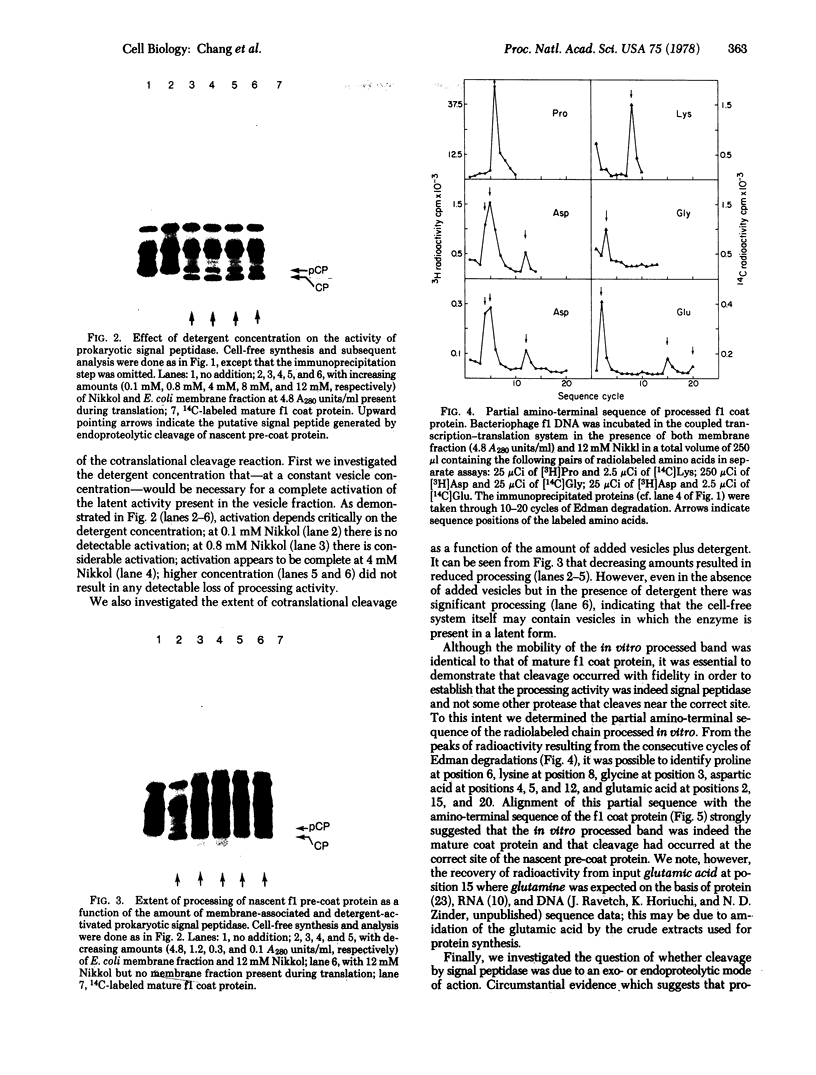
Abstract
An inverted membrane vesicle fraction isolated from uninfected Escherichia coli and largely derived from the inner membrane has been shown to contain an endoproteolytic activity that cleaves nascent bacteriophage f1 pre-coat protein into two identifiable products. The electrophoretic mobility on sodium dodecyl sulfate/urea/polyacrylamide gels and the partial amino-terminal sequence of the larger fragment were indistinguishable from those of the mature phage coat protein. Partial amino-terminal sequence analysis showed that the smaller fragment corresponds to the amino-terminal "signal peptide" of f1 pre-coat protein. Cleavage occurred only if the membrane fraction was present during in vitro synthesis, and was not observed if it was added after completion of pre-coat protein synthesis. The cleavage reaction was strongly stimulated when the membrane fraction was present together with the nonionic detergent Nikkol. These results are consistent with and discussed in terms of the signal hyothesis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey G. S., Gillett D., Hill D. F., Petersen G. B. Automated sequencing of insoluble peptides using detergent. Bacteriophage fl coat protein. J Biol Chem. 1977 Apr 10;252(7):2218–2225. [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. II. Reconstitution of functional rough microsomes from heterologous components. J Cell Biol. 1975 Dec;67(3):852–862. doi: 10.1083/jcb.67.3.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammack K. A., Wade H. E. The sedimentation behaviour of ribonuclease-active and -inactive ribosomes from bacteria. Biochem J. 1965 Sep;96(3):671–680. doi: 10.1042/bj0960671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. N-formyl-methionyl-sigma-ribonucleic acid and chain initiation in protein biosynthesis. Polypeptide synthesis directed by a bacteriophage ribonucleic acid in a cell-free system. Nature. 1966 Jul 23;211(5047):378–380. doi: 10.1038/211378a0. [DOI] [PubMed] [Google Scholar]
- Futai M. Orientation of membrane vesicles from Escherichia coli prepared by different procedures. J Membr Biol. 1974;15(1):15–28. doi: 10.1007/BF01870079. [DOI] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Genetic evidence on the nature of the repressor for alkaline phosphatase in E. coli. J Mol Biol. 1963 May;6:433–438. doi: 10.1016/s0022-2836(63)80054-6. [DOI] [PubMed] [Google Scholar]
- Inouye H., Beckwith J. Synthesis and processing of an Escherichia coli alkaline phosphatase precursor in vitro. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1440–1444. doi: 10.1073/pnas.74.4.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. C., Blobel G. Post-translational cleavage of presecretory proteins with an extract of rough microsomes from dog pancreas containing signal peptidase activity. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5598–5602. doi: 10.1073/pnas.74.12.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- Konings R. N., Hulsebos T., Van den Hondel C. A. Identification and characterization of the in vitro synthesized gene products of bacteriophage M13. J Virol. 1975 Mar;15(3):570–584. doi: 10.1128/jvi.15.3.570-584.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Devillers-Thiery A., Blobel G. Nascent prehormones are intermediates in the biosynthesis of authentic bovine pituitary growth hormone and prolactin. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2432–2436. doi: 10.1073/pnas.74.6.2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcker K. The formation of N-formyl-methionyl-sRNA. J Mol Biol. 1965 Nov;14(1):63–70. doi: 10.1016/s0022-2836(65)80230-3. [DOI] [PubMed] [Google Scholar]
- Model P., Zinder N. D. In vitro synthesis of bacteriophage f1 proteins. J Mol Biol. 1974 Feb 25;83(2):231–251. doi: 10.1016/0022-2836(74)90389-1. [DOI] [PubMed] [Google Scholar]
- Schwartz F. M., Zinder N. D. Morphological changes in Escherichia coli infected with the DNA bacteriophage fl. Virology. 1968 Feb;34(2):352–355. doi: 10.1016/0042-6822(68)90246-8. [DOI] [PubMed] [Google Scholar]
- Sekizawa J., Inouye S., Halegoua S., Inouye M. Precursors of major outer membrane proteins of Escherichia coli. Biochem Biophys Res Commun. 1977 Aug 8;77(3):1126–1133. doi: 10.1016/s0006-291x(77)80095-8. [DOI] [PubMed] [Google Scholar]
- Shields D., Blobel G. Cell-free synthesis of fish preproinsulin, and processing by heterologous mammalian microsomal membranes. Proc Natl Acad Sci U S A. 1977 May;74(5):2059–2063. doi: 10.1073/pnas.74.5.2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smilowitz H., Carson J., Robbins P. W. Association of newly synthesized major f1 coat protein with infected host cell inner membrane. J Supramol Struct. 1972;1(1):8–18. doi: 10.1002/jss.400010103. [DOI] [PubMed] [Google Scholar]
- Smith W. P., Tai P. C., Thompson R. C., Davis B. D. Extracellular labeling of nascent polypeptides traversing the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2830–2834. doi: 10.1073/pnas.74.7.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss A. W., Bennett C. D., Donohue A. M., Rodkey J. A., Alberts A. W. Rat liver pre-proalbumin: complete amino acid sequence of the pre-piece. Analysis of the direct translation product of albumin messenger RNA. J Biol Chem. 1977 Oct 10;252(19):6846–6855. [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. IV. The sequence of messenger RNA for the major coat protein gene. J Mol Biol. 1977 Apr 25;111(4):487–507. doi: 10.1016/s0022-2836(77)80065-x. [DOI] [PubMed] [Google Scholar]
- Wickner W. Asymmetric orientation of phage M13 coat protein in Escherichia coli cytoplasmic membranes and in synthetic lipid vesicles. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1159–1163. doi: 10.1073/pnas.73.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZINDER N. D., VALENTINE R. C., ROGER M., STOECKENIUS W. F1, A ROD-SHAPED MALE-SPECIFIC BACTERIOPHAGE THAT CONTAINS DNA. Virology. 1963 Aug;20:638–640. doi: 10.1016/0042-6822(63)90290-3. [DOI] [PubMed] [Google Scholar]