Abstract
Objective:
Early menopause is associated with an increased risk for developing rheumatoid arthritis (RA). The risk for cardiovascular disease (CVD) in women increases following menopause. Since RA is associated with an increased risk of CVD, this study was undertaken to determine if early menopause affects the risk of developing CVD in women with RA.
Methods:
A population-based inception cohort of 600 women with RA who fulfilled 1987 ACR criteria for RA between 1955 and 2007 and were age ≥ 45 years at diagnosis was assembled and followed. Age at menopause and duration of hormone replacement therapy (HRT), along with occurrence of CVD was ascertained by review of medical records. Cox proportional hazard models compared women who underwent early menopause (natural or artificial menopause at age ≤ 45 years) to those within the cohort who did not undergo early menopause.
Results:
Of 600 women, 79 exprienced early menopause. Women who underwent early menopause were at significantly higher risk for developing CVD when compared to women who did not (hazard ratio (HR): 1.56; 95% CI: 1.08-2.26).
Conclusion:
The risk of CVD in women with RA was higher in those who experience early menopause, and like other known risk factors should increase clinician concern for development of CVD in these patients.
Keywords: rheumatoid arthritis, menopause, cardiovascular disease
INTRODUCTION
Rheumatoid arthritis (RA) has a female predominance and multiple investigations in recent years have suggested that a woman’s lifetime exposure to female sex hormones may play a role in the development and severity of the disease, with higher hormone exposure being associated with lower risk of disease development (1-4). Specific evaluations of nulliparity, irregular menstrual cycles, breast feeding, oral contraceptive use and hormone replacement therapy have shed some further light on the role these hormones play in women with RA (5-9). Recent studies have also shown early menopause is associated with the development of RA (10), although early menopause has paradoxically been associated with a milder form of the disease (11).
It has also been suggested that lifetime exposure to female sex hormones may influence a woman’s risk of developing cardiovascular disease (CVD), with this risk being higher in women following menopause and increased even further by early menopause (12). Finally, RA has been associated with an increased risk of cardiovascular events, specifically sudden cardiac death, with these events occurring earlier in life in those with RA when compared to the general population (13-15).
In this study we investigated whether lower lifetime exposure to female sex hormones, with specific focus on age of menopause along with parity and hormone replacement exposure, is a predictor of CVD risk in women with RA. This was achieved by separately evaluating the relationship between early menopause (defined as natural or artificial menopause prior to age 45), parity and any hormone replacement exposure and CVD outcomes in a population based cohort study.
MATERIALS AND METHODS
This study was conducted within the population of Olmsted County, Minnesota, USA. This population is well suited for longitudinal, population-based cohort studies of patients with RA because comprehensive medical records for all residents seeking any medical care for over 55 years are available. The medical records linkage system of the Rochester Epidemiology Project (REP) allows access to the complete inpatient and outpatient records from all health care providers for the local population including the Mayo Clinic and its affiliated hospitals, the Olmsted Medical Center and its affiliated community hospital, local nursing homes and local private practitioners. The potential of this data system for population-based research studies has been previously described (16, 17) and assures virtually complete clinical information for cases of RA among Olmsted County, Minnesota residents.
In this case a historical population-based cohort study was designed. The study made use of a previously described (18,19) inception cohort of all subjects who first fulfilled 1987 ACR criteria for RA between 1955 and 2007 among Olmsted County, Minnesota residents ≥ 18 years of age at diagnosis. From 1955 to 1979, only Rochester, Minnesota residents (which are a subset of all Olmsted County residents) were included in the cohort. RA incidence date was defined as the first date of fulfillment of four (out of seven) classification criteria. For the purposes of our study’s objectives, this population was limited to women aged ≥ 45 years at RA incidence, and was then divided into subjects who experienced early menopause and subjects who did not, with early menopause being defined as natural or artificial menopause prior to 46 years of age. Artificial menopause was defined as hysterectomy with bilateral oophorectomy, bilateral oophorectomy alone, ovarian failure secondary to radiation and ovarian failure secondary to chemicals/medication.
The medical records of study subjects were reviewed by trained nurse abstractors and subjects were followed until death, migration, or December 31, 2008. Medical records were reviewed to ascertain the presence of CVD risk factors (i.e., body mass index, smoking status, presence of hypertension, dyslipidemia and diabetes mellitus, family history of coronary artery disease), RA disease characteristics (i.e., erythrocyte sedimentation rate both at RA incidence and at its highest level within the first year after RA diagnosis, presence of erosions and rheumatoid nodules during the first year following diagnosis with RA, rheumatoid factor positivity), female hormone related factors (i.e., parity, age at menopause, type of menopause, use of hormone replacement therapy) and to ascertain the development of CVD.
Ascertainment of CVD
The outcomes in this study included: coronary heart disease, heart failure, cerebrovascular disease and peripheral vascular disease. Data were collected regarding all coronary heart disease events within the study groups and included angina, revascularization procedures including percutaneous coronary interventions and coronary artery bypass grafting, myocardial infarction (MI) (including silent events) and physician diagnosis of coronary artery disease. MI was defined using standardized epidemiologic criteria (20), and Minnesota coding (21) of the electrocardiogram (ECG). Silent MI was considered as present at the date of the first documentation of a characteristic ECG or a recorded physician’s diagnosis in a patient with no documented history of MI. Heart failure was defined using the Framingham Heart Study criteria (22). Data were also collected regarding cerebrovascular events, which encompassed both hemorrhagic and non-hemorrhagic stroke, unspecified stroke and transient ischemic attacks, as were data regarding peripheral vascular events, which included aortic aneurysm, renal artery stenosis, peripheral vascular disease and arterial thromboembolism. Stroke, peripheral artery disease and arterial thromboembolism were verified with objective data (imaging and related examination) in addition to the clinical diagnosis (23). For conditions where there was no objective data (e.g. transient ischemic attack), we accepted the clinician diagnosis if the physician documented that this condition was present. A consensus discussion among the investigators with medical record review was held to resolve any unclear situations or discrepancies.
Statistical Methods
Descriptive statistics (means, percentages, etc.) were used to summarize the subject characteristics. Chi-square and rank sum tests were used to compare subject characteristics between women with and without early menopause. Cox proportional hazards models were used to examine the association between female hormone variables and the risk of cardiovascular disease outcomes, using age as a time scale and adjusted for calendar year of RA incidence. Individuals who died prior to the development of CVD were censored. Subjects were included in the analysis starting at the age of their index date and ending at the age of CVD, death, or last follow-up.
Additional adjustment for traditional cardiovascular risk factors including smoking status, hypertension, diabetes mellitus, and body mass index was also performed. Factors assessed throughout follow-up (i.e., hormone replacement therapy, hypertension, diabetes mellitus) were modeled as dichotomous time-dependent covariates. A subject's status changed from unexposed to exposed at the time of the diagnosis of a particular risk factor during follow-up. Smoothing splines were used to examine potential non-linear effects for parity. Person-year methods were used to estimate the rate of CVD according to early menopause status.
RESULTS
This study included 600 women with RA age ≥ 45 years at diagnosis, of whom 79 experienced early menopause. The mean age at menopause in those who experienced early menopause was 40.9 ± 5.0 years; while in those who did not experience early menopause the mean age was 50.7 ± 2.8 years. Table 1 delineates the baseline characteristics of the study population. No differences in RA disease characteristics (rheumatoid factor positivity, erythrocyte sedimentation rate at RA incidence, or presence of erosions on radiographs in the first year after RA incidence) were found for those with and without early menopause. However, women who experienced early menopause were more likely to develop rheumatoid nodules during the first year following their diagnosis of RA (24% vs. 13%). The number of women who experienced artificial menopause from surgery or secondary to radiation or chemical/medication exposure was significantly higher in those who underwent early menopause (42% vs. 6%). This group was also more likely to have been on hormone replacement therapy prior to diagnosis of RA (39% vs. 28%). The mean age at which RA was diagnosed, other female hormone related variables, cardiovascular risk factors, RA disease characteristics and prior CVD events were otherwise similar in both groups.
Table 1.
Characteristics of 600 women with rheumatoid arthritis (RA) according to presence or absence of early menopause.
|
No early
menopause (N=521) |
Early
menopause* (N=79) |
p value | |
|---|---|---|---|
| Mean age at index date, years (SD) | 63.6 (11.7) | 61.4 (11.0) | 0.195 |
| Mean age at menopause, years (SD) | 50.7 (2.8) | 40.9 (5.0) | <0.001 |
| Rheumatoid factor positivity | 331 (66) | 50 (66%) | 0.97 |
| Erythrocyte sedimentation rate at RA incidence, mm/hr |
33.9 (24.6) | 31.7 (24.5) | 0.36 |
| Presence of rheumatoid nodules in the first year after RA incidence (%) |
70 (13%) | 19 (24%) | 0.013 |
| Presence of erosions on radiographs in the first year after RA incidence (%) |
159 (30%) | 21 (27%) | 0.48 |
| Mean length of follow-up, years (SD) | 11.9 (8.9) | 11.0 (8.1) | -- |
| Natural menopause (%)** | 474 (93) | 46 (58) | <0.001 |
| Artificial menopause (%)** | 31 (6) | 33 (42) | <0.001 |
| Type of artificial menopause | 0.034 | ||
| Still menstruating (%) | 5 (14) | 0 (0.0) | |
| Hysterectomy/bilateral oophorectomy (%) | 24 (67) | 27 (82) | |
| Bilateral oophorectomy (%) | 2 (6) | 2 (6) | |
| Radiation (%) | 4 (11) | 0 (0.0) | |
| Chemical (%) | 1 (3) | 1 (3) | |
| Other (%) | 0 (0.0) | 3 (9) | |
| Mean gravidity (SD) | 3.1 (2.5) | 2.8 (2.6) | 0.392 |
| Mean parity (SD) | 2.6 (2.2) | 2.4 (2.1) | 0.568 |
| Any pregnancy or live birth (%) | 424 (81) | 61 (77) | 0.381 |
| Mean age at menarche (SD) | 13.0 (1.3) | 13.0 (1.5) | 0.470 |
| Hormone replacement therapy before RA incidence (%) |
144 (28) | 31 (39) | 0.035 |
Early menopause was defined as natural or artificial menopause at age ≤ 45 years.
The difference between the natural menopause and the artificial menopause variables is the 5 patients who are still menstruating at RA incidence.
Women with RA who did not experience early menopause were followed for an average of 11.9 years while women with RA who did experience early menopause were followed for an average of 11.0 years. Of the 521 women who did not undergo early menopause, 96 women without prior coronary heart disease experienced at least 1 coronary heart disease event during follow-up. Among women without prior events of each type, 117 developed heart failure, 62 experienced cerebrovascular events and 40 experienced peripheral vascular disease events. Of the 79 women with RA who underwent early menopause, 26 experienced at least 1 coronary heart disease event, 19 experienced heart failure, 12 experienced cerebrovascular events and 3 experienced peripheral vascular disease events during follow-up.
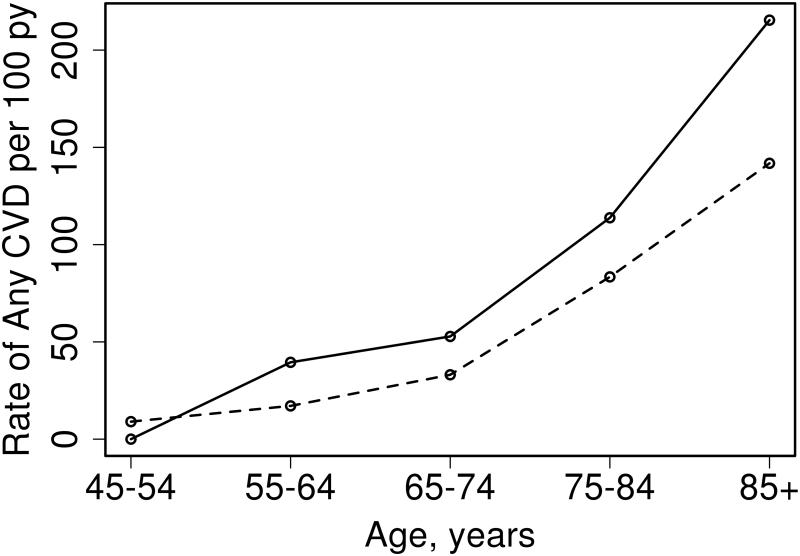
Overall, among women without prior CVD, 35 with early menopause and 170 without early menopause developed CVD during follow up. As can be seen in Figure 1, the rate of any CVD is similar in women age 45 to 54 who did not experience early menopause when compared to those who did, however the rate of CVD increased more in women who experienced early menopause as age progressed.
Figure 1.
Rate of any cardiovascular disease (CVD) according to age groups and early menopausal status (Solid line: early menopause, dashed line: no early menopause).
The association between characteristics influencing lifetime exposure to female sex hormones and CVD outcomes was assessed (table 2). Women who underwent early menopause had a higher risk of developing general CVD, including coronary heart disease, heart failure, cerebrovascular disease and/or peripheral vascular disease as described above (HR: 1.55, 95% CI 1.07-2.23). The risk of developing general CVD remained significant when the age of menopause was defined as the end of hormone replacement therapy for women with artificial menopause who started hormone replacement therapy at the time of artificial menopause (HR: 1.52, 95% CI 1.05-2.20). Of the 79 women who experienced early menopause, 26 were affected by this change in definition, however only 9 of these women no longer qualified as early menopause.
Table 2.
Association between characteristics of female sex hormones and cardiovascular disease outcomes in 600 women with rheumatoid arthritis (RA).
| Characteristic | Coronary Heart Disease |
Heart Failure | Cerebrovascular disease |
Peripheral Vascular disease |
General cardiovascular disease (incl CHD, HF, cerebrovascular disease & PVD) |
|---|---|---|---|---|---|
| Early Menopause |
1.42 (0.85, 2.39) | 1.14 (0.69, 1.87) | 1.41 (0.76, 2.62) | 0.51 (0.16, 1.64) | 1.55 (1.07, 2.23) |
| Artificial menopause |
0.71 (0.33, 1.53) | 0.82 (0.40, 1.69) | 1.19 (0.54, 2.60) | 0.24 (0.03, 1.74) | 0.80 (0.46, 1.39) |
| Any pregnancy/birth |
1.01 (0.65, 1.58) | 0.92 (0.62, 1.36) | 0.68 (0.41, 1.13) | 0.92 (0.46, 1.82) | 0.96 (0.68, 1.33) |
| Any HRT exposure |
1.02 (0.68, 1.53) | 0.72 (0.48, 1.07) | 1.26 (0.77, 2.07) | 0.84 (0.41, 1.72) | 1.05 (0.77, 1.43) |
Values in the table are hazard ratio (95% confidence interval). All models adjusted for age (as the time-scale) and calendar year of RA
CHD = coronary heart disease, HF = heart failure, PVD = peripheral vascular disease
The association between early menopause and the development of CVD did not differ among women with and without positive rheumatoid factor (interaction p=0.71). Similarly, there were no differences in the associations between artificial menopause, parity or any hormone replacement therapy exposure and the development of CVD among patients with and without positive rheumatoid factor.
After adjustment for CVD risk factors including smoking status, body mass index, as well as diagnosis of diabetes mellitus and hypertension, the association between early menopause and increased risk of CVD persisted (HR: 1.56, 95% CI 1.08-2.26). The risk of developing CVD continued to be significant when the age of menopause was defined as the end of hormone replacement therapy for women with artificial menopause who started hormone replacement therapy at the time of artificial menopause (HR: 1.53, 95% CI 1.06-2.23). As was found prior to adjustment for CVD risk factors, artificial menopause and exposure to hormone replacement at any time did not increase the risk of developing CVD.
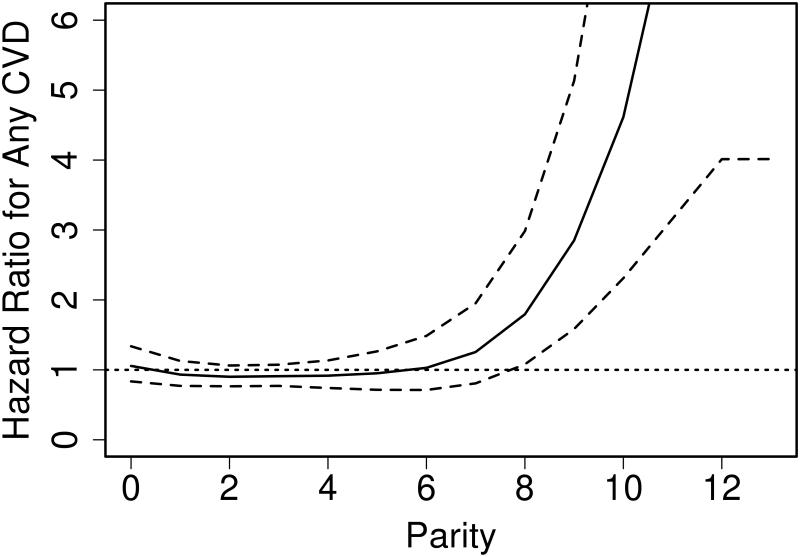
CVD risk was also increased in women with higher parity (in linear analyses, HR 1.07 per 1 birth increase, 95% CI 1.01-1.14). However, there was a strong non-linear relationship between parity and CVD outcomes, whereby the increased risk of CVD was detected only at very high values (>7) of parity (Figure 2).
Figure 2.
Association between parity and cardiovascular disease (CVD) outcomes in women with rheumatoid arthritis.
(Solid line depicts the hazard ratios according to parity and the dashed lines depict 95% confidence intervals for the hazard ratios. The dotted line depicts the reference value where the hazard ratio equals 1)
DISCUSSION
In this study, the overall risk for cardiovascular disease events was increased in women with RA who experienced early menopause. The risk for individual cardiovascular outcomes of coronary heart disease, heart failure and cerebrovascular disease were each increased, although none reached statistical significance.
In recent years, a number of studies have suggested that lower lifetime exposure to female sex hormones may play a role in the development and severity of RA in women. Decreased exposure to these hormones may also influence a woman’s risk of developing CVD. Further, RA has been associated with an increased risk of CVD events. In this study, we demonstrate that the risk of CVD in women with RA is significantly higher in those who experience early menopause.
It has long been recognized, that there is a female predominance for RA (1). A variety of investigations have also suggested that gonadal hormones may play a role in the development and progression of RA (2-9). However, what is less understood is what the contribution of these sex hormones might be on development of CVD in RA.
Our findings are in agreement with other reports in the literature that there is an increase in CVD risk in women following menopause (12), and this risk is further increased by early menopause. Indeed, women in the general population undergoing menopause prior to age 46 experience an approximately twofold increased risk of future coronary heart disease or stroke events (24). Our investigations also show that CVD risk is higher as parity increases. These findings are incongruous with the hypothesis that higher lifetime exposure to female sex hormones decreases the risk of CVD. However, further analysis shows that there is a strong nonlinear relationship between parity and CVD outcomes, with the increased risk of CVD only being detected at very high values (>7) of parity. This suggests that there may be a mechanism unrelated to hormone exposure leading to cardiovascular disease in these women, especially in the setting of the other physiologic changes that take place during pregnancy. In view of the small numbers of women in this study of high parity, this observation is tentative and would require further study in cohorts with more women of high parity.
The relationships between lifetime exposure to female sex hormones and RA, hormone exposure and CVD, as well as CVD in relation to RA, have recently all been examined individually in several investigations. However, this study is among the first to specifically examine all three variables, sex hormone exposure, RA and CVD, together and allowed us to conclude that early menopause, like other known CVD risk factors, increases the risk of CVD in women with RA.
Previous studies have suggested that RA may potentially contribute to early natural menopause. In an attempt to reduce this confounder only women diagnosed with RA at ages older than 45 were selected for this study. An additional strength of this study was the avoidance of selection bias with the population based approach. There were also several limitations to this study. First, the population of Olmsted County, Minnesota is predominantly white, thus while our findings should be reflective of the majority of patients with rheumatoid arthritis seen in Western countries, the generalizability of our findings to more ethnically diverse populations may be limited (25). Second, the retrospective study design necessitates the use of information documented in medical records to ascertain risk factors and outcomes. Therefore, risk factors and outcomes were dependent on physician observation and documentation. However, the use of the comprehensive population-based resources of Rochester Epidemiology Project likely minimized this bias. In addition, our analyses did not account for potential differences in RA disease severity or treatment regimens among women with and without early menopause. However, we did not find any significant differences in RA severity indicators measured at RA incidence. Furthermore, the number of peripheral disease events observed in our study population was low, so the study was underpowered to definitively assess this outcome. Finally, no menopause data are yet available on a comparison cohort of subjects without RA. Because of this it was not possible to determine whether the relationship between early menopause and CVD differs in the RA population compared to the general population of Olmsted County.
As an observational study, it is only possible to report an association between RA, early menopause and CVD. Our data suggests a significantly increased risk of CVD in women with RA who undergo early menopause. The underlying mechanism for this association remains unclear. Further studies will be needed to evaluate the role of female sex hormones in the increased inflammatory state of RA. It is possible this mechanism is related both to RA disease severity and activity as well as other CVD risk factors.
In conclusion, our findings demonstrate that the risk of developing CVD in women with RA is significantly higher in those who experience early menopause. It has previously been demonstrated that early menopause and RA each individually increase the risk of CVD in women. CVD has also been shown to occur earlier in those with RA. In aggregate, these findings suggest that early menopause, like other known risk factors, in women with RA should increase clinician concern for development of CVD in this population. These results suggest that optimal management of other known CVD risk factors may be especially important for women with both RA and early menopause. Further investigation is needed to determine the underlying mechanism by which female sex hormones act to protect against the development of CVD in women with RA.
Funding Source
This work was funded by research grants R01 AR46849 and PO1 AG04875-24 and made possible by the Rochester Epidemiology Project (R01 AG034676 from the National Institute on Aging of the National Institutes of Health). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
REFERENCES
- 1.Crowson CS, Matteson EL, Myasodedova E, Michet CJ, Ernste FC, Warrington KJ, Davis JM, Hunder G, Therneau TM, Gabriel SE. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:633–639. doi: 10.1002/art.30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Da Silva JA, Spector TD. The role of pregnancy in the course and aetiology of rheumatoid arthritis. Clin Rheumatol. 1992;11:189–194. doi: 10.1007/BF02207955. [DOI] [PubMed] [Google Scholar]
- 3.Silman A, Kay A, Brennan P. Timing of pregnancy in relation to the onset of rheumatoid arthritis. Arthritis Rheum. 1992;35:152–155. doi: 10.1002/art.1780350205. [DOI] [PubMed] [Google Scholar]
- 4.Hazes J, Dijkmans B, Vandenbroucke J, de Vries R, Cats A. Pregnancy and the risk of developing rheumatoid arthritis. Arthritis Rheum. 1990;33:1770–1775. doi: 10.1002/art.1780331203. [DOI] [PubMed] [Google Scholar]
- 5.Brun JG, Nilssen S, Kvale G. Breastfeeding, other reproductive factors and rheumatoid arthritis. Br J Rheumatol. 1995;34:452–546. doi: 10.1093/rheumatology/34.6.542. [DOI] [PubMed] [Google Scholar]
- 6.Pikwer M, Bergstrom I, Nilsson JA, Jacobsson L, Berglund G, Turesson C. Breastfeeding but not use of oral contraceptives is associated with a reduced risk of rheumatoid arthritis. Ann Rheumatic Dis. 2009;68:526–530. doi: 10.1136/ard.2007.084707. [DOI] [PubMed] [Google Scholar]
- 7.Karlson EW, Mandl LA, Hankinson SE, Grodstein F. Do breastfeeding and other reproductive factors influence future risk of rheumatoid arthritis? Results from the Nurse’s Health Study. Arthritis Rheum. 2004;50:2458–2467. doi: 10.1002/art.20621. [DOI] [PubMed] [Google Scholar]
- 8.Doran MF, Crowson CS, O MW, Gabriel SE. The effect of oral contraceptives and estrogen replacement therapy on the risk of rheumatoid arthritis: a population based study. J Rheumatol. 2004;31:207–213. [PubMed] [Google Scholar]
- 9.Spector TD, Roman E, Silman AJ. The pill, parity and rheumatoid arthritis. Arthritis Rheum. 1990;33:782–789. doi: 10.1002/art.1780330604. [DOI] [PubMed] [Google Scholar]
- 10.Pikwer M, Bergstrom U, Nilsson JA, Jacobsson L, Turesson C. Early menopause is an independent predictor of rheumatoid arthritis. Ann Rheumatic Dis. 2011;71:378–381. doi: 10.1136/ard.2011.200059. [DOI] [PubMed] [Google Scholar]
- 11.Pikwer M, Nilsson JA, Bergstrom U, Jacobsson L, Turesson C. Early menopause and severity of rheumatoid arthritis in women over 45 years of age. Arthritis Res Ther. 2012;14:R190. doi: 10.1186/ar4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sammaritano LR. Menopause in patients with autoimmune diseases. Autoimmunity Rev. 2012;11:A430–A436. doi: 10.1016/j.autrev.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 13.Bywaters EGL. 10-year follow-up study of rheumatoid arthritis. Ann Rheumatic Dis. 1961;20:198. [Google Scholar]
- 14.Maradit-Kremers H, et al. Increased unrecognized coronary heart disease and sudden deaths in rheumatoid arthritis: a population based cohort study. Arthritis Rheum. 2002;46:2010–2019. doi: 10.1002/art.20853. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez A, Maradit Kremers H, Crowson CS, Ballman KV, Roger VL, Jacobsen SJ, O’Fallon WM, Gabriel SE. Do cardiovascular risk factors confer the same risk for cardiovascular outcomes in rheumatoid arthritis patients as in non-rheumatoid arthritis patients? Ann Rheumatic Dis. 2008;67:64–69. doi: 10.1136/ard.2006.059980. [DOI] [PubMed] [Google Scholar]
- 16.Kurland L, Molgaard C. The patient record in epidemiology. Sci Am. 1981;245:54–63. doi: 10.1038/scientificamerican1081-54. [DOI] [PubMed] [Google Scholar]
- 17.Melton L. History of the Rochester Epidemiology Project. Mayo Clinic Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 18.Gabriel S, Crowson C, O’Fallon W. The epidemiology of rheumatoid arthritis in Rochester, Minnesota, 1955-1985. Arthritis Rheum. 1999;42:415–420. doi: 10.1002/1529-0131(199904)42:3<415::AID-ANR4>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 19.Doran M, Pond G, Crowson C, O’Fallon W, Gabriel S. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota over a forty-year period. Arthritis Rheum. 2002;46:625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- 20.Gillum RF, Fortmann SP, Prineas RJ, Kottke TE. International diagnostic criteria for acute myocardial infarction and acute stroke. Am Heart J. 1984;108:150–8. doi: 10.1016/0002-8703(84)90558-1. [DOI] [PubMed] [Google Scholar]
- 21.Prineas RCR, Blackburn H. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. Wright-PSG; Littleton (MA): 1982. [Google Scholar]
- 22.Ho K, Pinsky J, Kannel W, Levy D. The epidemiology of heart failure: the Framingham Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. 4 suppl A. [DOI] [PubMed] [Google Scholar]
- 23.Bacani AK, Gabriel SE, Crowson CS, Heit JA, Matteson EL. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum. 2012 Jan;64(1):53–61. doi: 10.1002/art.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Viadya D. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause. 2012;10:1081–1087. doi: 10.1097/gme.0b013e3182517bd0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauver JL, St., Grossardt BR, Yawn BP, Melton LJ, 3rd, Pankratz JJ, Brue SM, Rocca WA. Data Resource Profile: The Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41:1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]