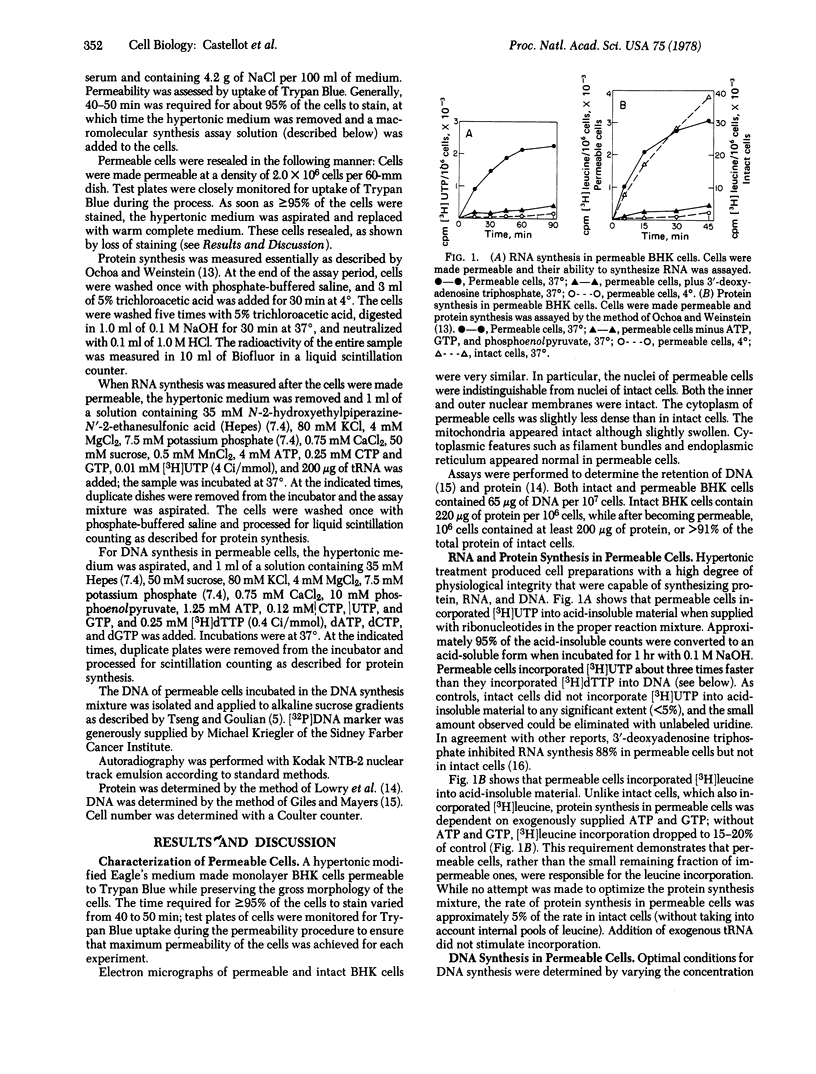
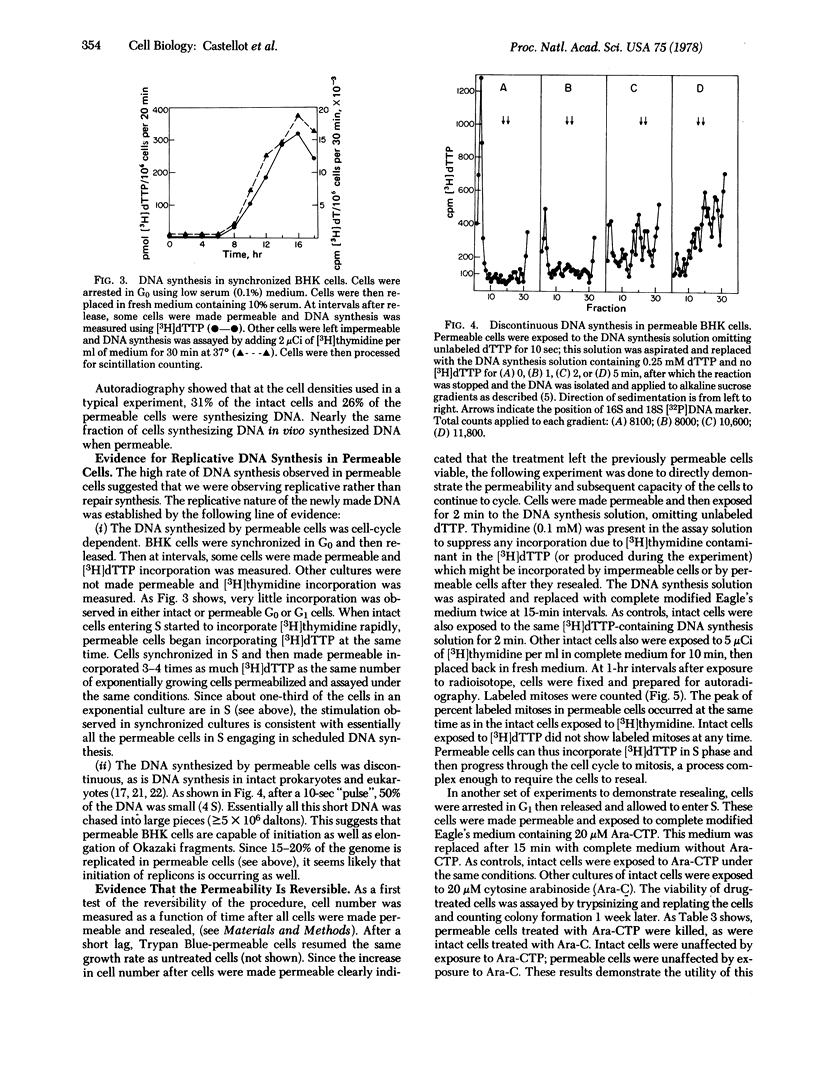
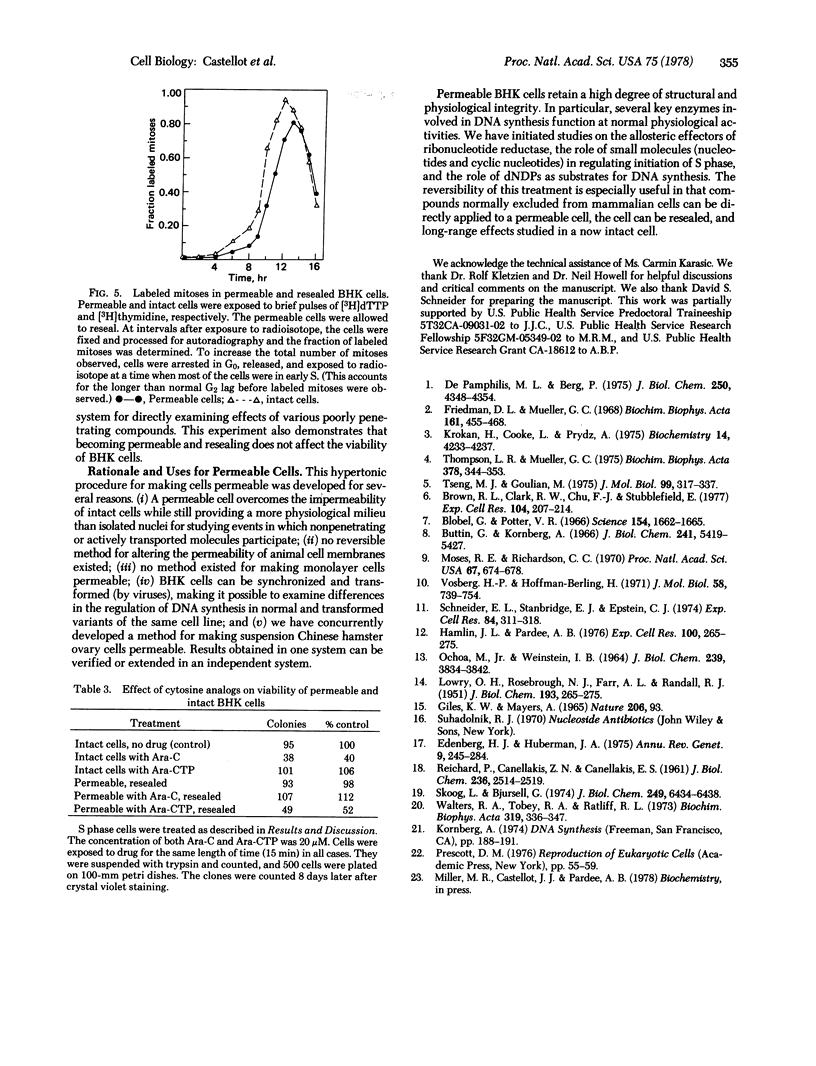
Abstract
A cell preparation, useful for studying the regulation of metabolism, was developed by making monolayer baby hamster kidney cells permeable. Hypertonically treated cells were permeable to nucleotides, but retained their gross cellular morphology, intact organelles, 100% of their DNA, and 91% of their total protein. The permeable cell synthesized DNA, RNA, and protein rapidly when supplied with the appropriate substrates and cofactors. They either could remain permeable or were able to “reseal” when replaced in complete medium under appropriate conditions. Optimal conditions for DNA synthesis were established for permeable cells, giving rates equal to those of intact cells. Replication rather than repair was shown by the cell-cycle dependence of DNA synthesis and its discontinuous nature. Ribonucleotide reductase was active in permeable cells, permitting equal rates of DNA synthesis when ribonucleotide diphosphates or deoxyribonucleotide triphosphates were provided. Hydroxyurea did not inhibit DNA synthesis in permeable cells supplied with deoxyribonucleotide di- or triphosphates, but drastically inhibited DNA synthesis when ribonucleotide diphosphates were supplied. Hydroxyurea is therefore primarily an inhibitor of ribonucleotide reductase.
Permeability was reversed, exposing permeable cells to [3H]thymidine triphosphate, which was incorporated, which labeled nuclei of cells that went on to mitosis. The reversible permeability procedure should prove especially useful in studying the functions of poorly penetrating compounds, such as drugs. Intact cells were unaffected by cytosine arabinoside triphosphate, while cells that had been made permeable and resealed were killed.
Keywords: DNA synthesis, ribonucleotide reductase, hydroxyurea, cytosine arabinosides, baby hamster kidney cells
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Brown R. L., Clark R. W., Chiu J. F., Stubblefield E. Protease activation of G1 nuclei isolated from Chinese hamster fibroblasts. Exp Cell Res. 1977 Jan;104(1):207–213. doi: 10.1016/0014-4827(77)90083-0. [DOI] [PubMed] [Google Scholar]
- Buttin G., Kornberg A. Enzymatic synthesis of deoxyribonucleic acid. XXI. Utilization of deoxyribonucleoside triphosphates by Escherichia coli cells. J Biol Chem. 1966 Nov 25;241(22):5419–5427. [PubMed] [Google Scholar]
- DePamphilis M. L., Berg P. Requirement of a Cytoplasmic Fraction for Synthesis of SV40 Deoxyribonucleic Acid in Isolated Nuclei*. J Biol Chem. 1975 Jun 10;250(11):4348–4354. [PubMed] [Google Scholar]
- Edenberg H. J., Huberman J. A. Eukaryotic chromosome replication. Annu Rev Genet. 1975;9:245–284. doi: 10.1146/annurev.ge.09.120175.001333. [DOI] [PubMed] [Google Scholar]
- Friedman D. L., Mueller G. C. A nuclear system for DNA replication from synchronized HeLa cells. Biochim Biophys Acta. 1968 Jul 23;161(2):455–468. doi: 10.1016/0005-2787(68)90122-6. [DOI] [PubMed] [Google Scholar]
- Hamlin J. L., Pardee A. B. S phase synchrony in monolayer CHO cultures. Exp Cell Res. 1976 Jul;100(2):265–275. doi: 10.1016/0014-4827(76)90147-6. [DOI] [PubMed] [Google Scholar]
- Krokan H., Cooke L., Prydz H. DNA synthesis in isolated HeLa cell nuclei. Evidence for in vitro initiation of synthesis of small pieces of DNA and their subsequent ligation. Biochemistry. 1975 Sep 23;14(19):4233–4237. doi: 10.1021/bi00690a013. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MASTER R. W. POSSIBLE SYNTHESIS OF POLYRIBONUCLEOTIDES OF KNOWN BASE-TRIPLET SEQUENCES. Nature. 1965 Apr 3;206:93–93. doi: 10.1038/206093b0. [DOI] [PubMed] [Google Scholar]
- Moses R. E., Richardson C. C. Replication and repair of DNA in cells of Escherichia coli treated with toluene. Proc Natl Acad Sci U S A. 1970 Oct;67(2):674–681. doi: 10.1073/pnas.67.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OCHOA M., Jr, WEINSTEIN I. B. POLYPEPTIDE SYNTHESIS IN A SUBCELLULAR SYSTEM DERIVED FROM THE L-1210 MOUSE ASCITES LEUKEMIA. J Biol Chem. 1964 Nov;239:3834–3842. [PubMed] [Google Scholar]
- REICHARD P., CANELLAKIS Z. N., CANELLAKIS E. S. Studies on a possible regulatory mechanism for the biosynthesis of deoxyribonucleic acid. J Biol Chem. 1961 Sep;236:2514–2519. [PubMed] [Google Scholar]
- Schneider E. L., Stanbridge E. J., Epstein C. J. Incorporation of 3H-uridine and 3H-uracil into RNA: a simple technique for the detection of mycoplasma contamination of cultured cells. Exp Cell Res. 1974 Mar 15;84(1):311–318. doi: 10.1016/0014-4827(74)90411-x. [DOI] [PubMed] [Google Scholar]
- Skoog L., Bjursell G. Nuclear and cytoplasmic pools of deoxyribonucleoside triphosphates in Chinese hamster ovary cells. J Biol Chem. 1974 Oct 25;249(20):6434–6438. [PubMed] [Google Scholar]
- Thompson L. R., Mueller G. C. DNA replication in nuclei isolated from bovine lymphocytes. Biochim Biophys Acta. 1975 Feb 10;378(3):344–353. doi: 10.1016/0005-2787(75)90179-3. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. DNA synthesis in human lymphocyts: intermediates in DNA synthesis, in vitro and in vivo. J Mol Biol. 1975 Dec 5;99(2):317–337. doi: 10.1016/s0022-2836(75)80149-5. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]
- Walters R. A., Tobey R. A., Ratliff R. L. Cell-cycle-dependent variations of deoxyribonucleoside triphosphate pools in Chinese hamster cells. Biochim Biophys Acta. 1973 Sep 7;319(3):336–347. doi: 10.1016/0005-2787(73)90173-1. [DOI] [PubMed] [Google Scholar]


