Abstract
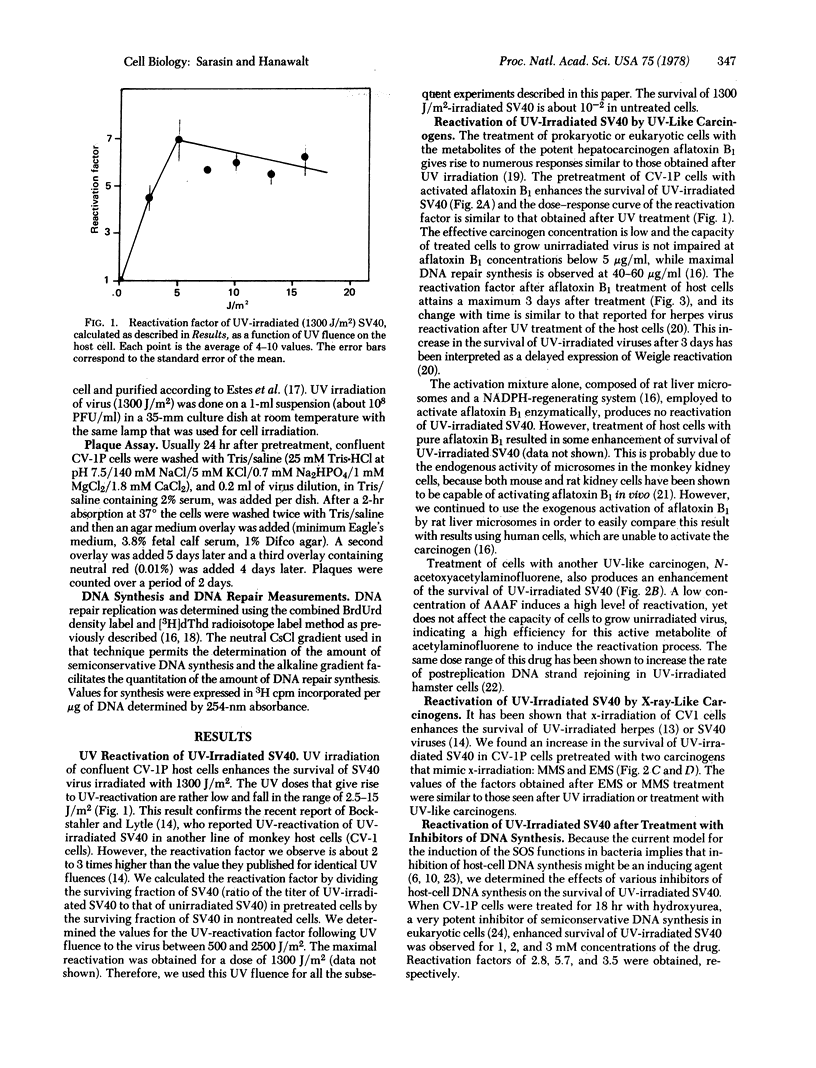
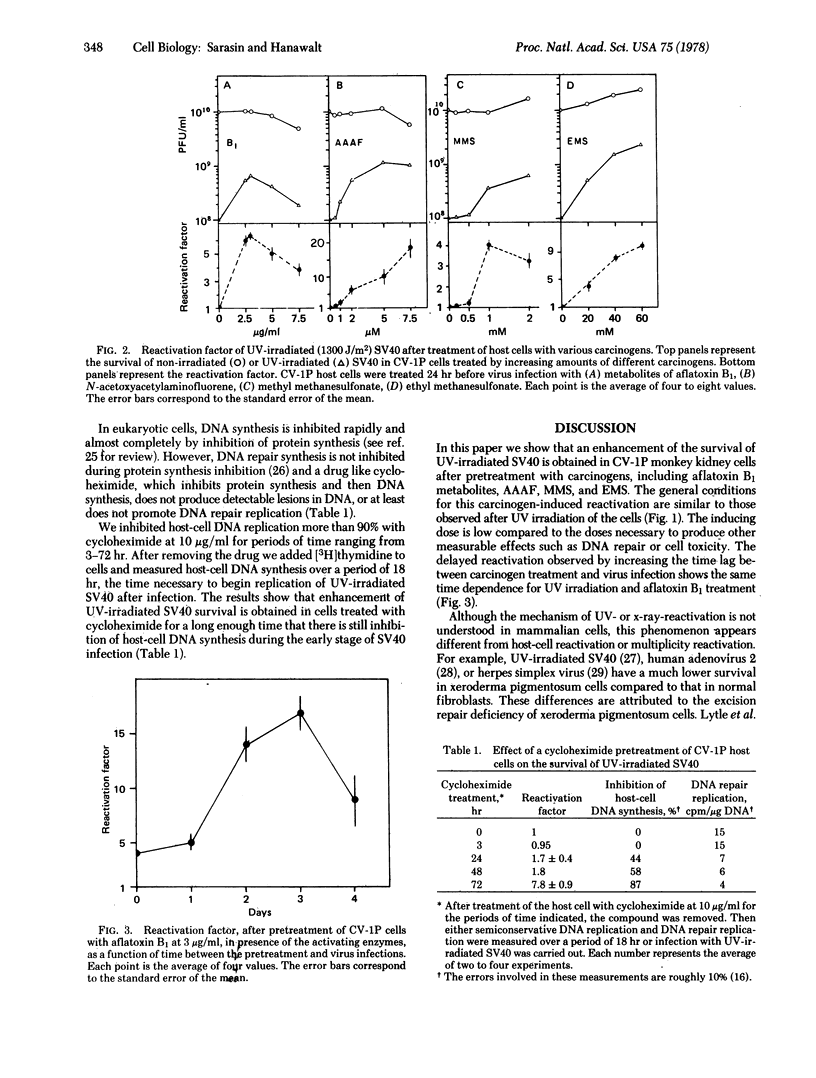
Treatment of monkey kidney cells with low doses of carcinogen enhances the survival of UV-irradiated simian virus 40 (SV40). This is true for compounds with UV-like effects (metabolites of aflatoxin B1, N-acetoxyacetylaminofluorene) and compounds with x-ray-like effects (methyl methanesulfonate, ethyl methanesulfonate). This phenomenon resembles the UV-reactivation of viruses in eukaryotic cells. The carcinogen-induced enhancement of the survival of UV-irradiated SV40 is correlated with the inhibition of host-cell DNA synthesis, suggesting that the inhibition is an inducing agent. An enhancement of UV-irradiated SV40 survival is also obtained in cells treated with hydroxyurea or cycloheximide for long enough that there is still inhibition of host DNA synthesis during the early stage of SV40 infection. We hypothesize that treatment of host cells with carcinogens induces a new recovery pathway that facilitates the replication of damaged DNA, bypassing the lesions and resulting in the enhanced survival of UV-irradiated SV40. This inducible process might represent the expression of "SOS repair" functions in eukaryotic cells analogous to the previously demonstrated induction of SOS repair in bacteria after UV or carcinogen treatment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Lytle C. D. Decreased host cell reactivation of irradiated SV40 virus in xeroderma pigmentosum. Nature. 1970 Oct 24;228(5269):359–361. doi: 10.1038/228359a0. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Lytle C. D. Radiation enhanced reactivation of nuclear replicating mammalian viruses. Photochem Photobiol. 1977 May;25(5):477–482. doi: 10.1111/j.1751-1097.1977.tb09173.x. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Lytle C. D., Stafford J. E., Haynes K. F. Ultraviolet enhanced reactivation of a human virus: effect of delayed infection. Mutat Res. 1976 Jun;35(2):189–198. doi: 10.1016/0027-5107(76)90184-6. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Lytle C. D. Ultraviolet light enhanced reactivation of a mammalian virus. Biochem Biophys Res Commun. 1970 Oct 9;41(1):184–189. doi: 10.1016/0006-291x(70)90486-9. [DOI] [PubMed] [Google Scholar]
- Bockstahler L. E., Lytle C. D. X-ray-enhanced reactivation of ultraviolet-irradiated human virus. J Virol. 1971 Oct;8(4):601–602. doi: 10.1128/jvi.8.4.601-602.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casto B. C., Pieczynski W. J., DiPaolo J. A. Enhancement of adenovirus transformation by treatment of hamster embryo cells with diverse chemical carcinogens. Cancer Res. 1974 Jan;34(1):72–78. [PubMed] [Google Scholar]
- Cleaver J. E. Defective repair replication of DNA in xeroderma pigmentosum. Nature. 1968 May 18;218(5142):652–656. doi: 10.1038/218652a0. [DOI] [PubMed] [Google Scholar]
- D'Ambrosio S. M., Setlow R. B. Enhancement of postreplication repair in Chinese hamster cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2396–2400. doi: 10.1073/pnas.73.7.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day R. S., 3rd Cellular reactivation of ultraviolet-irradiated human adenovirus 2 in normal and xeroderma pigmentosum fibroblasts. Photochem Photobiol. 1974 Jan;19(1):9–13. doi: 10.1111/j.1751-1097.1974.tb06467.x. [DOI] [PubMed] [Google Scholar]
- Estes M. K., Huang E. S., Pagano J. S. Structural polypeptides of simian virus 40. J Virol. 1971 May;7(5):635–641. doi: 10.1128/jvi.7.5.635-641.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautschi J. R., Young B. R., Cleaver J. E. Repair of damaged DNA in the absence of protein synthesis in mammalian cells. Exp Cell Res. 1973 Jan;76(1):87–94. doi: 10.1016/0014-4827(73)90422-9. [DOI] [PubMed] [Google Scholar]
- Gudas L. J., Pardee A. B. DNA synthesis inhibition and the induction of protein X in Escherichia coli. J Mol Biol. 1976 Mar 15;101(4):459–477. doi: 10.1016/0022-2836(76)90240-0. [DOI] [PubMed] [Google Scholar]
- Kaplan J. C., Wilbert S. M., Black P. H. Analysis of simian virus 40-induced transformation of hamster kidney tissue in vitro. 8. Induction of infectious simian virus 40 from virogenie transformed hamster cells by amino acid deprivation or cycloheximide treatment. J Virol. 1972 Mar;9(3):448–453. doi: 10.1128/jvi.9.3.448-453.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S., Ichikawa H., Iwo K., Kato T. Base-change mutagenesis and prophage induction in strains of Escherichia coli with different DNA repair capacities. Genetics. 1970 Oct;66(2):187–217. doi: 10.1093/genetics/66.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little J. W., Hanawalt P. C. Induction of protein X in Escherichia coli. Mol Gen Genet. 1977 Feb 15;150(3):237–248. doi: 10.1007/BF00268122. [DOI] [PubMed] [Google Scholar]
- Lytle C. D., Aaronson S. A., Harvey E. Host-cell reactivation in mammalian cells. II. Survival of herpes simplex virus and vaccinia virus in normal human and xeroderma pigmentosum cells. Int J Radiat Biol Relat Stud Phys Chem Med. 1972 Aug;22(2):159–165. [PubMed] [Google Scholar]
- Lytle C. D., Day R. S., 3rd, Hellman K. B., Bockstahler L. E. Infection of UV-irradiated xeroderma pigmentosum fibroblasts by herpes simplex virus: study of capacity and Weigle reactivation. Mutat Res. 1976 Sep;36(3):257–264. doi: 10.1016/0027-5107(76)90235-9. [DOI] [PubMed] [Google Scholar]
- McCann J., Choi E., Yamasaki E., Ames B. N. Detection of carcinogens as mutagens in the Salmonella/microsome test: assay of 300 chemicals. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5135–5139. doi: 10.1073/pnas.72.12.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy G. P., Weissbach A. HeLa cell DNA polymerases: the effect of cycloheximide in vivo and detection of a new form of DNA polymerase alpha. Biochim Biophys Acta. 1977 Jul 5;477(1):70–83. doi: 10.1016/0005-2787(77)90161-7. [DOI] [PubMed] [Google Scholar]
- Poon P. K., Parker J. W., O'Brien R. L. Faulty DNA repair following ultraviolet irradiation in Fanconi's anemia. Basic Life Sci. 1975;5B:821–824. doi: 10.1007/978-1-4684-2898-8_64. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., AURELIAN L., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. I. THE PROGRAMMING OF VIRAL DNA DUPLICATION IN HEP-2 CELLS. Virology. 1963 Nov;21:482–498. doi: 10.1016/0042-6822(63)90209-5. [DOI] [PubMed] [Google Scholar]
- Robbins J. H., Kraemer K. H., Lutzner M. A., Festoff B. W., Coon H. G. Xeroderma pigmentosum. An inherited diseases with sun sensitivity, multiple cutaneous neoplasms, and abnormal DNA repair. Ann Intern Med. 1974 Feb;80(2):221–248. doi: 10.7326/0003-4819-80-2-221. [DOI] [PubMed] [Google Scholar]
- STRAUSS B. S. Recovery of deoxyribonucleic acid from the effects of alkylation. J Gen Microbiol. 1963 Jan;30:89–103. doi: 10.1099/00221287-30-1-89. [DOI] [PubMed] [Google Scholar]
- STRAUSS B. S. Response of Escherichia coli auxotrophs to heat after treatment with mutagenic alkyl methanesulfonates. J Bacteriol. 1962 Feb;83:241–249. doi: 10.1128/jb.83.2.241-249.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarasin A. R., Smith C. A., Hanawalt P. C. Repair of DNA in human cells after treatment with activated aflatoxin B1. Cancer Res. 1977 Jun;37(6):1786–1793. [PubMed] [Google Scholar]
- Sarasin A., Goze A., Devoret R., Moulé Y. Induced reactivity of UV-damaged phage gamma in E. coli K12 host cells treated with aflatoxin B1 metabolites. Mutat Res. 1977 Feb;42(2):205–214. doi: 10.1016/s0027-5107(77)80024-9. [DOI] [PubMed] [Google Scholar]
- Sarasin A., Meunier-Rotival M. How chemicals may induce cancer. Biomedicine. 1976 Nov 10;24(5):306–316. [PubMed] [Google Scholar]
- Sarasin A., Moulé Y. Helical polysomes induced by aflatoxin B1 in vivo. A new hypothesis for helix formation by chemicals and carcinogens. Exp Cell Res. 1976 Feb;97(2):346–358. doi: 10.1016/0014-4827(76)90626-1. [DOI] [PubMed] [Google Scholar]
- Seale R. L., Simpson R. T. Effects of cycloheximide on chromatin biosynthesis. J Mol Biol. 1975 May 25;94(3):479–501. doi: 10.1016/0022-2836(75)90216-8. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Hanawalt P. C. Repair replication in human cells. Simplified determination utilizing hydroxyurea. Biochim Biophys Acta. 1976 May 19;432(3):336–347. doi: 10.1016/0005-2787(76)90143-x. [DOI] [PubMed] [Google Scholar]
- Timson J. Hydroxyurea. Mutat Res. 1975;32(2):115–132. doi: 10.1016/0165-1110(75)90002-0. [DOI] [PubMed] [Google Scholar]
- Todaro G. J., Huebner R. J. N.A.S. symposium: new evidence as the basis for increased efforts in cancer research. Proc Natl Acad Sci U S A. 1972 Apr;69(4):1009–1015. doi: 10.1073/pnas.69.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weigle J. J. Induction of Mutations in a Bacterial Virus. Proc Natl Acad Sci U S A. 1953 Jul;39(7):628–636. doi: 10.1073/pnas.39.7.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkin E. M. Ultraviolet mutagenesis and inducible DNA repair in Escherichia coli. Bacteriol Rev. 1976 Dec;40(4):869–907. doi: 10.1128/br.40.4.869-907.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto H., Shimojo H. Multiplicity reactivation of human adenovirus type 12 and simian virus 40 irradiated by ultraviolet light. Virology. 1971 Aug;45(2):529–531. doi: 10.1016/0042-6822(71)90355-2. [DOI] [PubMed] [Google Scholar]



