Abstract
In the past 50 years, thalidomide has undergone a remarkable metamorphosis from a notorious drug inducing birth defects into a highly effective therapy for treating leprosy and multiple myeloma. Today, most notably, thalidomide and its analogs have shown efficacy against a wide variety of diseases, including inflammation and cancer. The mechanism underlying its teratogenicity as well as its anticancer activities has been intensively studied. This review summarizes the biological effects and therapeutic uses of thalidomide and its analogs, and the underlying mechanisms of thalidomide’s action with a focus on its suppression of tumor growth.
Keywords: Thalidomide, teratogenesis, angiogenesis, cancer, multiple myeloma
1. INTRODUCTION
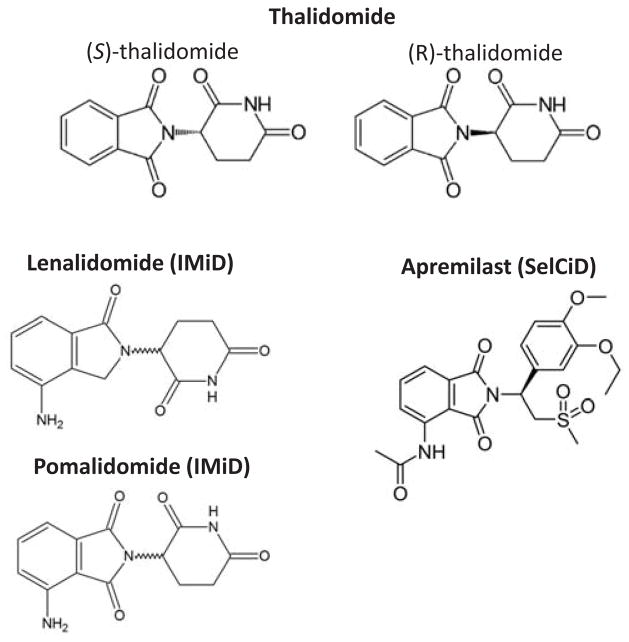
Thalidomide (Fig. 1) was originally synthesized by Chemische Industrie Basel (CIBA) in Switzerland in early 1950s without specific usage. In late 1950s, the discovery of its sedative effects led to the introduction of thalidomide to the public as a drug for treating morning sickness. However, the usage of thalidomide from the late 1950s to the early 1960s resulted in one of the biggest tragedy in the history of drug development. As a result of using thalidomide, it caused an estimated 10,000 children in 46 countries to be born with birth defects, marked by limb malformations and congenital defects affecting ears, eyes, heart, and kidney. Subsequently, thalidomide was withdrawn from the market in 1961 [1, 2]. This occurrence of devastating proportions acutely brought into focus the immense possible detrimental side effects of drugs. The investigation of its action mechanism is a root awakening for need of regulation in drug development as well as the development of systematic toxicity testing protocols in the United States and international regulatory agencies [3].
Fig. (1).
Structure of thalidomide and its analogs.
After removal of thalidomide from the market, an accidental discovery of its immunomodulatory effects was made in erythema nodosum leprosum (ENL) patients in 1965, thus defining a new indication of usage for thalidomide [4, 5]. Additional studies and clinical investigations further demonstrated the beneficial effects of thalidomide in human immunodeficiency virus (HIV) infection, and in autoimmune diseases [2, 6–9]. In 1994, Folkman and co-workers observed that thalidomide inhibited induction of the formation of new blood vessels from pre-existing ones, namely angiogenesis, by fibroblast growth factor 2 (FGF2), in rabbits [10]. Since angiogenesis is considered as an essential process that drives uncontrolled cell proliferation in the bone marrow of patients with myeloma, thalidomide was administered to the patients with refractory multiple myeloma and shown to be highly active [11]. This encouraging discovery also provided the impetus for launch of additional studies concerning efficacy of thalidomide for treating other cancer types, such as prostate cancer and glioblastoma [12–16]. The recognition of thalidomide as a therapeutic agent officially came in 1998 when it was approved by the US Food and Drug Administration (FDA) as a drug for treating ENL. In May 2006, the use of thalidomide in combination with dexamethasone for the treatment of newly diagnosed multiple myeloma received US FDA’s approval [17]. However, because of its serious side effects, the prescription of thalidomide in the USA is under stringent monitoring by the System for Thalidomide Education and Prescribing Safety (S.T.E.P.S) program [18].
The action mechanism of thalidomide has attracted considerable attention during the last decades. This review summarizes the proposed mechanism for the therapeutic effects of thalidomide and its analogs.
2. MECHANISM OF THALIDOMIDE
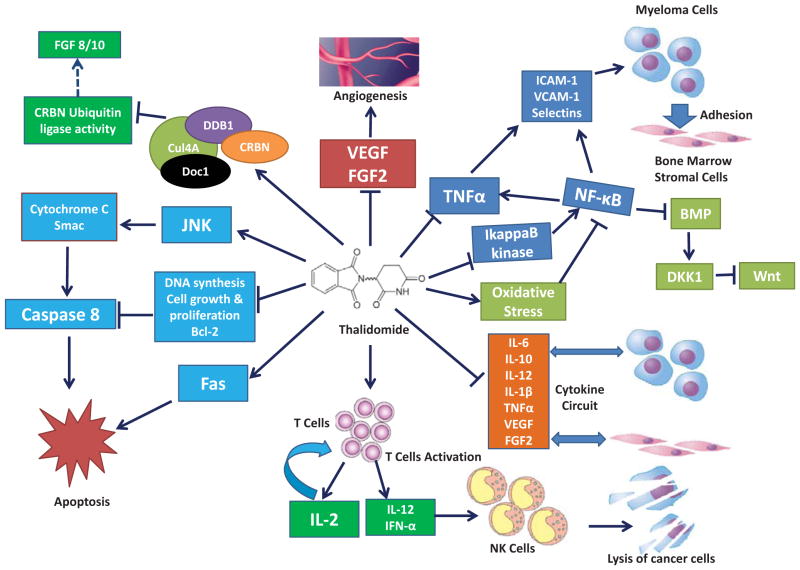
The mechanism of thalidomide’s action has been extensively studied for more than 50 years, during which more than 30 hypotheses had been proposed [19, 20]. Stephens and Fillmore classified six hypotheses of thalidomide, focusing on aberrations and/or dysfunctions in: 1) DNA replication and transcription, 2) synthesis and/or function of growth factors, 3) synthesis and/or function of integrins, 4) angiogenesis, 5) chondrogenesis, and 6) cell death or injury [20]. Among the six hypotheses presented on thalidomide, most support has been given to its anti-angiogenesis activities. For instance, the observation from D’Amato et al. agrees with the finding that thalidomide inhibits FGF2-induced angiogenesis in rabbits from Folkman’s group [10, 21]. Given the critical role of angiogenesis in the progression and metastasis of cancer, [22] and based on anti-angiogenesis effects in rabbit observed by Folkman’s group, thalidomide was first tested for treating patients with refractory myeloma [10, 11]. Later, the anti-angiogenesis potential of thalidomide was also demonstrated in humans and several other species [23–26]. Verheul et al. further demonstrated that thalidomide is capable of inhibiting the effects of FGF2 or vascular endothelial growth factor (VEGF), molecules essential for angiogenesis, on tumor growth in animal model [27]. Bertolini and colleagues further provided the clinical evidence that thalidomide decreased the plasma levels of these angiogenic growth factors in patients with multiple myeloma or myelodysplastic syndromes or histiocytosis [28]. Such effects were also observed in other cancer types, such as lung adenocarcinoma and head and neck squamous cell carcinoma [29, 30]. In addition, Steins and co-workers demonstrated a considerable efficacy of thalidomide against acute myeloid leukemia as evidenced by that the responders for thalidomide treatment experienced hematologic improvements with increased hemoglobin and platelet counts and this effect was linked to its significant anti-angiogenic effects [31]. Further, Stephens and co-workers showed that the effects of insulin-like growth factor 1 (IGF-1) and FGF2 can be reversed by thalidomide. They proposed that thalidomide intercalates into the GC box in the promoters of IGF-1 and FGF2 genes and abolishes their stimulation of the transcription of αv and β3 integrin subunit genes, which inhibit the angiogenic effects of the αvβ3 integrin dimer and eventually restrain the development of limb bud [19, 20]. It is also noteworthy that thalidomide is involved in the inhibition of cyclooxygenase-2 (COX-2), a key enzyme responsible for the formation of prostaglandins (PGs) [32]. COX-2 was shown to be required for the angiogenesis in a rat corneal model and COX-2-derived prostaglandin E2 (PGE2) is a potent inducer of angiogenic switch during mammary cancer progression [33, 34]. Though its anti-angiogenesis effect has been observed in in vitro and in vivo systems, the antitumor effects of thalidomide in patients did not always correlate with the block of angiogenic stimuli or reduction of microvessel in the patients [35]. Neben and colleagues found that the response to thalidomide in progressive multiple myeloma is not mediated by specific inhibition of angiogenic cytokine secretion in patients [36]. Moreover, even though a strong association between thalidomide and decreased microvessel density was observed in the xenografted primary human myeloma mice which responded to the treatment, Yaccoby and co-workers expressed reservation for the concept of thalidomide’s anti-angiogenic activity, as there was no changes at the density of microvessel in the mice which did not respond to the treatment [37]. Therefore, the mechanisms underlying thalidomide’s potent anticancer activities are complex as depicted in (Fig. 2) and yet to be completely elucidated [38].
Fig. (2). Therapeutic mechanism of thalidomide.
Thalidomide exerts its anticancer effects through the inhibition of angiogenesis, cell adhesion and cytokine circuit, the enhancement of host immune response, the induction of cancer cell apoptosis and oxidative stress, and the suppression on the activity of its binding targets. [22, 27, 39, 40, 42–45, 50–52, 64, 65, 67, 71]
Immunomodulatory properties of thalidomide have been the focus for its mechanistic study because therapeutic effects in patients with different types of cancer were linked with its immunomodulatory properties [39–41]. The potent immunomodulatory activity of thalidomide mainly lies in its capability of altering the secretion and activities of various cytokines including interleukin 6 (IL-6), interleukin 10 (IL-10), interleukin 12 (IL-12), interleukin 1β (IL-1β), and TNF-α [42–45]. Especially, as a pro-inflammatory cytokine, TNF-α plays a critical role in myeloma progression and is an important prognostic indicator for myeloma [46–48]. Thalidomide has been shown to inhibit TNF-α synthesis by increasing the TNF-α mRNA degradation [49]. The significant suppression of the production of TNF-α by thalidomide also resulted in decreased expression of multiple cell surface adhesion molecules, such as ICAM-1, VCAM-1, L-selectin, and E-selectin on endothelial cells [50, 51]. These broad effects have also been suggested to be partially attributed to the block of activation of Nuclear factor-κB (NF-κB), a key transcriptional regulator of inflammatory genes such as TNF-α and interleukin 8 (IL-8), by thalidomide through the suppression of IkappaB kinase activity [52]. By showing that thalidomide suppresses TNF-induced NF-κB activation in Jurkat cells, Majumdar et al. suggested that such modulations may contribute to thalidomide’s role in the suppression of proliferation, inflammation, angiogenesis, and the immune system [53]. The inactivation of NF-κB is observed in a variety of cell types including endothelial cells, epithelial cells, T cells and myeloid cells [52, 53]. In addition, thalidomide can also act via the co-stimulation of human CD8+ T cells and result in augmented production of interleukin 2 (IL-2), interleukin 12 (IL-12), and interferon α (IFN-α) [44]. IL-2 in turn enhances T-cell proliferation while IL-12 and IFN-α activate Natural killer (NK) cells to eliminate cancer cells [39, 40]. Moreover, the combination of anti-inflammatory drugs, such as sulindac and dexamethasone, with thalidomide can significantly enhance the anti-angiogenic and anti-tumor activities of thalidomide [27].
Free radical mediated DNA damage which has been associated with thalidomide teratogenicity, [54] may also play a role in the anti-cancer effects of thalidomide. The oxidative stress hypothesis of thalidomide derives from the induced oxidation of embryonic DNA concomitant with teratogenicity in rabbits, and the accompanying observation that the induced changes can be abolished by pre-treatment with alpha-phenyl-N-t-butyl-nitrone (PBN), a free radical spin trapping agent, as first advanced by Parman and co-workers [54]. Hansen et al. further postulated that thalidomide induced free radical production resulted in species-selective alteration in redox microenvironment subsequently attenuated the transcription factor NF-κB mediated gene expression [55]. Hanson and colleagues showed that thalidomide preferentially depletes glutathione and decreases the expression of NF-κB and regulatory genes involved in the initiation and maintenance of limb outgrowth and development, such as Twist, the transcription factor implicated in cell lineage determination and differentiation, and growth factors including FGF8 and FGF10, in rabbit embryos [56–58]. Knobloch and colleagues found that thalidomide initiated oxidative stress enhances signaling through bone morphogenetic proteins (BMPs), resulting in the elevation of BMP target gene and Wnt antagonist Dickkopf1 (Dkk1), and subsequently inhibits the Wnt/β-catenin signaling [59]. Since the abnormal Wnt/catenin signaling is often involved in tumor development in various cancers, perturbing the BMP/Dkk/Wnt signaling pathway by thalidomide induced oxidative stress is central to the effects of thalidomide.
Thalidomide also exhibits profound concentration-dependent inhibition of the proliferation of chemo-resistant cancer cells, e.g., multiple myeloma cells [60]. These effects may be related to its inhibition on the production of IL-6, which is a major growth factor for multiple myeloma cells, and DNA synthesis and cell cycle progression [61–63]. Moreover, thalidomide also increases the susceptibility of cancer cells to apoptosis by down-regulating the anti-apoptotic protein Bcl-2 and enhancing the sensitivity to Fas-induced apoptosis, and down-regulates NF-κB activity [64, 65]. Keller and colleagues demonstrated that thalidomide exerts its pharmacological activity at least in part via the inhibition of IL-1 and caspase-1 [66]. At the mitochondrial level, thalidomide is responsible for c-Jun terminal kinase (JNK)-dependent release of cytochrome-c and second mitochondria-derived activator of caspases (Smac) into the cytosol of cells and subsequently regulates the caspase 8 mediated apoptosis [67].
A significant breakthrough was made recently by Ito and colleagues on revealing the mechanism of thalidomide by successfully identified cereblon (CRBN) as the direct binding target of thalidomide [25]. By immobilized thalidomide onto a new affinity bead developed by their group called ferrite-glycidyl methacrylate (FG) beads, [68–70] Ito and colleagues recently purified two thalidomide binding molecules from human cell lines and identified that CRBN directly binds to thalidomide while damaged DNA binding protein 1 (DDB1) interacts with thalidomide through its binding with CRBN. The authors further found that CRBN forms an E3 ubiquitin ligase complex with DDB1, Cullin-4A (Cul4A) and Regulator of cullins 1(Roc1) and thalidomide functions as an E3 inhibitor to suppress the auto-ubiquitination of CRBN which is required for limb outgrowth. This hypothesis contributes to the understanding of thalidomide teratogenicity and may also link with its antitumor effects [25]. Recently, Zhu and colleagues tested the role of CRBN for thalidomide’s function in myeloma cells. Surprisingly, CRBN was shown to be an essential requirement for the anticancer properties of thalidomide analogs and the depletion of CRBN render myeloma cells resistance to these drugs [22, 71]. Very recently, Broyl and colleagues also provided clinical evidence that high levels of CRBN expression significantly associated with longer progression-free survival in patients with newly diagnosed multiple myeloma with thalidomide maintenance [72].
Therefore, numerous mechanisms underlie the anticancer effects of thalidomide, however, the manner by which these pathways are interconnected remains to be investigated. In addition, whether other mechanisms are integrally involved in the anti-tumor properties of thalidomide also await further investigation.
3. TALIDOMIDE ANALOGS
Thalidomide is a glutamic acid derivative. It is a racemic mixture of S (−) and R (+) enantiomers (Fig. 1). Interestingly, these two enantiomers are associated with distinct clinical properties: the S (−) enantiomer is responsible for the teratogenicity while the R (+) isoform is accountable for the sedative effects. However, the purification of R (+) isoform is not feasible owing to the rapid inter-conversion of the two enantiomers under physiological conditions [73, 74]. Because of this limitation, efforts were directed at synthesis of thalidomide derivatives with enhanced activity and limited side-effects. Thalidomide analogs can be classified into two categories: Immunomodulatory class (designated IMiDs) and Selective cytokine inhibitory class (designated SelCiDs).
3.1. IMiDs in Cancer Therapy
Structurally, IMiDs are thalidomide analogs with an amino group added to the fourth carbon of the phthaloyl ring of thalidomide (Fig. 1). Such modification results in enhanced immunomodulatory potency, exemplified by T-cell co-stimulation, TNF-α inhibition and mitogenic properties [42, 43]. Lenalidomide (Revlimid, CC-5013) and Pomalidomide (Pomalyst, CC-4047) are IMiDs that possess 500 and 5,000 times more potency with respect to inhibition of TNF-α synthesis compared to parent molecule, thalidomide [45, 75]. Lenalidomide has been approved by the US FDA in June 2006 for the treatment of relapsed or refractory multiple myeloma; its use in combination with dexamethasone was approved by the European Medicines Agency (EMA) in 2007 [76]. Notably, although side effects, such as neutropenia, remain an issue, patients treated with lenalidomide show substantially less frequent adverse effects commonly seen by administration of thalidomide [77, 78]. Lenalidomide is currently undergoing clinical trial for treating Hodgkin’s lymphoma, non-Hodgkin’s lymphoma, follicular lymphoma, chronic lymphocytic leukemia and glioma [79–83]. In February 8, 2013, pomalidomide received US FDA’s approval for the treatment of relapsed and refractory multiple myeloma. [84] By targeting inhibition of tumor growth and angiogenesis, pomalidomide is more efficacious than both thalidomide and lenalidomide [85, 86]. This drug is available under prescription as “Pomalyst”.
3.2. Therapeutic Effects of SelCiDs
The predominant difference between IMiDs and SelCiDs, N-Phthaloyl 3-amino-3-arylpropionic acid analogs of thalidomide, [87] is that SelCiDs lack of T-cell co-stimulatory effects [42]. In addition, SelCiDs class of thalidomide derivatives is not only a potent inhibitor of TNF-α but also an effective inhibitor of phosphodiesterase type 4 (PDE4) [87]. The inhibition of PDE4 by SelCiDs increases the intracellular level of cyclic adenosine monophosphate (cAMP), altering lipopolysaccharide induced cytokines, such as inhibition of TNF-α, by as yet unknown mechanisms. A specific Sel-CiD analog called SelCiD-3 was recently shown to reduce tumor cell viability in a variety of solid tumor lines but had no effect on non-neoplastic cells [65]. Apremilast, another SelCiD analog (Fig. 1), is being extensively tested in clinical trials for efficacy in treating chronic inflammatory diseases, such as rheumatoid arthritis and psoriasis [88, 89].
CONCLUSION AND FUTURE DIRECTIONS
Over the past half century, thalidomide underwent a remarkable transformation, changing from a worldwide infamous teratogen into a valuable compound for the treatment of ENL and multiple myeloma. Moreover, important mechanistic leads have emerged from the studies of its analogs. Collectively, they point to a drug prototype harnessing promising potential in providing beneficial effects in inflammatory and malignant diseases and conditions.
Despite the multiple mechanisms of action for thalidomide’s therapeutic effects discussed previously, the exact mode of thalidomide’s action remains to be completely unraveled. None of the hypotheses advanced to explain thalidomide’s teratogenicity can satisfactorily resolve the tissue specificity of thalidomide’s action. Similarly, while several mechanisms have been proposed for the action mode of thalidomide in cancer, interconnections between the different proposed pathways for thalidomide’s therapeutic effects in cancer require further investigation. Recent identification of the binding target of thalidomide for both teratogenicity and anticancer effects has shed some additional lights for new directions on its mechanistic study. An unanswered question of considerable importance is how thalidomide inhibits the ubiquitination of CRBN. Development of new analogs of thalidomide possessing improved efficacy and reduced toxicity is also imperative. In this connection, it is notable that Shoji and co-workers have recently synthesized a modified DNA aptamer that can enantio-selectively bind to the(R)-thalidomide derivative only. Such aptamer may have the potential to be used as a biochemical tool for the analysis and study of the biological action of thalidomide enantiomers [90]. Future studies regarding the inter-conversion and action modes of the two isomers of thalidomide is also of paramount significance as this may enable the use of thalidomide in safer and more effective ways.
Acknowledgments
This study was supported by pilot project grants from Program Project Grants the NCRR (P20 RR020151) and the NIGMS (P20 GM103505 and P30 GM103332-01) from the NIH.
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the NIH, National Center for Research Resources (NCRR), or National Institute of General Medical Sciences (NIGMS).
References
- 1.McBride WG. Thalidomide embryopathy. Teratology. 1977;16(1):79–82. doi: 10.1002/tera.1420160113. [DOI] [PubMed] [Google Scholar]
- 2.Zwingenberger K, Wnendt S. Immunomodulation by thalidomide: systematic review of the literature and of unpublished observations. J Inflamm (Lond) 1995;46(4):177–211. [PubMed] [Google Scholar]
- 3.Kelsey FO. Thalidomide update: regulatory aspects. Teratology. 1988;38(3):221–226. doi: 10.1002/tera.1420380305. [DOI] [PubMed] [Google Scholar]
- 4.Sheskin J. The treatment of lepra reaction in lepromatous leprosy. Fifteen years’ experience with thalidomide. Int J Dermatol. 1980;19(6):318–322. doi: 10.1111/j.1365-4362.1980.tb00342.x. [DOI] [PubMed] [Google Scholar]
- 5.Sheskin J. Thalidomide in the Treatment of Lepra Reactions. Clin Pharmacol Ther (St Louis) 1965;6:303–306. doi: 10.1002/cpt196563303. [DOI] [PubMed] [Google Scholar]
- 6.Lovell CR, Hawk JL, Calnan CD, Magnus IA. Thalidomide in actinic prurigo. Brit J Dermatol. 1983;108(4):467–471. doi: 10.1111/j.1365-2133.1983.tb04601.x. [DOI] [PubMed] [Google Scholar]
- 7.Calabrese L, Fleischer AB. Thalidomide: current and potential clinical applications. Am J Med. 2000;108(6):487–495. doi: 10.1016/s0002-9343(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 8.Mujagic H, Chabner BA, Mujagic Z. Mechanisms of action and potential therapeutic uses of thalidomide. Croat Med J. 2002;43(3):274–285. [PubMed] [Google Scholar]
- 9.Reyes-Teran G, Sierra-Madero JG, Martinez del Cerro V, Arroyo-Figueroa H, Pasquetti A, Calva JJ, Ruiz-Palacios GM. Effects of thalidomide on HIV-associated wasting syndrome: a randomized, double-blind, placebo-controlled clinical trial. AIDS. 1996;10(13):1501–1507. doi: 10.1097/00002030-199611000-00007. [DOI] [PubMed] [Google Scholar]
- 10.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci USA. 1994;91(9):4082–4085. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singhal S, Mehta J, Desikan R, Ayers D, Roberson P, Eddlemon P, Munshi N, Anaissie E, Wilson C, Dhodapkar M, Zeddis J, Barlogie B. Antitumor activity of thalidomide in refractory multiple myeloma. New Engl J Med. 1999;341(21):1565–1571. doi: 10.1056/NEJM199911183412102. [DOI] [PubMed] [Google Scholar]
- 12.Drake MJ, Robson W, Mehta P, Schofield I, Neal DE, Leung HY. An open-label phase II study of low-dose thalidomide in androgen-independent prostate cancer. Brit J Cancer. 2003;88(6):822–827. doi: 10.1038/sj.bjc.6600817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marx GM, Pavlakis N, McCowatt S, Boyle FM, Levi JA, Bell DR, Cook R, Biggs M, Little N, Wheeler HR. Phase II study of thalidomide in the treatment of recurrent glioblastoma multiforme. J Neuro-Oncol. 2001;54(1):31–38. doi: 10.1023/a:1012554328801. [DOI] [PubMed] [Google Scholar]
- 14.Figg WD, Dahut W, Duray P, Hamilton M, Tompkins A, Steinberg SM, Jones E, Premkumar A, Linehan WM, Floeter MK, Chen CC, Dixon S, Kohler DR, Kruger EA, Gubish E, Pluda JM, Reed E. A randomized phase II trial of thalidomide an angiogenesis inhibitor in patients with androgen-independent prostate cancer. Clin Cancer Res. 2001;7(7):1888–1893. [PubMed] [Google Scholar]
- 15.Eleutherakis-Papaiakovou V, Bamias A, Dimopoulos MA. Thalidomide in cancer medicine. Ann Oncol. 2004;15(8):1151–1160. doi: 10.1093/annonc/mdh300. [DOI] [PubMed] [Google Scholar]
- 16.Kesari S, Schiff D, Henson JW, Muzikansky A, Gigas DC, Doherty L, Batchelor TT, Longtine JA, Ligon KL, Weaver S, Laforme A, Ramakrishna N, Black PM, Drappatz J, Ciampa A, Folkman J, Kieran M, Wen PY. Phase II study of temozolomide, thalidomide, and celecoxib for newly diagnosed glioblastoma in adults. J Neuro-Oncol. 2008;10(3):300–308. doi: 10.1215/15228517-2008-005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute, N.C. information, C.D, editor. 2006 http://www.cancer.gov/cancertopics/druginfo/fda-thalidomide.
- 18.Zeldis JB, Williams BA, Thomas SD, Elsayed ME. S.T.E.P.S.: a comprehensive program for controlling and monitoring access to thalidomide. Clin Ther. 1999;21(2):319–330. doi: 10.1016/s0149-2918(00)88289-2. [DOI] [PubMed] [Google Scholar]
- 19.Stephens TD, Bunde CJ, Fillmore BJ. Mechanism of action in thalidomide teratogenesis. Biochem Pharmacol. 2000;59(12):1489–1499. doi: 10.1016/s0006-2952(99)00388-3. [DOI] [PubMed] [Google Scholar]
- 20.Stephens TD, Fillmore BJ. Hypothesis: thalidomide embryopathy-proposed mechanism of action. Teratology. 2000;61(3):189–195. doi: 10.1002/(SICI)1096-9926(200003)61:3<189::AID-TERA6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 21.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1(1):27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 22.Folkman J. Seminars in Medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. New Engl J Med. 1995;333(26):1757–1763. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]
- 23.Joussen AM, Germann T, Kirchhof B. Effect of thalidomide and structurally related compounds on corneal angiogenesis is comparable to their teratological potency. Graefes Arch Clin Exp Ophthalmol. 1999;237(12):952–961. doi: 10.1007/s004170050330. [DOI] [PubMed] [Google Scholar]
- 24.Therapontos C, Erskine L, Gardner ER, Figg WD, Vargesson N. Thalidomide induces limb defects by preventing angiogenic outgrowth during early limb formation. Proc Natl Acad Sci USA. 2009;106(21):8573–8578. doi: 10.1073/pnas.0901505106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ito T, Ando H, Suzuki T, Ogura T, Hotta K, Imamura Y, Yamaguchi Y, Handa H. Identification of a primary target of thalidomide teratogenicity. Science. 2010;327(5971):1345–1350. doi: 10.1126/science.1177319. [DOI] [PubMed] [Google Scholar]
- 26.Yabu T, Tomimoto H, Taguchi Y, Yamaoka S, Igarashi Y, Okazaki T. Thalidomide-induced antiangiogenic action is mediated by ceramide through depletion of VEGF receptors, and is antagonized by sphingosine-1-phosphate. Blood. 2005;106(1):125–134. doi: 10.1182/blood-2004-09-3679. [DOI] [PubMed] [Google Scholar]
- 27.Verheul HM, Panigrahy D, Yuan J, D’Amato RJ. Combination oral antiangiogenic therapy with thalidomide and sulindac inhibits tumour growth in rabbits. Brit J Cancer. 1999;79(1):114–118. doi: 10.1038/sj.bjc.6690020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bertolini F, Mingrone W, Alietti A, Ferrucci PF, Cocorocchio E, Peccatori F, Cinieri S, Mancuso P, Corsini C, Burlini A, Zucca E, Martinelli G. Thalidomide in multiple myeloma, myelodysplastic syndromes and histiocytosis. Analysis of clinical results and of surrogate angiogenesis markers. Ann Oncol. 2001;12(7):987–990. doi: 10.1023/a:1011141009812. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Liu X, Wang J, Wang Z, Jiang W, Reed E, Zhang Y, Liu Y, Li QQ. Effects of thalidomide on the expression of angiogenesis growth factors in human A549 lung adenocarcinoma cells. Int J Mol Med. 2003;11(6):785–790. [PubMed] [Google Scholar]
- 30.Vasvari GP, Dyckhoff G, Kashfi F, Lemke B, Lohr J, Helmke BM, Schirrmacher V, Plinkert PK, Beckhove P, Herold-Mende CC. Combination of thalidomide and cisplatin in an head and neck squamous cell carcinomas model results in an enhanced antiangiogenic activity in vitro and in vivo. Int J Cancer. 2007;121(8):1697–1704. doi: 10.1002/ijc.22867. [DOI] [PubMed] [Google Scholar]
- 31.Steins MB, Bieker R, Padro T, Kessler T, Kienast J, Berdel WE, Mesters RM. Thalidomide for the treatment of acute myeloid leukemia. Leukemia lymphoma. 2003;44(9):1489–1493. doi: 10.3109/10428190309178769. [DOI] [PubMed] [Google Scholar]
- 32.Fujita J, Mestre JR, Zeldis JB, Subbaramaiah K, Dannenberg AJ. Thalidomide and its analogs inhibit lipopolysaccharide-mediated Iinduction of cyclooxygenase-2. Clin Cancer Res. 2001;7(11):3349–3355. [PubMed] [Google Scholar]
- 33.Chang SH, Liu CH, Conway R, Han DK, Nithipatikom K, Trifan OC, Lane TF, Hla T. Role of prostaglandin E2-dependent angiogenic switch in cyclooxygenase 2-induced breast cancer progression. Proc Natl Acad Sci USA. 2004;101(2):591–596. doi: 10.1073/pnas.2535911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamada M, Kawai M, Kawai Y, Mashima Y. The effect of selective cyclooxygenase-2 inhibitor on corneal angiogenesis in the rat. Curr Eye Res. 1999;19(4):300–304. doi: 10.1076/ceyr.19.4.300.5301. [DOI] [PubMed] [Google Scholar]
- 35.Strasser K, Ludwig H. Thalidomide treatment in multiple myeloma. Blood Rev. 2002;16(4):207–215. doi: 10.1016/s0268-960x(02)00031-0. [DOI] [PubMed] [Google Scholar]
- 36.Neben K, Moehler T, Kraemer A, Benner A, Egerer G, Ho AD, Goldschmidt H. Response to thalidomide in progressive multiple myeloma is not mediated by inhibition of angiogenic cytokine secretion. Brit J Haematol. 2001;115(3):605–608. doi: 10.1046/j.1365-2141.2001.03142.x. [DOI] [PubMed] [Google Scholar]
- 37.Yaccoby S, Johnson CL, Mahaffey SC, Wezeman MJ, Barlogie B, Epstein J. Antimyeloma efficacy of thalidomide in the SCID-hu model. Blood. 2002;100(12):4162–4168. doi: 10.1182/blood-2002-03-0939. [DOI] [PubMed] [Google Scholar]
- 38.Kumar V, Chhibber S. Thalidomide: an old drug with new action. J Chemother. 2011;23(6):326–334. doi: 10.1179/joc.2011.23.6.326. [DOI] [PubMed] [Google Scholar]
- 39.Davies FE, Raje N, Hideshima T, Lentzsch S, Young G, Tai YT, Lin B, Podar K, Gupta D, Chauhan D, Treon SP, Richardson PG, Schlossman RL, Morgan GJ, Muller GW, Stirling DI, Anderson KC. Thalidomide and immunomodulatory derivatives augment natural killer cell cytotoxicity in multiple myeloma. Blood. 2001;98(1):210–216. doi: 10.1182/blood.v98.1.210. [DOI] [PubMed] [Google Scholar]
- 40.Kawamata A, Ito D, Odani T, Isobe T, Iwase M, Hatori M, Nagumo M. Thalidomide suppresses melanoma growth by activating natural killer cells in mice. Oncol Rep. 2006;16(6):1231–1236. [PubMed] [Google Scholar]
- 41.von Moos R, Stolz R, Cerny T, Gillessen S. Thalidomide: from tragedy to promise. Swiss Med Wkly. 2003;133(5–6):77–87. doi: 10.4414/smw.2003.09947. [DOI] [PubMed] [Google Scholar]
- 42.Corral LG, Haslett PA, Muller GW, Chen R, Wong LM, Ocampo CJ, Patterson RT, Stirling DI, Kaplan G. Differential cytokine modulation and T cell activation by two distinct classes of thalidomide analogs that are potent inhibitors of TNF-alpha. J Immunol. 1999;163(1):380–386. [PubMed] [Google Scholar]
- 43.Corral LG, Kaplan G. Immunomodulation by thalidomide and thalidomide analogs. Ann Rheum Dis. 1999;58(Suppl 1):I107–113. doi: 10.1136/ard.58.2008.i107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haslett PA, Klausner JD, Makonkawkeyoon S, Moreira A, Metatratip P, Boyle B, Kunachiwa W, Maneekarn N, Vongchan P, Corral LG, Elbeik T, Shen Z, Kaplan G. Thalidomide stimulates T cell responses and interleukin 12 production in HIV-infected patients. AIDS Res Hum Retrov. 1999;15(13):1169–1179. doi: 10.1089/088922299310269. [DOI] [PubMed] [Google Scholar]
- 45.Muller GW, Chen R, Huang SY, Corral LG, Wong LM, Patterson RT, Chen Y, Kaplan G, Stirling DI. Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production. Bioorg Med Chem Lett. 1999;9(11):1625–1630. doi: 10.1016/s0960-894x(99)00250-4. [DOI] [PubMed] [Google Scholar]
- 46.Beckner ME. Factors promoting tumor angiogenesis. Cancer Invest. 1999;17(8):594–623. doi: 10.3109/07357909909032845. [DOI] [PubMed] [Google Scholar]
- 47.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104(4):487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 48.Thompson MA, Witzig TE, Kumar S, Timm MM, Haug J, Fonseca R, Greipp PR, Lust JA, Rajkumar SV. Plasma levels of tumour necrosis factor alpha and interleukin-6 predict progression-free survival following thalidomide therapy in patients with previously untreated multiple myeloma. Brit J Haematol. 2003;123(2):305–308. doi: 10.1046/j.1365-2141.2003.04605.x. [DOI] [PubMed] [Google Scholar]
- 49.Moreira AL, Sampaio EP, Zmuidzinas A, Frindt P, Smith KA, Kaplan G. Thalidomide exerts its inhibitory action on tumor necrosis factor alpha by enhancing mRNA degradation. J Exp Med. 1993;177(6):1675–1680. doi: 10.1084/jem.177.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geitz H, Handt S, Zwingenberger K. Thalidomide selectively modulates the density of cell surface molecules involved in the adhesion cascade. Immunopharmacology. 1996;31(2–3):213–221. doi: 10.1016/0162-3109(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 51.Nogueira AC, Neubert R, Helge H, Neubert D. Thalidomide and the immune system. 3. Simultaneous up- and down-regulation of different integrin receptors on human white blood cells. Life sciences. 1994;55(2):77–92. doi: 10.1016/0024-3205(94)90099-x. [DOI] [PubMed] [Google Scholar]
- 52.Keifer JA, Guttridge DC, Ashburner BP, Baldwin AS., Jr Inhibition of NF-kappa B activity by thalidomide through suppression of IkappaB kinase activity. J Biol Chem. 2001;276(25):22382–22387. doi: 10.1074/jbc.M100938200. [DOI] [PubMed] [Google Scholar]
- 53.Majumdar S, Lamothe B, Aggarwal BB. Thalidomide suppresses NF-kappa B activation induced by TNF and H2O2 but not that activated by ceramide lipopolysaccharides or phorbol ester. J Immunol. 2002;168(6):2644–2651. doi: 10.4049/jimmunol.168.6.2644. [DOI] [PubMed] [Google Scholar]
- 54.Parman T, Wiley MJ, Wells PG. Free radical-mediated oxidative DNA damage in the mechanism of thalidomide teratogenicity. Nat Med. 1999;5(5):582–585. doi: 10.1038/8466. [DOI] [PubMed] [Google Scholar]
- 55.Hansen JM, Harris C. A novel hypothesis for thalidomide-induced limb teratogenesis: redox misregulation of the NF-kappaB pathway. Antioxid Redox Sign. 2004;6(1):1–14. doi: 10.1089/152308604771978291. [DOI] [PubMed] [Google Scholar]
- 56.Hansen JM, Gong SG, Philbert M, Harris C. Misregulation of gene expression in the redox-sensitive NF-kappab-dependent limb outgrowth pathway by thalidomide. Dev Dynam. 2002;225(2):186–194. doi: 10.1002/dvdy.10150. [DOI] [PubMed] [Google Scholar]
- 57.Hansen JM, Carney EW, Harris C. Differential alteration by thalidomide of the glutathione content of rat vs. rabbit conceptuses in vitro. Reprod Toxicol. 1999;13(6):547–554. doi: 10.1016/s0890-6238(99)00053-2. [DOI] [PubMed] [Google Scholar]
- 58.Lee MS, Lowe GN, Strong DD, Wergedal JE, Glackin CA. TWIST a basic helix-loop-helix transcription factor can regulate the human osteogenic lineage. J Cell Biochem. 1999;75(4):566–577. doi: 10.1002/(sici)1097-4644(19991215)75:4<566::aid-jcb3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 59.Knobloch J, Shaughnessy JD, Jr, Ruther U. Thalidomide induces limb deformities by perturbing the Bmp/Dkk1/Wnt signaling pathway. FASEB J. 2007;21(7):1410–1421. doi: 10.1096/fj.06-7603com. [DOI] [PubMed] [Google Scholar]
- 60.Lentzsch S, LeBlanc R, Podar K, Davies F, Lin B, Hideshima T, Catley L, Stirling DI, Anderson KC. Immunomodulatory analogs of thalidomide inhibit growth of Hs Sultan cells and angiogenesis in vivo. Leukemia. 2003;17(1):41–44. doi: 10.1038/sj.leu.2402745. [DOI] [PubMed] [Google Scholar]
- 61.Hitzler JK, Martinez-Valdez H, Bergsagel DB, Minden MD, Messner HA. Role of interleukin-6 in the proliferation of human multiple myeloma cell lines OCI-My 1 to 7 established from patients with advanced stage of the disease. Blood. 1991;78(8):1996–2004. [PubMed] [Google Scholar]
- 62.Hideshima T, Chauhan D, Shima Y, Raje N, Davies FE, Tai YT, Treon SP, Lin B, Schlossman RL, Richardson P, Muller G, Stirling DI, Anderson KC. Thalidomide and its analogs overcome drug resistance of human multiple myeloma cells to conventional therapy. Blood. 2000;96(9):2943–2950. [PubMed] [Google Scholar]
- 63.Hideshima T, Chauhan D, Podar K, Schlossman RL, Richardson P, Anderson KC. Novel therapies targeting the myeloma cell and its bone marrow microenvironment. Semin Oncol. 2001;28(6):607–612. doi: 10.1016/s0093-7754(01)90033-8. [DOI] [PubMed] [Google Scholar]
- 64.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Richardson PG, Hideshima T, Munshi NC, Treon SP, Anderson KC. Apoptotic signaling induced by immunomodulatory thalidomide analogs in human multiple myeloma cells: therapeutic implications. Blood. 2002;99(12):4525–4530. doi: 10.1182/blood.v99.12.4525. [DOI] [PubMed] [Google Scholar]
- 65.Marriott JB, Clarke IA, Czajka A, Dredge K, Childs K, Man HW, Schafer P, Govinda S, Muller GW, Stirling DI, Dalgleish AG. A novel subclass of thalidomide analog with anti-solid tumor activity in which caspase-dependent apoptosis is associated with altered expression of bcl-2 family proteins. Cancer Res. 2003;63(3):593–599. [PubMed] [Google Scholar]
- 66.Keller M, Sollberger G, Beer HD. Thalidomide inhibits activation of caspase-1. J Immunol. 2009;183(9):5593–5599. doi: 10.4049/jimmunol.0900476. [DOI] [PubMed] [Google Scholar]
- 67.Anderson KC. Lenalidomide and thalidomide: mechanisms of action--similarities and differences. Semin Hematol. 2005;42(4 Suppl 4):S3–S8. doi: 10.1053/j.seminhematol.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 68.Sakamoto S, Kabe Y, Hatakeyama M, Yamaguchi Y, Handa H. Development and application of high-performance affinity beads: toward chemical biology and drug discovery. Chem Rec. 2009;9(1):66–85. doi: 10.1002/tcr.20170. [DOI] [PubMed] [Google Scholar]
- 69.Shimizu N, Sugimoto K, Tang J, Nishi T, Sato I, Hiramoto M, Aizawa S, Hatakeyama M, Ohba R, Hatori H, Yoshikawa T, Suzuki F, Oomori A, Tanaka H, Kawaguchi H, Watanabe H, Handa H. High-performance affinity beads for identifying drug receptors. Nat Biotechnol. 2000;18(8):877–881. doi: 10.1038/78496. [DOI] [PubMed] [Google Scholar]
- 70.Nishio K, Masaike Y, Ikeda M, Narimatsu H, Gokon N, Tsubouchi S, Hatakeyama M, Sakamoto S, Hanyu N, Sandhu A, Kawaguchi H, Abe M, Handa H. Development of novel magnetic nano-carriers for high-performance affinity purification. Colloid Surface B. 2008;64(2):162–169. doi: 10.1016/j.colsurfb.2008.01.013. [DOI] [PubMed] [Google Scholar]
- 71.Zhu YX, Braggio E, Shi CX, Bruins LA, Schmidt JE, Van Wier S, Chang XB, Bjorklund CC, Fonseca R, Bergsagel PL, Orlowski RZ, Stewart AK. Cereblon expression is required for the antimyeloma activity of lenalidomide and pomalidomide. Blood. 2011;118(18):4771–4779. doi: 10.1182/blood-2011-05-356063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Broyl A, Kuiper R, van Duin M, van der Holt B, el Jarari L, Bertsch U, Zweegman S, Buijs A, Hose D, Lokhorst HM, Goldschmidt H, Sonneveld P. High cereblon expression is associated with better survival in patients with newly diagnosed multiple myeloma treated with thalidomide maintenance. Blood. 2013;121(4):624–627. doi: 10.1182/blood-2012-06-438101. [DOI] [PubMed] [Google Scholar]
- 73.Eriksson T, Bjorkman S, Roth B, Fyge A, Hoglund P. Stereospecific determination, chiral inversion in vitro and pharmacokinetics in humans of the enantiomers of thalidomide. Chirality. 1995;7(1):44–52. doi: 10.1002/chir.530070109. [DOI] [PubMed] [Google Scholar]
- 74.Bartlett JB, Dredge K, Dalgleish AG. The evolution of thalidomide and its IMiD derivatives as anticancer agents. Nat Rev Cancer. 2004;4(4):314–322. doi: 10.1038/nrc1323. [DOI] [PubMed] [Google Scholar]
- 75.Muller GW, Corral LG, Shire MG, Wang H, Moreira A, Kaplan G, Stirling DI. Structural modifications of thalidomide produce analogs with enhanced tumor necrosis factor inhibitory activity. J Med Chem. 1996;39(17):3238–3240. doi: 10.1021/jm9603328. [DOI] [PubMed] [Google Scholar]
- 76.Institue, N.C. Health, N.I.o., editor 2006 http://www.cancer.gov/cancertopics/druginfo/fda-lenalidomide.
- 77.Sharma RA, Steward WP, Daines CA, Knight RD, O’Byrne KJ, Dalgleish AG. Toxicity profile of the immunomodulatory thalidomide analog, lenalidomide: phase I clinical trial of three dosing schedules in patients with solid malignancies. Eur J Cancer. 2006;42(14):2318–2325. doi: 10.1016/j.ejca.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 78.McCarthy PL, Owzar K, Hofmeister CC, Hurd DD, Hassoun H, Richardson PG, Giralt S, Stadtmauer EA, Weisdorf DJ, Vij R, Moreb JS, Callander NS, Van Besien K, Gentile T, Isola L, Maziarz RT, Gabriel DA, Bashey A, Landau H, Martin T, Qazilbash MH, Levitan D, McClune B, Schlossman R, Hars V, Postiglione J, Jiang C, Bennett E, Barry S, Bressler L, Kelly M, Seiler M, Rosenbaum C, Hari P, Pasquini MC, Horowitz MM, Shea TC, Devine SM, Anderson KC, Linker C. Lenalidomide after stem-cell transplantation for multiple myeloma. New Engl J Med. 2012;366(19):1770–1781. doi: 10.1056/NEJMoa1114083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Boll B, Borchmann P, Topp MS, Hanel M, Reiners KS, Engert A, Naumann R. Lenalidomide in patients with refractory or multiple relapsed Hodgkin lymphoma. Brit J Haematol. 2010;148(3):480–482. doi: 10.1111/j.1365-2141.2009.07963.x. [DOI] [PubMed] [Google Scholar]
- 80.Wiernik PH, Lossos IS, Tuscano JM, Justice G, Vose JM, Cole CE, Lam W, McBride K, Wride K, Pietronigro D, Takeshita K, Ervin-Haynes A, Zeldis JB, Habermann TM. Lenalidomide monotherapy in relapsed or refractory aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2008;26(30):4952–4957. doi: 10.1200/JCO.2007.15.3429. [DOI] [PubMed] [Google Scholar]
- 81.Chiappella A, Vitolo U. Lenalidomide in diffuse large B-cell lymphomas. Adv Hematol. 2012;2012:498342. doi: 10.1155/2012/498342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Giannopoulos K, Mertens D, Stilgenbauer S. Treating chronic lymphocytic leukemia with thalidomide and lenalidomide. Expert Opin Pharmacother. 2011;12(18):2857–2864. doi: 10.1517/14656566.2011.635644. [DOI] [PubMed] [Google Scholar]
- 83.Fine HA, Kim L, Albert PS, Duic JP, Ma H, Zhang W, Tohnya T, Figg WD, Royce C. A phase I trial of lenalidomide in patients with recurrent primary central nervous system tumors. Clin Cancer Res. 2007;13(23):7101–7106. doi: 10.1158/1078-0432.CCR-07-1546. [DOI] [PubMed] [Google Scholar]
- 84.Institute, N.C. Health, N.I.o, editor. 2013 http://www.cancer.gov/cancertopics/druginfo/fda-pomalidomide.
- 85.Lentzsch S, Rogers MS, LeBlanc R, Birsner AE, Shah JH, Treston AM, Anderson KC, D’Amato RJ. S-3-Amino-phthalimido-glutarimide inhibits angiogenesis and growth of B-cell neoplasias in mice. Cancer Res. 2002;62(8):2300–2305. [PubMed] [Google Scholar]
- 86.D’Amato RJ, Lentzsch S, Anderson KC, Rogers MS. Mechanism of action of thalidomide and 3-aminothalidomide in multiple myeloma. Semin Oncol. 2001;28(6):597–601. doi: 10.1053/sonc.2001.28601. [DOI] [PubMed] [Google Scholar]
- 87.Muller GW, Shire MG, Wong LM, Corral LG, Patterson RT, Chen Y, Stirling DI. Thalidomide analogs and PDE4 inhibition. Bioorg Med Chem Lett. 1998;8(19):2669–2674. doi: 10.1016/s0960-894x(98)00475-2. [DOI] [PubMed] [Google Scholar]
- 88.Schett G, Wollenhaupt J, Papp K, Joos R, Rodrigues JF, Vessey A, Hu A, Stevens R, de Vlam KL. Oral apremilast in the treatment of active psoriatic arthritis: Results of a multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2012 doi: 10.1002/art.34627. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 89.Schett G, Sloan VS, Stevens RM, Schafer P. Apremilast: a novel PDE4 inhibitor in the treatment of autoimmune and inflammatory diseases. Ther Adv Musculoskelet Dis. 2010;2(5):271–278. doi: 10.1177/1759720X10381432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shoji A, Kuwahara M, Ozaki H, Sawai H. Modified DNA aptamer that binds the (R)-isomer of a thalidomide derivative with high enantioselectivity. J Am Chem Soc. 2007;129(5):1456–1464. doi: 10.1021/ja067098n. [DOI] [PubMed] [Google Scholar]