Abstract
Population-based, longitudinal studies spanning decades linking risk factors in childhood, adolescence and early adulthood to incident clinical interstitial lung disease (ILD) events in late adulthood have not been performed. In addition, no observational or randomized clinical trials have been conducted; therefore, there is presently no evidence to support the notion that reduction of risk factor levels in early life prevents ILD events in adult life. Primary prevention strategies are host-directed interventions designed to modify adverse risk factors (i.e., smoking) with the goal of preventing the development of ILD, whereas primordial prevention for ILD can be defined as the elimination of external risk factors (i.e., environmental pollutants). As no ILD primary prevention studies have been previously conducted, we propose that research studies that promote implementation of primary prevention strategies could, over time, make a subset of ILD preventable. Herein, we provide a number of initial steps required for the future implementation of prevention strategies; this statement discusses the rationale and available evidence that support potential opportunities for primordial and primary prevention, as well as fertile areas for future research of preventive intervention in ILD.
Keywords: pulmonary fibrosis, prevention
The State of the Science in Primary Prevention of Interstitial Lung Disease
Primary prevention requires the identification of risk factors for the disease of interest followed by public health interventions targeting these risk factors. To date, the vast majority of interstitial lung disease (ILD) studies has employed cross-sectional or case–control designs, and have restricted the clinical phenotype or case status to symptomatic disease. These studies have led to the identification of a number of risk factors, including age, cigarette smoking, autoimmunity, inhaled occupational and environmental exposures, drug toxicity, radiation exposure, family history, and genetic polymorphisms. Despite extensive literature examining ILD risk factors, many ILD cases occur in the absence of one or more clearly delineated risk factors.
Much of the work done to date in this field is reminiscent of work done in the first half of the 20th century to examine risk factors for cardiovascular disease, which, at that time, was considered to be symptomatic angina, acute myocardial infarction, or sudden cardiac death. The Framingham Heart Study (FHS) firmly established hypertension, diabetes mellitus, and hyperlipidemia as risk factors for cardiovascular disease by studying adults without symptomatic disease in a cohort study design, an approach that seemed unusual at the time. ILD investigators can learn two important lessons from the FHS and subsequent population-based cardiovascular cohort studies, such as the Multi-Ethnic Study of Atherosclerosis. First, the study of nondiseased individuals is essential to identify risk factors for disease. Second, the study of subclinical disease cannot only help identify risk factors, but also permits the study of early biological processes that contribute to disease development long before “end-stage” disease (myocardial infarction or symptomatic pulmonary fibrosis) develops. On the other hand, the rarity of these disorders makes an FHS-like study difficult to conceptualize practically. As such, more targeted approaches in groups at higher risk seem more reasonable, and will be described in subsequent sections.
A broader definition of ILD is required to enable the development of primary prevention strategies. Most broadly defined, subclinical ILD encompasses groups of individuals that have specific radiologic, physiologic, molecular, and, in some cases, histopathologic abnormalities, but are either asymptomatic or have symptoms that have not been attributed to ILD (1). More generally, we refer to subclinical ILD in undiagnosed individuals who have interstitial lung abnormalities (ILAs) defined by high-resolution computed tomography (HRCT), that are consistent with, but subtler than, those observed in patients with established clinical ILD (2). It is important to recognize that ILD occurs in children as well as adults, and that ILD has been associated with considerable morbidity and mortality in the pediatric age group (3, 4). As there is no uniform definition of ILD that encompasses the spectrum of symptomatic and subclinical disease, we propose the following working definition of ILD: Interstitial lung disease is the presence of cellular proliferation, cellular infiltration, and/or fibrosis of the lung parenchyma not due to infection or neoplasia. This definition does not require the presence of symptoms, adventitious breath sounds, or abnormal pulmonary function testing. It is important to note that this definition includes “subclinical” pathological processes of uncertain clinical significance, such as ILAs. The inclusion of asymptomatic pathological changes in the definition of a “disease” is consistent with the principles of primary prevention, but presents certain challenges in the practice of clinical medicine. Health care practitioners should be careful to distinguish asymptomatic “disease” from symptomatic “illness” when clinically silent ILD is detected. The clinical and prognostic significance of subclinical ILD is unknown at this time.
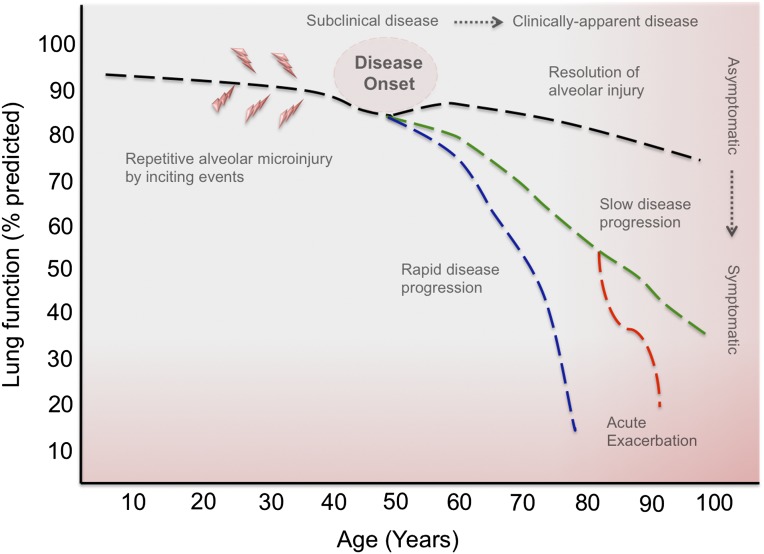
A number of investigators have begun examining cohorts at risk of developing ILD (1). These proof-of-concept studies, in mostly undiagnosed, asymptomatic individuals, have focused on defining changes that precede the development of clinically significant ILD, and have improved our understanding of the natural history of pulmonary fibrosis (Figure 1). HRCT scanning is the radiographic standard in the evaluation of ILD. The widespread use of HRCT in clinical and research settings has increased the detection of ILAs in asymptomatic individuals. In these studies, HRCT abnormalities are defined as nondependent visual changes affecting greater than 5% of the lung parenchyma. The most common radiographic changes include ground-glass, reticular abnormalities, diffuse centrilobular nodularity, honeycombing, traction bronchiectasis, and nonemphysematous cysts (2). Alternative approaches quantify area of increased CT lung attenuation (high-attenuation areas [HAAs]) or use novel techniques to quantify structural abnormalities (5, 6). The significance of these radiographic subtypes is unknown, but they may represent early stages of distinct idiopathic interstitial pneumonias (IIPs) or ILD associated with connective tissue disease (CTD), with differing rates of progression and/or prognosis. Interestingly, these radiographic findings have been associated with spirometric restriction (7, 8) and reductions in 6-minute walk distance (9), suggesting physiological correlates of abnormal lung parenchyma. These recent findings suggest that visual or automated scoring of radiographic abnormalities could be used to screen at-risk populations and facilitate serial measurements of disease progression. However, before we implement these methods, the natural history of ILAs or HAAs and several technical limitations need to be addressed.
Figure 1.
The natural history of patients affected by idiopathic interstitial pneumonia has been well characterized. Clinical progression is heterogeneous, with a rate of decline in lung function that can be slowly or rapidly progressive, with a subset of patients that will develop periods of rapid decline or acute disease exacerbation. What remain poorly defined are the inciting events, disease onset, and progression from asymptomatic to symptomatic interstitial lung disease (ILD). The widespread use of high-resolution computed tomography in clinical and research settings has increased the detection of subclinical ILD in family members of affected individuals with familial pulmonary fibrosis, smokers and subjects with connective tissue disease. A better understanding of the phenotypic and molecular characteristics of subclinical ILD will increase our ability to identify at-risk populations and implement preventive strategies.
Risk Factors Related to the Development of ILD
A significant number of patients develop diffuse bilateral interstitial pneumonia without a clear precipitating cause. Idiopathic pulmonary fibrosis (IPF) is the most common IIP. It carries a poor prognosis, and has so far proven refractory to drug treatment. Aging is clearly an important risk factor (10), as are male sex, gastroesophageal reflux disease (11), and diabetes mellitus (12). A number of environmental risk factors for IPF have been investigated, including occupational exposure to silica, wood dust, metal dust, textile dust, livestock, and agricultural activities (13, 14). A large percentage of subjects with familial pulmonary fibrosis are current or former smokers, suggesting that gene–environment interactions play a key role in the pathogenesis of lung fibrosis (15, 16). Studies of risk factors for development of ILD in children are currently lacking (3).
Aging
Fibrotic ILDs, including IPF, are more prevalent in aging populations, with a sharp increase in incidence for those older than 50 years (17–19). With the exception of familial interstitial pneumonia (FIP), populations affected with subclinical ILD are significantly older than control subjects, suggesting that pulmonary fibrosis is a disease of aging. Aging is associated with reduced mitochondrial energy metabolism, enhanced mitochondrial oxidative stress, increased production of mitochondrial reactive oxygen species, and accumulated mitochondrial DNA mutations (20–23). Premature aging has been invoked as a possible contributor to IPF (24–31). Telomere biology has recently been implicated in the pathogenesis of a variety of lung diseases (32). Telomeres are DNA–protein structures that cap the ends of chromosomes; telomerase is the enzyme that ensures their integrity. Subsets of patients with IPF have telomerase mutations; these mutations could be involved in accelerated cellular aging associated with short telomeres, and contribute to the pathogenesis of pulmonary fibrosis (33). In addition, evidence suggests that telomeres may be more prone to DNA damage (34), which could link smoke-induced and or dysregulated antioxidant defense mechanism with telomere-specific DNA damage, and contribute to the development of chronic lung diseases (35).
Sex
The incidence and prevalence of ILD is higher in males than in females, and female sex is associated with improved survival (10). In addition, Han and colleagues (36) demonstrated that IPF in males is associated with a more rapid clinical progression and reduced survival. Similar statistics have been reported for populations at risk of developing ILD. Murine models suggest that hormones could play a role in exacerbating experimental fibrosis; however, results are inconsistent across species (37, 38). Clearly, additional studies are required to better understand the role of sex in the development and progression of ILD.
Tobacco Smoking and Other Environmental Exposures
In the general population, a history of chronic tobacco smoke exposure is a risk factor for the development of several IIPs, including IPF (39). Multiple past and present studies have demonstrated that ILAs observed in the chest X-rays or chest CT scans of smokers represent a heterogeneous group of parenchymal lung diseases. These observations led to the hypothesis that ILAs are present in cohorts of ever-smokers screened for the development of chronic obstructive pulmonary disease (2), cardiovascular disease (8), or lung cancer (40, 41). These recent studies demonstrate that ILAs are present in a significant proportion of smokers (42), and are associated with reduction in 6-minute-walk distance (9). Although recent evidence suggests that these radiographic changes may be progressive (41, 43), long-term follow up in the research setting is required to determine the significance of ILAs in smokers.
Risk Factors in CTD-associated ILD
In systemic sclerosis (SSc), early disease duration, and scleroderma-specific autoantibodies are associated with progressive ILD. Presence of nucleolar pattern on anti-nuclear antibody (representing anti-Th/To, anti-U3-ribonucleoprotein, and anti-Pm-Scl) and anti-topoisomerase antibodies are associated with worse SSc-ILD (44); 16–27% develop severe ILD. Conversely, anti-centromere antibodies appear to have a protective effect on developing severe ILD (44, 45). Other predictors include African American race (46) and severe gastroesophageal reflux disease (47). These features provide clues to pathogenesis, and offer insights to identifying higher risk patients for ILD prevention research. Given the high prevalence of ILD in rheumatoid arthritis (RA) and its relationship to increased morbidity and mortality (48), there has been a recent interest in defining risk factors, including phenotypic features, biomarkers, and genetic markers that can help define which patients with RA are at highest risk for ILD, and thus serve as a focus for primary prevention. Recent data suggest that age (49), male sex (50, 51), smoking (52), high rheumatoid factor, cyclic citrullinated peptide antibodies, and genetic factors, such as major histocompatibility complex, class II, DR beta 1 status (53), confer higher risk of ILD in RA, and point the way toward a risk profile that may be clinically applicable in primary prevention strategies.
Mechanisms of Disease
The molecular mechanisms that drive the development of pulmonary fibrosis have not been fully elucidated; however, major pathogenic determinants that contribute to the development of IPF include alveolar epithelial cell injury and pneumocyte apoptosis. Epithelial cellular injury is likely followed by dysregulated repair characterized by fibroblast/myofibroblast activation and extracellular matrix deposition, which ultimately results in lung remodeling and progressive loss of lung function (54). It has been suggested that aberrant activation of developmental pathways that are usually suppressed in adult tissues may be central to the pathogenesis of IPF (55). Additional molecular mechanisms that contribute to aberrant wound healing include dysregulated alveolar coagulation, cell stress responses, and alveolar epithelial cell senescence (56–58). In SSc-ILD, microvascular injury and damage to endothelial cells appear to be the initiating factors. Endothelial damage leads to the production of thrombin, endothelin-1, vascular endothelial growth factor, and adhesion molecules, which, in turn, may promote inflammation (59, 60). Recent animal data also suggest that injury to epithelial cells may also play an important role in the pathogenesis of SSc-ILD (61). A number of genetic disorders provide insight into molecular mechanisms that contribute to the development of IPF. Certain mutations in surfactant (SFTPA, SFTPB, SFTPC) (62–64) and ABC transporter (ABCA3) (64) proteins are associated with alveolar epithelial type ll endoplasmic reticulum stress and apoptosis, resulting in interstitial pneumonia and respiratory failure in children and young adults. In contrast, mutations in telomerase genes (TERT/hTR) are more frequently observed in adults with FIP (27, 65). More recently, a common polymorphism in the airway mucin gene, MUC5B, was associated with development of both familial and sporadic pulmonary fibrosis (66). Although it remains unclear how the MUC5B promoter polymorphism could contribute to disease pathogenesis, a recent study demonstrates an association between this polymorphism and ILAs in the general population (7).
Windows of Opportunity for Lung Primary Prevention Research
Although there are numerous different subtypes of ILD that occur in different clinical contexts, several have been selected for particular focus based on information suggesting promising windows of opportunity for primary prevention research (Table 1).
Table 1.
Imaging, physiological, and pathological abnormalities within context of genetics and potential biomarkers
| HRCT Imaging Abnormalities | Lung Function Abnormalities | Pathologic Changes | Genetics | Biomarkers | |
|---|---|---|---|---|---|
| Familial pulmonary fibrosis | Subpleural reticulation, GGO’s | TLC, DLCO, CPET | UIP, NSIP, COP, RBILD | TERT/hTR, SPTC, ABCA3 | MMP7 |
| Smokers | Subpleural reticulation, GGO’s, centrilobular nodules | TLC, DLCO, 6MWD | SRIF | MUC1, SPA, SPD | |
| Connective tissue disease | Subpleural reticulation, GGO’s | FVC, DLCO | NSIP | TGF-B1, PDGF | |
| General population | Subpleural reticulation, GGO’s | TLC, DLCO 6MWD | MUC5B |
Definition of abbreviations: 6MWD = 6-minute-walk distance; ABCA = ATP-binding cassette transporter; COP = cryptogenic organizing pneumonia; CPET = cardiopulmonary exercise testing; GGO = ground glass opacities; MMP = matrix metalloproteinase; MUC = mucin gene; NSIP = nonspecific interstitial pneumonia; PDGF = platelet-derived growth factor; RBILD = respiratory bronchiolitis interstitial lung disease; SPTC = surfactant protein C; SRIF = smoking-related interstitial fibrosis; TGF = transforming growth factor; TLC = total lung capacity; UIP = usual interstitial pneumonia.
Subjects with subclinical interstitial lung disease demonstrate histopathologic changes, physiologic and functional abnormalities, and genetic/genomic profiles similar to those observed in patients with clinically significant interstitial lung disease; these similarities indicate that subclinical interstitial lung disease may precede the development of clinically apparent interstitial lung disease.
Numerous studies suggest that characterization of subclinical ILD in at-risk subjects is feasible. These findings suggest that familial ILD may provide a promising opportunity for prevention efforts. A study of 111 families with interstitial pneumonia documented a high risk for ILD among asymptomatic family members, and estimated that approximately 8% of self-reported unaffected family members have subclinical ILD (15). In a second study, 143 asymptomatic subjects from 18 kindreds affected with FIP were studied to determine clinical, radiographic, and pathologic features of subclinical ILD in patients at risk of developing IPF (16). Using HRCT, subclinical ILD was identified in 22% of unaffected relatives; these subjects were, on average, 20 years younger than their relatives with established disease. A history of smoking was significantly more common in both subjects with ILAs and relatives with established disease when compared with unaffected relatives. Several recent studies have identified subclinical ILD in subjects with known genetic mutations (27, 67, 68). For instance, radiographic evidence of subclinical ILD has been described in asymptomatic subjects with heterozygous TERT mutations (67). Additional rare genetic disorders, such as SFTPC mutations and Hermansky-Pudlak syndrome, which are associated with the development of ILD, also provide windows of opportunity to intervene early in at-risk patients (69).
Several autoimmune diseases, such as RA, SSc, polymyositis/dermatomyositis, lupus, and Sjogren’s syndrome, have a high prevalence of ILD, and thus also provide windows of opportunity for lung primary prevention research in at-risk populations. Pulmonary complications are an important cause of morbidity and mortality in patients with SSc (70–73). Approximately 70–80% of adult patients with SSc have evidence of ILD (74), and ILD occurs in both limited and diffuse cutaneous SSc (75, 76). Although the majority of these patients remain relatively stable with respect to their SSc-ILD, 40% develop moderate-to-severe restrictive disease (FVC ≤ 50% of predicted). When decline in lung function occurs in early disease stages (45, 77), it is associated with a higher mortality (45). Chest CT scans have been successfully used to identify occult ILD in patients with SSc. In a study of patients with SSc with normal lung volumes, 56% of subjects had HRCT abnormalities suggesting ILD (71). These data, coupled with knowledge of risk factors and disease biology (discussed subsequently here), provide strong rationale for studies to attempt to prevent development of ILD in individuals with SSc.
RA is another CTD associated with a significant ILD burden. An estimated 10% of patients with RA have RA-associated ILD (RA-ILD), and one-third have subclinical ILD on chest CT (78–80). These findings are clinically relevant, as, in a prospective study, 34% of patients with RA with subclinical ILD had evidence of disease progression (81). Furthermore, the presence of interstitial pneumonia, particularly biopsy-proven usual interstitial pneumonia, is associated with a poor prognosis, which may approach that seen in IPF (48, 82, 83). The high prevalence of ILD, availability of relatively effective immunosuppressive therapy, and increased mortality associated with cardiopulmonary involvement (84) suggest that detection of subclinical ILD and early intervention, or strategies aimed at primary prevention of ILD, could improve outcomes in patients with RA.
Recommendations for Primary Prevention Research
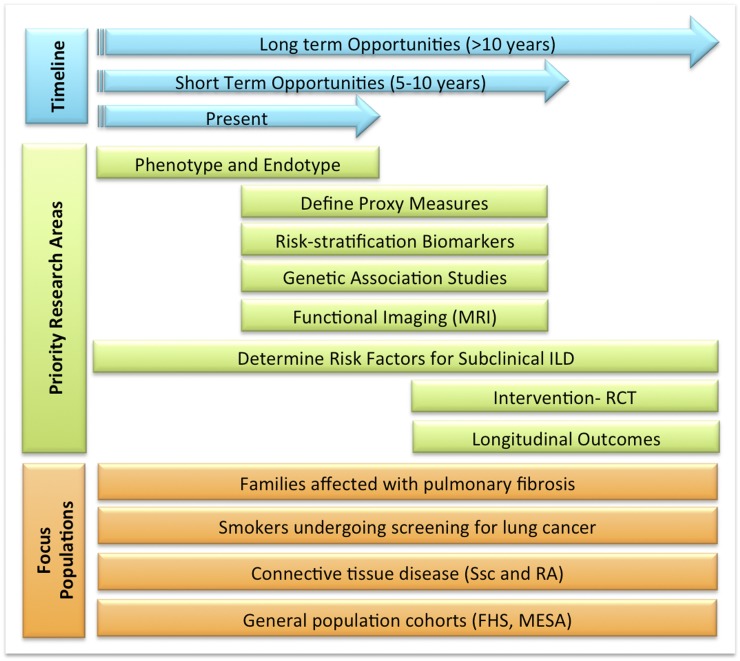
It is evident that prevention strategies in ILD remain at an early stage of development but with clear opportunities for a rapid expansion. The previously described studies in IIPs and CTD-associated ILD suggest short-term (next 5 yr) and long-term opportunities (next 10 yr) are available to develop appropriate primary prevention strategies (Figure 2). A number of research topics need to be addressed before conducting primary prevention trials, and these priority research areas include:
-
1.
Improve strategies for early detection of disease (phenotype and endotype).
-
2.
Define proxy measures and risk-stratification biomarkers (genomics and imaging).
-
3.
Determine risk factors for the development of subclinical ILD.
-
4.
Determine the natural history of different forms of ILD (longitudinal outcomes), so as to enable interpretation of potential outcome.
-
5.
Design safe interventions that can be tested in individuals affected with subclinical ILD (i.e., smoking cessation).
Figure 2.
Priority research areas proposed for at-risk populations. Ongoing studies have focused on defining the phenotype and endotype of populations affected with subclinical interstitial lung disease (ILD). In the short term, observational studies will enhance our ability to identify proxy measures and risk factors, which will be required to design therapeutic interventions and, in the long term, determine the longitudinal outcomes of subjects affected with subclinical ILD. FHS = Framingham Heart Study; MESA = Multi-Ethnic Study of Atherosclerosis; MRI = magnetic resonance imaging; RA = rheumatoid arthritis; RCT = randomized controlled trial; Ssc = systemic sclerosis.
The design of robust predictive models that identify those individuals at highest risk for the development of clinically relevant disease will provide opportunities for preventive strategies. To achieve this goal, we need to develop sensitive, high-throughput methods to screen large populations at risk for future development of pulmonary fibrosis. Advances in this priority area are highlighted by recent reports of ILAs in large cohorts of smokers and in population-based samples of older adults who were screened using CT scans. Visual inspection of ILAs is based on conventional imaging interpretation, and is therefore a robust and reliable method to qualify ILAs; however, there is a clear need to develop methods that objectively measure HRCT scan features. Several groups have recently shown that automated assessment of HRCT scans can detect and quantify ILAs in FIP, patients with RA-ILD, and smokers (85, 86). The use of HAAs successfully identified ground-glass opacities and reticular abnormalities in the Multi-Ethnic Study of Atherosclerosis–Lung Study, a population-based cohort of U.S. adults (8). Finally, objective quantification of structural abnormalities predicts survival in established IPF (87). Overall, these results suggest that quantification of HRCT scans may be an endpoint that complements other study parameters (i.e., biomarkers) for detection of subclinical ILD. Despite this progress, it is clear that development of advanced functional imaging techniques (functional magnetic resonance imaging) that use molecular markers and reduce radiation exposure are required to effectively and safely screen at-risk populations. Defining the impact of ILAs on the natural history of imaging, as well as physiological and clinical features, will be important to determine what subjects will go on to develop ILD and could potentially benefit from interventions, such as removal from an environmental exposure or smoking cessation.
We anticipate that genomic biomarkers (peripheral blood and skin tissue in SSc-ILD [88]) will help identify individuals at risk of developing subclinical ILD, improve our ability to monitor disease progression, and serve as clinical outcome measures in intervention trials. Lung-based protein biomarkers can be detected in the peripheral blood, but, although some biomarkers have been shown to correlate with disease progression and survival, the use of biomarkers in IPF remains experimental (89–91). Several peripheral blood biomarkers have been independently validated in the research setting, and should be investigated as potential risk-stratification tools (92–94). Although these data are promising, additional investigation, particularly into the development of a panel of biomarkers that have additive value to established clinical parameters, will be necessary to establish which biomarkers will be useful to detect subclinical disease or risk stratify populations at risk of developing ILD.
Short-Term Opportunities
There are potential target populations already at risk for ILD who have undergone extensive baseline and longitudinal evaluations.
FIP.
Although differing disease mechanisms are potentially involved in the development of sporadic interstitial pneumonia and FIP, detection of subclinical ILD in genetically susceptible individuals is both feasible and informative (16). We anticipate that future research in this population at risk of developing pulmonary fibrosis will increase our understanding of the natural history of the IIPs. A significant challenge to implementing preventive measures is the absence of proven effective therapies in IIPs, which limits the current ability to design and conduct randomized clinical trials. Furthermore, the lack of predictive markers (genetic, genomic) and longitudinal studies limit our ability to risk stratify at-risk individuals, which is required to effectively design primary prevention trials. However, given the anticipated results of a series of large, well conducted, phase III studies over the next year (95, 96), these agents may provide viable therapeutic strategies for primary prevention studies in well defined at-risk populations. In addition, future availability of genetic testing (i.e., telomerase mutations, MUC5B polymorphism) will enhance the predictive value of ILAs in FIP in other at-risk populations. Due to the complexities associated with performing genetic testing, providing genetic counseling, and proposing enrollment in experimental interventions, these studies should occur in the context of clinical trials. Additional consideration should be given to performing such research studies in the pediatric age group.
Patients with CTD.
The National Heart, Lung, and Blood Institute–funded Scleroderma Lung Study I and II have generated significant knowledge regarding risk factors for the development of SSc-ILD, and developed a successful national network for clinical trials. We know that the majority of patients develop ILD within the first 3 years of SSc disease onset (97). Recommendations for a primary prevention trial may include all patients with very early diagnosis of SSc (preferably within a few months to <2–3 yr from first non-Raynaud sign or symptom) without the presence of ILD on HRCT. In this proposed trial, the randomization would be stratified by autoantibodies (nucleolar pattern and anti-topoisomerase), and subjects would be randomized to immunosuppressive therapies (e.g., mycophenolate mofietil, methotrexate, or cyclophosphamide [98]) versus placebo, and followed longitudinally with physiological testing and semiquantitative or quantitative HRCT of lungs. Primary outcome would be the time to develop ILD during the follow-up period. Patients who develop new ILD and/or have worsening of skin disease would be initiated on standard-of-care immunosuppressive therapies, and laboratory data included to assess safety of the therapy. In addition, the longitudinal cohort of very early SSc in Europe will provide further insight into natural history of SSc-ILD (97). Similarly, there are staging systems for RA-ILD based on imaging, lung physiology and disease progression. The addition of biomarkers to this phenotypic information, as has been successfully completed in SSc-associated ILD, could aid in refining predictive models to define which patients with RA are at highest risk for ILD. The current gaps in knowledge regarding early diagnosis and identification of subclinical disease, risk stratification, development of prediction models, and management of CTDs could be potentially addressed over the next 5 years. In fact, efforts to formulate and validate risk-stratification models using phenotype, imaging, biomarkers, and genetic markers are well underway, and will serve in the near future as a basis for a primary prevention trial using known agents, such as mycophenolate or emerging biologic agents. We expect that these advances will lead to improvements in the lives of patients with these debilitating conditions.
Long-Term Opportunities
General population.
In the long term (next 10 yr), we will need to design prospective cohort studies to determine risk factors for ILD in the general population. The ideal scenario would be the creation of a Framingham-like prospective, observational cohort study. This approach would carefully study a representative cohort with extensive baseline and standardized longitudinal assessments. The main limitation of such a research strategy is the expense and complexity required for such an approach.
An alternative approach is to target high-risk populations, such as older adults with a history of cigarette smoking. An estimated 94 million U.S. adults who are current or former smokers have an increased risk of developing lung cancer or parenchymal lung diseases (i.e., ILD and chronic obstructive pulmonary disease) (99). The U.S. Preventive Services Task Force issued a draft recommendation for annual lung cancer screening using low-dose CT in individuals considered at high risk based on age and smoking history (100). The widespread use of HRCT in clinical and research settings will increase our ability to detect subclinical ILD. Although the long-term benefits, risks, and costs associated with implementing chest CT screening remain to be determined, this represents an opportunity to determine the long-term significance of ILAs in smokers and identify risk factors for the development of ILD.
Footnotes
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Doyle TJ, Hunninghake GM, Rosas IO. Subclinical interstitial lung disease: why you should care. Am J Respir Crit Care Med. 2012;185:1147–1153. doi: 10.1164/rccm.201108-1420PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Washko GR, Lynch DA, Matsuoka S, Ross JC, Umeoka S, Diaz A, Sciurba FC, Hunninghake GM, San José Estépar R, Silverman EK, et al. Identification of early interstitial lung disease in smokers from the COPDGene Study. Acad Radiol. 2010;17:48–53. doi: 10.1016/j.acra.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurland G, Deterding RR, Hagood JS, Young LR, Brody AS, Castile RG, Dell S, Fan LL, Hamvas A, Hilman BC, et al. American Thoracic Society Committee on Childhood Interstitial Lung Disease (chILD) and the chILD Research Network. An official American Thoracic Society clinical practice guideline: classification, evaluation, and management of childhood interstitial lung disease in infancy. Am J Respir Crit Care Med. 2013;188:376–394. doi: 10.1164/rccm.201305-0923ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Deutsch GH, Young LR, Deterding RR, Fan LL, Dell SD, Bean JA, Brody AS, Nogee LM, Trapnell BC, Langston C, et al. Pathology Cooperative Group; ChILD Research Co-operative. Diffuse lung disease in young children: application of a novel classification scheme. Am J Respir Crit Care Med. 2007;176:1120–1128. doi: 10.1164/rccm.200703-393OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartholmai BJ, Raghunath S, Karwoski RA, Moua T, Rajagopalan S, Maldonado F, Decker PA, Robb RA. Quantitative computed tomography imaging of interstitial lung diseases. J Thorac Imaging. 2013;28:298–307. doi: 10.1097/RTI.0b013e3182a21969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xu Y, van Beek EJ, Hwanjo Y, Guo J, McLennan G, Hoffman EA. Computer-aided classification of interstitial lung diseases via MDCT: 3D adaptive multiple feature method (3D AMFM) Acad Radiol. 2006;13:969–978. doi: 10.1016/j.acra.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Hunninghake GM, Hatabu H, Okajima Y, Gao W, Dupuis J, Latourelle JC, Nishino M, Araki T, Zazueta OE, Kurugol S, et al. MUC5B promoter polymorphism and interstitial lung abnormalities. N Engl J Med. 2013;368:2192–2200. doi: 10.1056/NEJMoa1216076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lederer DJ, Enright PL, Kawut SM, Hoffman EA, Hunninghake G, van Beek EJ, Austin JH, Jiang R, Lovasi GS, Barr RG. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)–lung study. Am J Respir Crit Care Med. 2009;180:407–414. doi: 10.1164/rccm.200812-1966OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doyle TJ, Washko GR, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Divo MJ, Celli BR, Sciurba FC, Silverman EK, et al. COPDGene Investigators. Interstitial lung abnormalities and reduced exercise capacity. Am J Respir Crit Care Med. 2012;185:756–762. doi: 10.1164/rccm.201109-1618OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810–816. doi: 10.1164/rccm.200602-163OC. [DOI] [PubMed] [Google Scholar]
- 11.Raghu G, Freudenberger TD, Yang S, Curtis JR, Spada C, Hayes J, Sillery JK, Pope CE, 2nd, Pellegrini CA. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. Eur Respir J. 2006;27:136–42. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 12.Enomoto T, Usuki J, Azuma A, Nakagawa T, Kudoh S. Diabetes mellitus may increase risk for idiopathic pulmonary fibrosis. Chest. 2003;123:2007–2011. doi: 10.1378/chest.123.6.2007. [DOI] [PubMed] [Google Scholar]
- 13.Taskar VS, Coultas DB. Is idiopathic pulmonary fibrosis an environmental disease? Proc Am Thorac Soc. 2006;3:293–298. doi: 10.1513/pats.200512-131TK. [DOI] [PubMed] [Google Scholar]
- 14.Pinheiro GA, Antao VC, Wood JM, Wassell JT. Occupational risks for idiopathic pulmonary fibrosis mortality in the United States. Int J Occup Environ Health. 2008;14:117–123. doi: 10.1179/oeh.2008.14.2.117. [DOI] [PubMed] [Google Scholar]
- 15.Steele MP, Speer MC, Loyd JE, Brown KK, Herron A, Slifer SH, Burch LH, Wahidi MM, Phillips JA, III, Sporn TA, et al. Clinical and pathologic features of familial interstitial pneumonia. Am J Respir Crit Care Med. 2005;172:1146–1152. doi: 10.1164/rccm.200408-1104OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosas IO, Ren P, Avila NA, Chow CK, Franks TJ, Travis WD, McCoy JP, Jr, May RM, Wu HP, Nguyen DM, et al. Early interstitial lung disease in familial pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:698–705. doi: 10.1164/rccm.200702-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navaratnam V, Fleming KM, West J, Smith CJ, Jenkins RG, Fogarty A, Hubbard RB. The rising incidence of idiopathic pulmonary fibrosis in the UK. Thorax. 2011;66:462–467. doi: 10.1136/thx.2010.148031. [DOI] [PubMed] [Google Scholar]
- 18.Naik PK, Moore BB. Viral infection and aging as cofactors for the development of pulmonary fibrosis. Expert Rev Respir Med. 2010;4:759–771. doi: 10.1586/ers.10.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Araki T, Katsura H, Sawabe M, Kida K. A clinical study of idiopathic pulmonary fibrosis based on autopsy studies in elderly patients. Intern Med. 2003;42:483–489. doi: 10.2169/internalmedicine.42.483. [DOI] [PubMed] [Google Scholar]
- 20.Gomez-Cabrera MC, Sanchis-Gomar F, Garcia-Valles R, Pareja-Galeano H, Gambini J, Borras C, Viña J. Mitochondria as sources and targets of damage in cellular aging. Clin Chem Lab Med. 2012;50:1287–1295. doi: 10.1515/cclm-2011-0795. [DOI] [PubMed] [Google Scholar]
- 21.Moslehi J, DePinho RA, Sahin E. Telomeres and mitochondria in the aging heart. Circ Res. 2012;110:1226–1237. doi: 10.1161/CIRCRESAHA.111.246868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee HC, Wei YH. Mitochondria and aging. Adv Exp Med Biol. 2012;942:311–327. doi: 10.1007/978-94-007-2869-1_14. [DOI] [PubMed] [Google Scholar]
- 23.Marchi S, Giorgi C, Suski JM, Agnoletto C, Bononi A, Bonora M, De Marchi E, Missiroli S, Patergnani S, Poletti F, et al. Mitochondria-ROS crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alder JK, Guo N, Kembou F, Parry EM, Anderson CJ, Gorgy AI, Walsh MF, Sussan T, Biswal S, Mitzner W, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184:904–912. doi: 10.1164/rccm.201103-0520OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aoshiba K, Nagai A. Senescence hypothesis for the pathogenetic mechanism of chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2009;6:596–601. doi: 10.1513/pats.200904-017RM. [DOI] [PubMed] [Google Scholar]
- 26.Aoshiba K, Zhou F, Tsuji T, Nagai A. DNA damage as a molecular link in the pathogenesis of COPD in smokers. Eur Respir J. 2012;39:1368–1376. doi: 10.1183/09031936.00050211. [DOI] [PubMed] [Google Scholar]
- 27.Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, Lawson WE, Xie M, Vulto I, Phillips JA, III, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med. 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 28.Borie R, Crestani B, Bichat H. Prevalence of telomere shortening in familial and sporadic pulmonary fibrosis is increased in men. Am J Respir Crit Care Med. 2009;179:1073. doi: 10.1164/ajrccm.179.11.1073. [DOI] [PubMed] [Google Scholar]
- 29.Cronkhite JT, Xing C, Raghu G, Chin KM, Torres F, Rosenblatt RL, Garcia CK. Telomere shortening in familial and sporadic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178:729–737. doi: 10.1164/rccm.200804-550OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito K, Barnes PJ. COPD as a disease of accelerated lung aging. Chest. 2009;135:173–180. doi: 10.1378/chest.08-1419. [DOI] [PubMed] [Google Scholar]
- 31.MacNee W. Accelerated lung aging: a novel pathogenic mechanism of chronic obstructive pulmonary disease (COPD) Biochem Soc Trans. 2009;37:819–823. doi: 10.1042/BST0370819. [DOI] [PubMed] [Google Scholar]
- 32.Gansner JM, Rosas IO. Telomeres in lung disease. Transl Res. 2013;162:343–352. doi: 10.1016/j.trsl.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 33.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13:693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fumagalli M, Rossiello F, Clerici M, Barozzi S, Cittaro D, Kaplunov JM, Bucci G, Dobreva M, Matti V, Beausejour CM, et al. Telomeric DNA damage is irreparable and causes persistent DNA-damage-response activation. Nat Cell Biol. 2012;14:355–365. doi: 10.1038/ncb2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Savale L, Chaouat A, Bastuji-Garin S, Marcos E, Boyer L, Maitre B, Sarni M, Housset B, Weitzenblum E, Matrat M, et al. Shortened telomeres in circulating leukocytes of patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:566–571. doi: 10.1164/rccm.200809-1398OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han MK, Murray S, Fell CD, Flaherty KR, Toews GB, Myers J, Colby TV, Travis WD, Kazerooni EA, Gross BH, et al. Sex differences in physiological progression of idiopathic pulmonary fibrosis. Eur Respir J. 2008;31:1183–1188. doi: 10.1183/09031936.00165207. [DOI] [PubMed] [Google Scholar]
- 37.Voltz JW, Card JW, Carey MA, Degraff LM, Ferguson CD, Flake GP, Bonner JC, Korach KS, Zeldin DC. Male sex hormones exacerbate lung function impairment after bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol. 2008;39:45–52. doi: 10.1165/rcmb.2007-0340OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gharaee-Kermani M, Hatano K, Nozaki Y, Phan SH. Gender-based differences in bleomycin-induced pulmonary fibrosis. Am J Pathol. 2005;166:1593–1606. doi: 10.1016/S0002-9440(10)62470-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antoniou KM, Hansell DM, Rubens MB, Marten K, Desai SR, Siafakas NM, Nicholson AG, du Bois RM, Wells AU. Idiopathic pulmonary fibrosis: outcome in relation to smoking status. Am J Respir Crit Care Med. 2008;177:190–194. doi: 10.1164/rccm.200612-1759OC. [DOI] [PubMed] [Google Scholar]
- 40.Tsushima K, Sone S, Yoshikawa S, Yokoyama T, Suzuki T, Kubo K. The radiological patterns of interstitial change at an early phase: over a 4-year follow-up. Respir Med. 2010;104:1712–1721. doi: 10.1016/j.rmed.2010.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Sverzellati N, Guerci L, Randi G, Calabrò E, La Vecchia C, Marchianò A, Pesci A, Zompatori M, Pastorino U. Interstitial lung diseases in a lung cancer screening trial. Eur Respir J. 2011;38:392–400. doi: 10.1183/09031936.00201809. [DOI] [PubMed] [Google Scholar]
- 42.Washko GR, Hunninghake GM, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estépar RS, Lynch DA, Brehm JM, et al. COPDGene Investigators. Lung volumes and emphysema in smokers with interstitial lung abnormalities. N Engl J Med. 2011;364:897–906. doi: 10.1056/NEJMoa1007285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jin GY, Lynch D, Chawla A, Garg K, Tammemagi MC, Sahin H, Misumi S, Kwon KS. Interstitial lung abnormalities in a CT lung cancer screening population: prevalence and progression rate. Radiology. 2013;268:563–571. doi: 10.1148/radiol.13120816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Steen VD. Autoantibodies in systemic sclerosis. Semin Arthritis Rheum. 2005;35:35–42. doi: 10.1016/j.semarthrit.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Steen VD, Conte C, Owens GR, Medsger TA., Jr Severe restrictive lung disease in systemic sclerosis. Arthritis Rheum. 1994;37:1283–1289. doi: 10.1002/art.1780370903. [DOI] [PubMed] [Google Scholar]
- 46.Steen V, Domsic RT, Lucas M, Fertig N, Medsger TA., Jr A clinical and serologic comparison of African American and Caucasian patients with systemic sclerosis. Arthritis Rheum. 2012;64:2986–2994. doi: 10.1002/art.34482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christmann RB, Wells AU, Capelozzi VL, Silver RM. Gastroesophageal reflux incites interstitial lung disease in systemic sclerosis: clinical, radiologic, histopathologic, and treatment evidence. Semin Arthritis Rheum. 2010;40:241–249. doi: 10.1016/j.semarthrit.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, Murphy J, Cohen M, Raghu G, Brown KK. Rheumatoid arthritis–interstitial lung disease–associated mortality. Am J Respir Crit Care Med. 2011;183:372–378. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106:1591–1599. doi: 10.1016/j.rmed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 50.Furukawa H, Oka S, Shimada K, Sugii S, Ohashi J, Matsui T, Ikenaka T, Nakayama H, Hashimoto A, Takaoka H, et al. Association of human leukocyte antigen with interstitial lung disease in rheumatoid arthritis: a protective role for shared epitope. PLoS ONE. 2012;7:e33133. doi: 10.1371/journal.pone.0033133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aubart F, Crestani B, Nicaise-Roland P, Tubach F, Bollet C, Dawidowicz K, Quintin E, Hayem G, Palazzo E, Meyer O, et al. High levels of anti-cyclic citrullinated peptide autoantibodies are associated with co-occurrence of pulmonary diseases with rheumatoid arthritis. J Rheumatol. 2011;38:979–982. doi: 10.3899/jrheum.101261. [DOI] [PubMed] [Google Scholar]
- 52.Gochuico BR, Avila NA, Chow CK, Novero LJ, Wu HP, Ren P, MacDonald SD, Travis WD, Stylianou MP, Rosas IO. Progressive preclinical interstitial lung disease in rheumatoid arthritis. Arch Intern Med. 2008;168:159–166. doi: 10.1001/archinternmed.2007.59. [DOI] [PubMed] [Google Scholar]
- 53.Migita K, Nakamura T, Koga T, Eguchi K. HLA-DRB1 alleles and rheumatoid arthritis–related pulmonary fibrosis. J Rheumatol. 2010;37:205–207. doi: 10.3899/jrheum.090303. [DOI] [PubMed] [Google Scholar]
- 54.Chapman HA. Epithelial–mesenchymal interactions in pulmonary fibrosis. Annu Rev Physiol. 2011;73:413–435. doi: 10.1146/annurev-physiol-012110-142225. [DOI] [PubMed] [Google Scholar]
- 55.Selman M, Pardo A, Kaminski N. Idiopathic pulmonary fibrosis: aberrant recapitulation of developmental programs? PLoS Med. 2008;5:e62. doi: 10.1371/journal.pmed.0050062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scotton CJ, Krupiczojc MA, Königshoff M, Mercer PF, Lee YC, Kaminski N, Morser J, Post JM, Maher TM, Nicholson AG, et al. Increased local expression of coagulation factor X contributes to the fibrotic response in human and murine lung injury. J Clin Invest. 2009;119:2550–2563. doi: 10.1172/JCI33288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–1081. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lawson WE, Cheng DS, Degryse AL, Tanjore H, Polosukhin VV, Xu XC, Newcomb DC, Jones BR, Roldan J, Lane KB, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci USA. 2011;108:10562–10567. doi: 10.1073/pnas.1107559108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Beon M, Harley RA, Wessels A, Silver RM, Ludwicka-Bradley A. Myofibroblast induction and microvascular alteration in scleroderma lung fibrosis. Clin Exp Rheumatol. 2004;22:733–742. [PubMed] [Google Scholar]
- 60.Ludwicka-Bradley A, Silver RM, Bogatkevich GS. Coagulation and autoimmunity in scleroderma interstitial lung disease. Semin Arthritis Rheum. 2011;41:212–222. doi: 10.1016/j.semarthrit.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hoyles RK, Khan K, Shiwen X, Howat SL, Lindahl GE, Leoni P, du Bois RM, Wells AU, Black CM, Abraham DJ, et al. Fibroblast-specific perturbation of transforming growth factor beta signaling provides insight into potential pathogenic mechanisms of scleroderma-associated lung fibrosis: exaggerated response to alveolar epithelial injury in a novel mouse model. Arthritis Rheum. 2008;58:1175–1188. doi: 10.1002/art.23379. [DOI] [PubMed] [Google Scholar]
- 62.Wang Y, Kuan PJ, Xing C, Cronkhite JT, Torres F, Rosenblatt RL, DiMaio JM, Kinch LN, Grishin NV, Garcia CK. Genetic defects in surfactant protein A2 are associated with pulmonary fibrosis and lung cancer. Am J Hum Genet. 2009;84:52–59. doi: 10.1016/j.ajhg.2008.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nogee LM, Wert SE, Proffit SA, Hull WM, Whitsett JA. Allelic heterogeneity in hereditary surfactant protein B (SP-B) deficiency. Am J Respir Crit Care Med. 2000;161:973–981. doi: 10.1164/ajrccm.161.3.9903153. [DOI] [PubMed] [Google Scholar]
- 64.Nogee LM, Dunbar AE, III, Wert SE, Askin F, Hamvas A, Whitsett JA. A mutation in the surfactant protein C gene associated with familial interstitial lung disease. N Engl J Med. 2001;344:573–579. doi: 10.1056/NEJM200102223440805. [DOI] [PubMed] [Google Scholar]
- 65.Tsakiri KD, Cronkhite JT, Kuan PJ, Xing C, Raghu G, Weissler JC, Rosenblatt RL, Shay JW, Garcia CK. Adult-onset pulmonary fibrosis caused by mutations in telomerase. Proc Natl Acad Sci USA. 2007;104:7552–7557. doi: 10.1073/pnas.0701009104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seibold MA, Wise AL, Speer MC, Steele MP, Brown KK, Loyd JE, Fingerlin TE, Zhang W, Gudmundsson G, Groshong SD, et al. A common MUC5B promoter polymorphism and pulmonary fibrosis. N Engl J Med. 2011;364:1503–1512. doi: 10.1056/NEJMoa1013660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Diaz de Leon A, Cronkhite J, Yilmaz C, Brewington C, Wang R, Xing C, Hsia CC, Garcia CK. Subclinical lung disease, macrocytosis and premature graying in kindreds with telomerase (TERT) mutations. Chest. 2011;140:753–763. doi: 10.1378/chest.10-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Crossno PF, Polosukhin VV, Blackwell TS, Johnson JE, Markin C, Moore PE, Worrell JA, Stahlman MT, Phillips JA, III, Loyd JE, et al. Identification of early interstitial lung disease in an individual with genetic variations in ABCA3 and SFTPC. Chest. 2010;137:969–973. doi: 10.1378/chest.09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Anikster Y, Huizing M, White J, Shevchenko YO, Fitzpatrick DL, Touchman JW, Compton JG, Bale SJ, Swank RT, Gahl WA, et al. Mutation of a new gene causes a unique form of Hermansky-Pudlak syndrome in a genetic isolate of central Puerto Rico. Nat Genet. 2001;28:376–380. doi: 10.1038/ng576. [DOI] [PubMed] [Google Scholar]
- 70.Arroliga AC, Podell DN, Matthay RA. Pulmonary manifestations of scleroderma. J Thorac Imaging. 1992;7:30–45. doi: 10.1097/00005382-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 71.Launay D, Remy-Jardin M, Michon-Pasturel U, Mastora I, Hachulla E, Lambert M, Delannoy V, Queyrel V, Duhamel A, Matran R, et al. High resolution computed tomography in fibrosing alveolitis associated with systemic sclerosis. J Rheumatol. 2006;33:1789–1801. [PubMed] [Google Scholar]
- 72.Strollo D, Goldin J. Imaging lung disease in systemic sclerosis. Curr Rheumatol Rep. 2010;12:156–161. doi: 10.1007/s11926-010-0095-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Steen VD, Medsger TA. Changes in causes of death in systemic sclerosis, 1972–2002. Ann Rheum Dis. 2007;66:940–944. doi: 10.1136/ard.2006.066068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, Corte TJ, Sander CR, Ratoff J, Devaraj A, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–1254. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 75.Clements PJ, Roth MD, Elashoff R, Tashkin DP, Goldin J, Silver RM, Sterz M, Seibold JR, Schraufnagel D, Simms RW, et al. Scleroderma Lung Study Group. Scleroderma lung study (SLS): differences in the presentation and course of patients with limited versus diffuse systemic sclerosis. Ann Rheum Dis. 2007;66:1641–1647. doi: 10.1136/ard.2007.069518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Walker UA, Tyndall A, Czirják L, Denton C, Farge-Bancel D, Kowal-Bielecka O, Müller-Ladner U, Bocelli-Tyndall C, Matucci-Cerinic M. Clinical risk assessment of organ manifestations in systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group database. Ann Rheum Dis. 2007;66:754–763. doi: 10.1136/ard.2006.062901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Plastiras SC, Karadimitrakis SP, Ziakas PD, Vlachoyiannopoulos PG, Moutsopoulos HM, Tzelepis GE. Scleroderma lung: initial forced vital capacity as predictor of pulmonary function decline. Arthritis Rheum. 2006;55:598–602. doi: 10.1002/art.22099. [DOI] [PubMed] [Google Scholar]
- 78.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Fibrosing alveolitis in patients with rheumatoid arthritis as assessed by high resolution computed tomography, chest radiography, and pulmonary function tests. Thorax. 2001;56:622–627. doi: 10.1136/thorax.56.8.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cervantes-Perez P, Toro-Perez AH, Rodriguez-Jurado P. Pulmonary involvement in rheumatoid arthritis. JAMA. 1980;243:1715–1719. [PubMed] [Google Scholar]
- 80.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, Lake FR. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156:528–535. doi: 10.1164/ajrccm.156.2.9609016. [DOI] [PubMed] [Google Scholar]
- 81.Dawson JK, Fewins HE, Desmond J, Lynch MP, Graham DR. Predictors of progression of HRCT diagnosed fibrosing alveolitis in patients with rheumatoid arthritis. Ann Rheum Dis. 2002;61:517–521. doi: 10.1136/ard.61.6.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim EJ, Elicker BM, Maldonado F, Webb WR, Ryu JH, Van Uden JH, Lee JS, King TE, Jr, Collard HR. Usual interstitial pneumonia in rheumatoid arthritis–associated interstitial lung disease. Eur Respir J. 2010;35:1322–1328. doi: 10.1183/09031936.00092309. [DOI] [PubMed] [Google Scholar]
- 83.Kim EJ, Collard HR, King TE., Jr Rheumatoid arthritis–associated interstitial lung disease: the relevance of histopathologic and radiographic pattern. Chest. 2009;136:1397–1405. doi: 10.1378/chest.09-0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, Vassallo R, Gabriel SE, Matteson EL. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62:1583–1591. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rosas IO, Yao J, Avila NA, Chow CK, Gahl WA, Gochuico BR. Automated quantification of high-resolution CT scan findings in individuals at risk for pulmonary fibrosis. Chest. 2011;140:1590–1597. doi: 10.1378/chest.10-2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yilmaz C, Watharkar S, Diaz de Leon A, Garcia CK, Patel NC, Jordan KG, Hsia CC. Quantification of regional interstitial lung disease from CT-derived fractional tissue volume: a Lung Tissue Research Consortium study. Acad Rad. 2011;18:1014–1023. doi: 10.1016/j.acra.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maldonado F, Moua T, Rajagopalan S, Karwoski RA, Raghunath S, Decker PA, Hartman TE, Bartholmai BJ, Robb RA, Ryu JH. Automated quantification of radiologic patterns predicts survival in idiopathic pulmonary fibrosis. Eur Respir J. 2014;43:204–212. doi: 10.1183/09031936.00071812. [DOI] [PubMed] [Google Scholar]
- 88.Assassi S, Wu M, Tan FK, Chang J, Graham TA, Furst DE, Khanna D, Charles J, Ferguson EC, Feghali-Bostwick C, et al. Skin gene expression correlates of severity of interstitial lung disease in systemic sclerosis. Arthritis Rheum. 2013;65:2917–2927. doi: 10.1002/art.38101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Doyle TJ, Pinto-Plata V, Morse D, Celli BR, Rosas IO. The expanding role of biomarkers in the assessment of smoking-related parenchymal lung diseases. Chest. 2012;142:1027–1034. doi: 10.1378/chest.12-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Vij R, Noth I. Peripheral blood biomarkers in idiopathic pulmonary fibrosis. Transl Res. 2012;159:218–227. doi: 10.1016/j.trsl.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang Y, Kaminski N. Biomarkers in idiopathic pulmonary fibrosis. Curr Opin Pulm Med. 2012;18:441–446. doi: 10.1097/MCP.0b013e328356d03c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rosas IO, Richards TJ, Konishi K, Zhang Y, Gibson K, Lokshin AE, Lindell KO, Cisneros J, Macdonald SD, Pardo A, et al. MMP1 and MMP7 as potential peripheral blood biomarkers in idiopathic pulmonary fibrosis. PLoS Med. 2008;5:e93. doi: 10.1371/journal.pmed.0050093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Richards TJ, Kaminski N, Baribaud F, Flavin S, Brodmerkel C, Horowitz D, Li K, Choi J, Vuga LJ, Lindell KO, et al. Peripheral blood proteins predict mortality in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2012;185:67–76. doi: 10.1164/rccm.201101-0058OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Prasse A, Probst C, Bargagli E, Zissel G, Toews GB, Flaherty KR, Olschewski M, Rottoli P, Müller-Quernheim J. Serum CC-chemokine ligand 18 concentration predicts outcome in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:717–723. doi: 10.1164/rccm.200808-1201OC. [DOI] [PubMed] [Google Scholar]
- 95.Richeldi L, Costabel U, Selman M, Kim DS, Hansell DM, Nicholson AG, Brown KK, Flaherty KR, Noble PW, Raghu G, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med. 2011;365:1079–1087. doi: 10.1056/NEJMoa1103690. [DOI] [PubMed] [Google Scholar]
- 96.Noble PW, Richeldi L, Kaminski N. End of an ERA: lessons from negative clinical trials in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;184:4–5. doi: 10.1164/rccm.201105-0813ED. [DOI] [PubMed] [Google Scholar]
- 97.Khanna D, Denton CP. Evidence-based management of rapidly progressing systemic sclerosis. Best Pract Res Clin Rheumatol. 2010;24:387–400. doi: 10.1016/j.berh.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kowal-Bielecka O, Landewé R, Avouac J, Chwiesko S, Miniati I, Czirjak L, Clements P, Denton C, Farge D, Fligelstone K, et al. EUSTAR Co-Authors. EULAR recommendations for the treatment of systemic sclerosis: a report from the EULAR Scleroderma Trials and Research group (EUSTAR) Ann Rheum Dis. 2009;68:620–628. doi: 10.1136/ard.2008.096677. [DOI] [PubMed] [Google Scholar]
- 99.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults and trends in smoking cessation—United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58:1227–1232. [PubMed] [Google Scholar]
- 100.Humphrey LL, Deffebach M, Pappas M, Baumann C, Artis K, Mitchell JP, Zakher B, Fu R, Slatore CG. Screening for lung cancer with low-dose computed tomography: a systematic review to update the US Preventive Services Task Force recommendation. Ann Intern Med. 2013;159:411–420. doi: 10.7326/0003-4819-159-6-201309170-00690. [DOI] [PubMed] [Google Scholar]