, Su GH, Frucht H. Head and neck squamous cell carcinoma in fammm syndrome. Head Neck 2009;31:1524–7 [DOI] [PubMed] [Google Scholar]
- 20.Bishop DT, Demenais F, Goldstein AM, Bergman W, Bishop JN, Bressac-de Paillerets B, Chompret A, Ghiorzo P, Gruis N, Hansson J, Harland M, Hayward N, Holland EA, Mann GJ, Mantelli M, Nancarrow D, Platz A, Tucker MA, Melanoma Genetics C. Geographical variation in the penetrance of cdkn2a mutations for melanoma. J Natl Cancer Inst 2002;94:894–903 [DOI] [PubMed] [Google Scholar]
- 21.Hansson J, Bergenmar M, Hofer PA, Lundell G, Mansson-Brahme E, Ringborg U, Synnerstad I, Bratel AT, Wennberg AM, Rosdahl I. Monitoring of kindreds with hereditary predisposition for cutaneous melanoma and dysplastic nevus syndrome: Results of a swedish preventive program. J Clin Oncol 2007;25:2819–24 [DOI] [PubMed] [Google Scholar]
- 22.Goldstein AM, Chan M, Harland M, Hayward NK, Demenais F, Bishop DT, Azizi E, Bergman W, Bianchi-Scarra G, Bruno W, Calista D, Albright LA, Chaudru V, Chompret A, Cuellar F, Elder DE, Ghiorzo P, Gillanders EM, Gruis NA, Hansson J, Hogg D, Holland EA, Kanetsky PA, Kefford RF, Landi MT, Lang J, Leachman SA, MacKie RM, Magnusson V, Mann GJ, Bishop JN, Palmer JM, Puig S, Puig-Butille JA, Stark M, Tsao H, Tucker MA, Whitaker L, Yakobson E, Lund Melanoma Study G, Melanoma Genetics C. Features associated with germline cdkn2a mutations: A genomel study of melanoma-prone families from three continents. J Med Genet 2007;44:99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Statistics Sweden BF, Population and Welfare Statistics 2012:1, Multi-generation register 2011. A description of contents and quality. http://www.scb.se/statistik/_publikationer/BE9999_2011A01_BR_BE96BR1202.pdf.
- 24. Socialstyrelsen; Swedish cancer registry. http://www.Socialstyrelsen.Se/register/halsodataregister/cancerregistret/inenglish.
- 25.Goldstein AM. Familial melanoma, pancreatic cancer and germline cdkn2a mutations. Hum Mutat 2004;23:630. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher O, Easton D, Anderson K, Gilham C, Jay M, Peto J. Lifetime risks of common cancers among retinoblastoma survivors. J Natl Cancer Inst 2004;96:357–63 [DOI] [PubMed] [Google Scholar]
- 27.Hwang SJ, Cheng LS, Lozano G, Amos CI, Gu X, Strong LC. Lung cancer risk in germline p53 mutation carriers: Association between an inherited cancer predisposition, cigarette smoking, and cancer risk. Hum Genet 2003;113:238–43 [DOI] [PubMed] [Google Scholar]
- 28.Kleinerman RA, Tarone RE, Abramson DH, Seddon JM, Li FP, Tucker MA. Hereditary retinoblastoma and risk of lung cancer. J Natl Cancer Inst 2000;92:2037–9 [DOI] [PubMed] [Google Scholar]
- 29.Sadler TW, Thomas W, Langman J. Langman's medical embryology. 11th edn Philadelphia: Wolters Kluwer Lippincott Williams & Wilkins, 2010:275–319, 363–402 [Google Scholar]
- 30.Kuper H, Boffetta P, Adami HO. Tobacco use and cancer causation: Association by tumour type. J Intern Med 2002;252:206–24 [DOI] [PubMed] [Google Scholar]
- 31.Pelucchi C, Tramacere I, Boffetta P, Negri E, La Vecchia C. Alcohol consumption and cancer risk. Nutr Cancer 2011;63:983–90 [DOI] [PubMed] [Google Scholar]
- 32.McWilliams RR, Wieben ED, Rabe KG, Pedersen KS, Wu Y, Sicotte H, Petersen GM. Prevalence of cdkn2a mutations in pancreatic cancer patients: Implications for genetic counseling. Eur J Hum Genet 2011;19:472–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dulak AM, Stojanov P, Peng S, Lawrence MS, Fox C, Stewart C, Bandla S, Imamura Y, Schumacher SE, Shefler E, McKenna A, Carter SL, Cibulskis K, Sivachenko A, Saksena G, Voet D, Ramos AH, Auclair D, Thompson K, Sougnez C, Onofrio RC, Guiducci C, Beroukhim R, Zhou Z, Lin L, Lin J, Reddy R, Chang A, Landrenau R, Pennathur A, Ogino S, Luketich JD, Golub TR, Gabriel SB, Lander ES, Beer DG, Godfrey TE, Getz G, Bass AJ. Exome and whole-genome sequencing of esophageal adenocarcinoma identifies recurrent driver events and mutational complexity. Nat Genet 2013;45:478–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carter H, Samayoa J, Hruban RH, Karchin R. Prioritization of driver mutations in pancreatic cancer using cancer-specific high-throughput annotation of somatic mutations (chasm). Cancer Biol Ther 2010;10:582–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J, van Hummelen P, Go C, Palescandolo E, Jang J, Park HY, Kang SY, Park JO, Kang WK, MacConaill L, Kim KM. High-throughput mutation profiling identifies frequent somatic mutations in advanced gastric adenocarcinoma. PLoS ONE 2012;7:e38892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ding L, Getz G, Wheeler DA, Mardis ER, McLellan MD, Cibulskis K, Sougnez C, Greulich H, Muzny DM, Morgan MB, Fulton L, Fulton RS, Zhang Q, Wendl MC, Lawrence MS, Larson DE, Chen K, Dooling DJ, Sabo A, Hawes AC, Shen H, Jhangiani SN, Lewis LR, Hall O, Zhu Y, Mathew T, Ren Y, Yao J, Scherer SE, Clerc K, Metcalf GA, Ng B, Milosavljevic A, Gonzalez-Garay ML, Osborne JR, Meyer R, Shi X, Tang Y, Koboldt DC, Lin L, Abbott R, Miner TL, Pohl C, Fewell G, Haipek C, Schmidt H, Dunford-Shore BH, Kraja A, Crosby SD, Sawyer CS, Vickery T, Sander S, Robinson J, Winckler W, Baldwin J, Chirieac LR, Dutt A, Fennell T, Hanna M, Johnson BE, Onofrio RC, Thomas RK, Tonon G, Weir BA, Zhao X, Ziaugra L, Zody MC, Giordano T, Orringer MB, Roth JA, Spitz MR, Wistuba II, Ozenberger B, Good PJ, Chang AC, Beer DG, Watson MA, Ladanyi M, Broderick S, Yoshizawa A, Travis WD, Pao W, Province MA, Weinstock GM, Varmus HE, Gabriel SB, Lander ES, Gibbs RA, Meyerson M, Wilson RK. Somatic mutations affect key pathways in lung adenocarcinoma. Nature 2008;455:1069–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim DH, Nelson HH, Wiencke JK, Zheng S, Christiani DC, Wain JC, Mark EJ, Kelsey KT. P16(ink4a) and histology-specific methylation of cpg islands by exposure to tobacco smoke in non-small cell lung cancer. Cancer Res 2001;61:3419–24 [PubMed] [Google Scholar]
- 38.Kraunz KS, McClean MD, Nelson HH, Peters E, Calderon H, Kelsey KT. Duration but not intensity of alcohol and tobacco exposure predicts p16ink4a homozygous deletion in head and neck squamous cell carcinoma. Cancer Res 2006;66:4512–15 [DOI] [PubMed] [Google Scholar]
- 39.Tam KW, Zhang W, Soh J, Stastny V, Chen M, Sun H, Thu K, Rios JJ, Yang C, Marconett CN, Selamat SA, Laird-Offringa IA, Taguchi A, Hanash S, Shames D, Ma X, Zhang MQ, Lam WL, Gazdar A. Cdkn2a/p16 inactivation mechanisms and their relationship to smoke exposure and molecular features in non-small-cell lung cancer. J Thorac Oncol 2013;8:1378–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Belinsky SA, Nikula KJ, Palmisano WA, Michels R, Saccomanno G, Gabrielson E, Baylin SB, Herman JG. Aberrant methylation of p16(ink4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci USA 1998;95:11891–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alexandrov LB, Nik-Zainal S, Wedge DC, Aparicio SA, Behjati S, Biankin AV, Bignell GR, Bolli N, Borg A, Borresen-Dale AL, Boyault S, Burkhardt B, Butler AP, Caldas C, Davies HR, Desmedt C, Eils R, Eyfjord JE, Foekens JA, Greaves M, Hosoda F, Hutter B, Ilicic T, Imbeaud S, Imielinsk M, Jager N, Jones DT, Jones D, Knappskog S, Kool M, Lakhani SR, Lopez-Otin C, Martin S, Munshi NC, Nakamura H, Northcott PA, Pajic M, Papaemmanuil E, Paradiso A, Pearson JV, Puente XS, Raine K, Ramakrishna M, Richardson AL, Richter J, Rosenstiel P, Schlesner M, Schumacher TN, Span PN, Teague JW, Totoki Y, Tutt AN, Valdes-Mas R, van Buuren MM, van 't Veer L, Vincent-Salomon A, Waddell N, Yates LR, Australian Pancreatic Cancer Genome I, Consortium IBC, Consortium IM-S, PedBrain I, Zucman-Rossi J, Futreal PA, McDermott U, Lichter P, Meyerson M, Grimmond SM, Siebert R, Campo E, Shibata T, Pfister SM, Campbell PJ, Stratton MR. Signatures of mutational processes in human cancer. Nature 2013;500:415–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hocker T, Tsao H. Ultraviolet radiation and melanoma: A systematic review and analysis of reported sequence variants. Hum Mutat 2007;28:578–88 [DOI] [PubMed] [Google Scholar]
- 43.Hoiom V, Tuominen R, Kaller M, Linden D, Ahmadian A, Mansson-Brahme E, Egyhazi S, Sjoberg K, Lundeberg J, Hansson J. Mc1r variation and melanoma risk in the swedish population in relation to clinical and pathological parameters. Pigment Cell Melanoma Res 2009;22:196–204 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.