Abstract
Objectives
To evaluate incidence trends and timing of large-vessel (LV) manifestations in patients with giant cell arteritis (GCA), and, to examine the influence of LV manifestations on survival.
Methods
A population-based incident cohort of patients diagnosed with GCA between 1950 and 2004 was used. LV involvement was defined as large artery stenosis or aortic aneurysm/dissection that developed in the 1 year prior to GCA diagnosis or any time thereafter. Patients were followed until death or December 31, 2009.
Results
The study included 204 patients, 80% women, mean age at diagnosis of GCA 76.0 years (± 8.2 years). Median length of follow-up was 8.8 years. The cumulative incidence of any LV manifestation at 10 years was 24.9% for patients diagnosed with GCA between 1980–2004 compared to 8.3% for patients diagnosed with GCA between 1950 and 1979. The incidence of any LV event was high within the first year of GCA diagnosis. The incidence of aortic aneurysm/dissection increased 5 years after GCA diagnosis.
Compared to the general population, survival was decreased in patients with an aortic aneurysm/dissection (p<0.001) but not in patients with large-artery stenosis (p=0.11). Patients with GCA and aortic manifestations had higher than expected number of deaths from cardiovascular and pulmonary causes compared to the general population. Among patients with GCA, aortic manifestations were associated with increased mortality (HR: 3.4; 95% CI: 2.2, 5.4).
Conclusions
Screening for aortic aneurysms should be considered in all patients with GCA with vigilance 5 years after incidence. Aortic aneurysm/dissection is associated with increased mortality in GCA.
Keywords: Giant cell arteritis, aortic aneurysm, aortic dissection, large-artery stenosis, survival
INTRODUCTION
Giant cell arteritis (GCA) is a chronic, granulomatous medium-size and large-vessel vasculitis that affects the aorta and its branches. The incidence of any large-vessel manifestation such as large-artery stenosis, aortic aneurysm, or aortic dissection is estimated at 30.5 events per 1,000 person-years at risk.[1] Patients with GCA have a 17-fold increased risk of developing thoracic aortic aneurysm compared to the general population.[2] It has been recommended that patients with GCA be screened for this manifestation but the optimal imaging modality which should be used and frequency with which screening should be performed are unknown.[2,3] With the increased use of imaging modalities, involvement of the aorta and, or its branches is frequently detected in patients with newly diagnosed GCA with estimates ranging from 22% to 85%.[4–9] Predictors of large-vessel involvement in GCA remain poorly understood. While overall survival in patients with GCA is similar to the general population, a previous study found that patients with thoracic aortic dissection have higher mortality.[10–16]
Our study aims were to: 1) to evaluate predictors, time-trends and timing of LV manifestations in a well defined cohort of patients with GCA; 2) to assess survival and cause-specific mortality in the patients with GCA and LV disease compared to the general population and 3) to evaluate the association between the different LV manifestations in patients with GCA and survival.
PATIENTS AND METHODS
This was a retrospective, population-based cohort study utilizing the resources of the Rochester Epidemiology Project (REP), a unique records-linkage system which allows ready access to the medical records of all health care providers for the population of Olmsted County, Minnesota.[17] A cohort of patients with GCA diagnosed between 1950–2004 has already been established and previously described.[18,19] All patients in this cohort met the 1990 American College of Rheumatology (ACR) classification criteria for GCA.[20] The complete (inpatient and outpatient) medical records for all patients in this cohort were reviewed. All patients were followed until migration, death or December 31, 2009 (end of study). The study was approved by the institutional review boards at Mayo Clinic and Olmsted Medical Center.
Standardized case report forms were used to abstract data. We collected information on date of diagnosis of GCA, symptoms and laboratory findings at GCA diagnosis, presence of LV involvement, date of diagnosis of LV involvement, method of diagnosis and arteries affected. Incident LV involvement or LV manifestation was defined as large-vessel complications including large-artery stenosis, aortic aneurysm or aortic dissection/rupture detected within 1 year prior to diagnosis of GCA or any time thereafter.[21] “Aortic manifestations” were defined as aortic aneurysm or aortic dissection/rupture. Previously used definitions for large-artery stenosis and aortic aneurysm, rupture and dissection were used for this study as well. The diagnosis of large-vessel disease required confirmation by imaging studies, histopathology or autopsy.[21]
Vital status at the end of the study (December 31, 2009) was recorded for each patient. All subjects (irrespective of residency status) were tracked nationally to ascertain vital status, and death certificates were obtained from the respective states for subjects who died outside of Minnesota. If the patient was deceased, death certificates were reviewed to ascertain the physician-designated causes of death.
Statistical analysis
The cumulative incidence of each LV event adjusted for the competing risk of death was estimated. Poisson regression models were used to model the rates of LV involvement over disease duration. Smoothing splines were used to allow for non-linear time trends. Cox proportional hazards models were used to assess the association of risk factors on the development of large-vessel events. To evaluate secular trends, we divided the cohort into two different study periods: those diagnosed with GCA between 1950 and 1979 and those diagnosed with GCA from 1980 to 2004. The cumulative incidence rates for the two time-periods were compared using methods by Gray.[22]
The distribution of survival times following GCA incidence date was estimated using the Kaplan-Meier method. The expected number of deaths was determined from the National Center for Health Statistics life tables for the United States population, according to the age, sex and calendar year of the GCA cohort. Standardized mortality ratio (SMR) was estimated by dividing the observed number of deaths by the expected number of deaths. Ninety-five percent confidence intervals (CI) for the SMR were calculated assuming that the expected rates were fixed and the observed rates followed a Poisson distribution.
The underlying cause of death was coded from national mortality statistics and grouped according to International Classification of Diseases, 9th Revision (ICD-9) and ICD-10 chapters. Cause-specific expected mortality rates were estimated by applying the age-, sex-, and calendar-year-specific mortality rates from the Minnesota Caucasian population (1980–2002) to the GCA cohort. Cause-specific Minnesota life tables were available until the end of 2002. Therefore, we carried forward the 2002 expected mortality rates to 2009. American Heart Association (AHA) classification was used to categorize death from cardiovascular disease (CVD) into coronary heart disease (CHD), non-CHD diseases of the heart, and non-cardiac circulatory diseases.[23] Survival following LV disease was estimated with each Kaplan-Meier curve starting at date of diagnosis of LV involvement instead of at GCA incidence date. Cox proportional hazards models were used to examine the association between LV involvement and mortality. Time-dependent covariates were used to represent LV involvement, which occurred throughout follow-up.
RESULTS
The cohort
The study population included 204 patients diagnosed with GCA between January 1, 1950 and December 31, 2004. Temporal artery biopsy was positive in 176 (86%) patients. All subjects met the 1990 ACR classification criteria for GCA. Mean age (±SD) at diagnosis of GCA was 76 (±8.2) years. The cohort was predominantly female (163 subjects; 80%). Median duration of follow-up was 8.8 years (total 1,996 person-years). Baseline demographics for the cohort are available in the online Supplementary Table S1.
LV manifestations and predictors
Fifty-six patients developed 63 LV events - 36 aortic manifestations and 27 large-artery stenosis. Aortic aneurysms were diagnosed in 33 patients (isolated thoracic aneurysm in 14 cases, isolated abdominal aneurysm in 6 cases and both thoracic and abdominal aneurysms in 13 cases). The cumulative incidence (± standard error) of any clinically evident LV manifestation, after adjusting for competing risk of death, was 19.9% (±2.9) at 10 years. Clinical and treatment variables as predictors of large artery stenosis or aortic aneurysm/dissection are evaluated in Table 1.
Table 1.
Clinical variables and their association with large-artery stenosis or aortic aneurysm/dissection in 204 patients with giant cell arteritis (GCA)*.
| Variable | Large-artery stenosis Hazard ratio (95% CI)** |
Aortic aneurysm/ dissection Hazard ratio (95% CI)** |
|---|---|---|
| Age at diagnosis GCA, per 10 year increase |
0.7 (0.4, 1.2) | 1.4 (0.9, 2.1) |
| Sex, female | 1.5 (0.5, 4.2) | 1.2 (0.5, 3.2) |
| Smoking, ever | 2.4 (1.04, 5.4) | 1.8 (0.9, 3.8) |
| Headache at diagnosis GCA | 0.7 (0.3, 1.6) | 0.9 (0.4, 2.0) |
| Jaw claudication at diagnosis GCA | 0.9 (0.4, 1.9) | 1.1 (0.6, 2.2) |
| Scalp tenderness at diagnosis GCA | 1.3 (0.6, 2.9) | 1.6 (0.8, 3.1) |
| Tender temporal artery at diagnosis GCA | 1.4 (0.6, 3.1) | 1.0 (0.4, 2.3) |
| Bruit at diagnosis GCA | 11.7 (3.6, 37.4) | 0.8 (0.1, 6.0) |
| PMR symptoms at diagnosis GCA | 0.5 (0.2, 1.4) | 1.1 (0.5, 2.2) |
| Hemoglobin at diagnosis GCA, per 1 g/dL decrease |
0.9 (0.7, 1.2) | 0.9 (0.7, 1.3) |
| ESR at diagnosis GCA, per 10 mm/hour increase |
1.0 (0.9, 1.1) | 1.0 (0.9, 1.1) |
| Start dose glucocorticoids, per 10 mg increase |
0.9 (0.7, 1.2) | 0.9 (0.7, 1.1) |
| Hypertension before GCA incidence | 1.5 (0.7, 3.4) | 0.8 (0.4, 1.7) |
| Coronary artery disease before GCA incidence |
1.4 (0.5, 4.4) | 5.3 (2.2, 13.1) |
| Hyperlipidemia before GCA incidence | 1.2 (0.5, 2.9) | 1.6 (0.7, 3.5) |
| TIA/stroke before GCA incidence | 3.5 (1.3, 9.6) | 0.8 (0.2, 3.2) |
| Cumulative glucocorticoid dose † | ||
| Low tertile | 1(reference) | 1(reference) |
| Medium tertile | 0.8 (0.3, 2.4) | 4.3 (1.2, 15.7) |
| High tertile | 2.2 (0.5, 9.5) | 3.8 (0.8, 17.4) |
| Number of relapses | 1.2 (1.0, 1.5) | 1.0 (0.9, 1.2) |
Statistically significant values have been bolded.
Adjusted for age, sex and calendar year
CI=confidence intervals; PMR=polymyalgia rheumatica; ESR=erythrocyte sedimentation rate; TIA=transient ischemic attack
for cumulative glucocorticoid exposure, low tertile:≤ 5,000 mg, mid tertile:> 5,000 to ≤ 15,000 mg, and high tertile: > 15,000 mg
Time-trends and timing of LV manifestations
The cumulative incidence of any LV manifestations at 10 years increased significantly, from 8.3% in the cohort diagnosed between 1950–1979 to 24.9% for patients diagnosed between 1980–2004 (p=0.004). While the cumulative incidence of large-artery stenosis and aortic aneurysms increased for patients diagnosed in the earlier decades compared to those diagnosed from 1980–2004, the cumulative incidence of aortic dissection remained unchanged (Table 2). A greater proportion of patients with GCA diagnosed from 1980–2004 underwent echocardiography (p<0.001), abdominal ultrasonography (p = 0.03) and angiography, CT or MRI (p <0.001).
Table 2.
Time-trends in cumulative incidence rates at 10 years of LV involvement in 204 patients with GCA.
| Event | Number of events |
Cumulative incidence* (%) for 1950–1979 patients (± SE) |
Cumulative incidence* (%) for 1980–2004 patients (± SE) |
p-value | Cumulative incidence* (%) for all patients (± SE) |
|---|---|---|---|---|---|
| Any LV manifestation | 56 | 8.3 ± 3.6 | 24.9 ± 3.7 | 0.004 | 19.9 ± 2.9 |
| Large-artery stenosis | 27 | 3.4 ± 2.4 | 14.4 ± 3.0 | 0.026 | 11.1 ± 2.2 |
| Aortic aneurysm | 33 | 3.3 ± 2.3 | 12.0 ± 2.8 | 0.026 | 9.3 ± 2.1 |
| Aortic dissection | 12 | 3.3 ± 2.3 | 5.2 ± 1.9 | 0.53 | 4.6 ± 1.5 |
LV= large vessel; SE= Standard error
All cumulative incidences are at 10 years; events that occurred greater than 1 year prior to incidence of GCA were excluded from the cumulative incidence analysis of that particular event only.
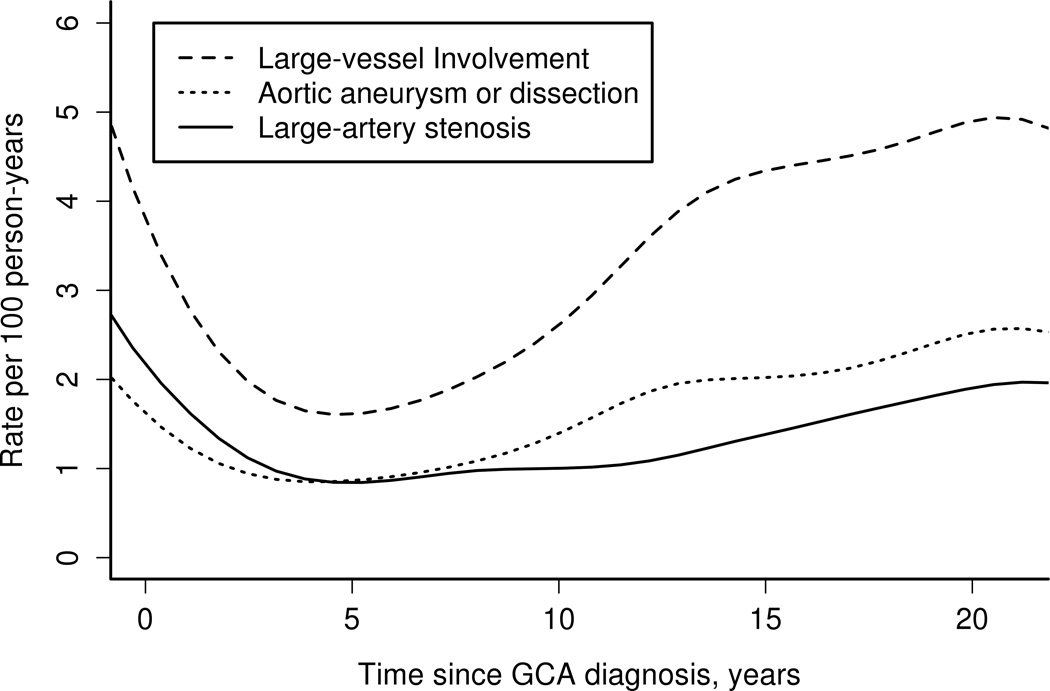
The incidence of LV disease by follow-up from diagnosis of GCA is depicted in Figure 1. The rate of occurrence of any LV disease was high within the first year of GCA incidence (5 events per 100 person-years). The incidence of large-artery stenosis remained relatively constant beyond 5 years from diagnosis of GCA (p-value for trend = 0.77) but the incidence of aortic aneurysm/dissection increased after 5 years (p-value for trend = 0.009).
Figure 1.
Incidence rates of large-vessel involvement by disease duration in patients with giant cell arteritis (GCA).
Survival in patients with GCA compared to the general population
There were 154 deaths. Overall survival for the cohort of 204 patients with GCA was similar to that expected in the general population (log-rank p=0.25). Cause-specific mortality in patients with GCA compared to the general population are available in Table 3.
Table 3.
Cause-specific mortality compared to the general population in the cohort of patients with GCA diagnosed between 1950 and 2004, and, cause-specific mortality compared to the general population in the subset of patients with GCA and aortic manifestations*.
| Cause | Entire cohort of patients with GCA SMR* (95% CI) |
Subset of patients with aortic manifestations SMR* (95% CI) |
|---|---|---|
| All Causes** | 1.0 (0.9, 1.2) | 5.1 (3.4, 7.4) |
| Circulatory System | 1.1 (0.9, 1.4) | 8.7 (5.2, 13.8) |
| CHD | 1.3 (0.95, 1.8) | 4.9 (1.6, 11.3) |
| Non-CHD | 0.9 (0.5, 1.5) | 7.0 (1.9, 18.0) |
| Noncardiac circulatory | 1.1 (0.7, 1.6) | 11.7 (5.3, 22.2) |
| Respiratory System | 1.4 (0.8, 2.2) | 10.7 (3.9, 23.3) |
| Neoplasms | 0.8 (0.5, 1.2) | 1.9 (0.2, 6.8) |
| Digestive System | 2.1 (1.02, 3.9) | 5.6 (0.1, 31.0) |
| Vascular diseases | 4.9 (1.01, 14.4) | |
| GI ulcer/hemorrhage | 3.1 (0.9, 8.0) | |
| Other | 1.1 (0.2, 3.1) | |
| Infectious and Parasitic | 0.4 (0.0, 2.8) | |
| Hematological | 1.3 (0.0, 7.4) | |
| Nervous System | 0.8 (0.2, 1.8) |
Standardized mortality ratio (SMR) was estimated by dividing the observed number of deaths by the expected number of deaths. Expected based on cause-specific mortality of the MN White population in 1980–2002.
CHD=coronary heart disease; SMR=standardized mortality ratio; CI=Confidence interval; GI=gastrointestinal
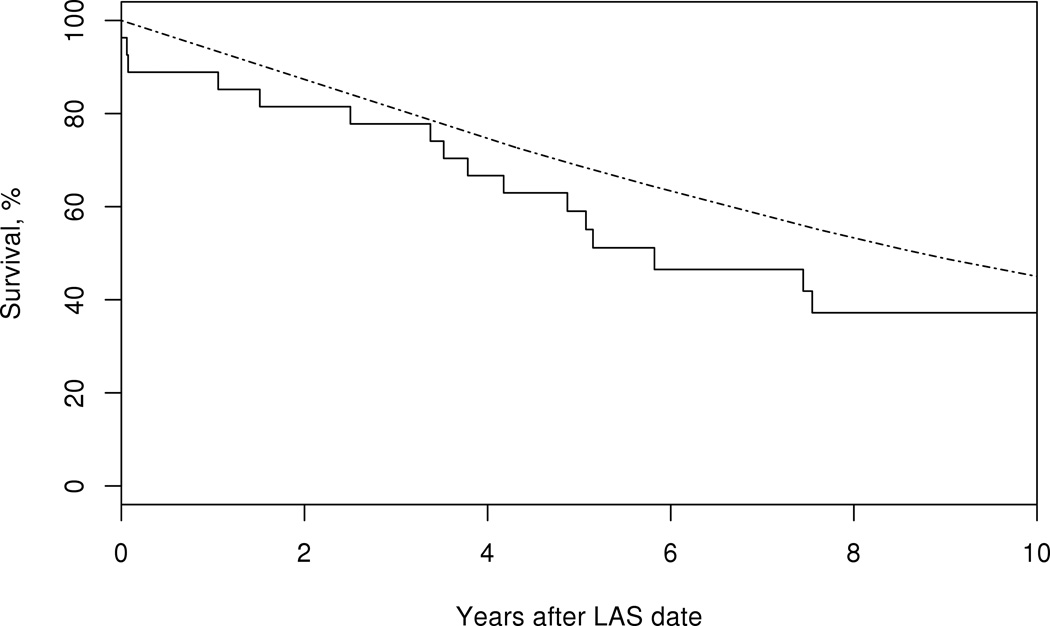
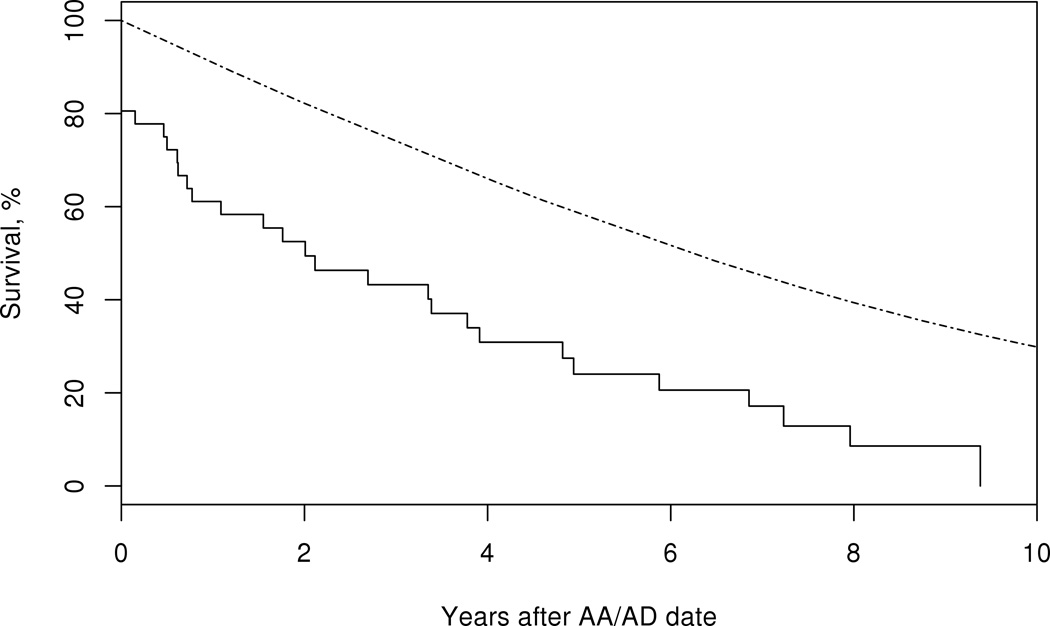
Patients with GCA who developed any LV involvement had decreased survival compared to the general population (logrank p<0.001). While survival in patients with large-artery stenosis was similar to that expected in the general population, patients with aortic aneurysm/dissection experienced a significantly reduced survival (Figure 2). Evaluation of cause-specific mortality in patients with GCA and aortic aneurysm/dissection showed an excess mortality attributed to the circulatory system compared to the general population (Table 3).
Figure 2.
Survival in patients with giant cell arteritis (solid line) who develop large-artery stenosis (LAS) (top panel) logrank p=0.11, or, aortic aneurysm/dissection (AA/AD) (bottom panel) compared to the general population (dotted line), logrank p<0.001.
The influence of LV manifestations on survival among patients with GCA
Among patients with GCA, LV manifestations were associated with increased mortality compared to patients with GCA without LV involvement (HR 2.4; 95% CI 1.6, 3.6). Aortic aneurysm/dissection was associated with increased mortality (HR 3.4; 95% CI 2.2, 5.4). The mortality in patients with GCA and large-artery stenosis was similar to GCA patients without this manifestation (HR 1.5; 95% CI 0.9, 2.5).
Time-trends in mortality were evaluated in patients with large-vessel manifestations and showed no difference in mortality for patients diagnosed with GCA between 1950–1979 compared to those diagnosed from 1980–2004 (Table 4).
Table 4.
Time-trends in mortality in patients with GCA and large-vessel manifestations comparing those diagnosed with GCA between 1980–2004 to those diagnosed between 1950–1979
| Hazard ratio (95% CI) | p-value | |
|---|---|---|
| Any large-vessel manifestation | 0.8 (0.3, 1.8) | 0.56 |
| Large-artery stenosis | 1.4 (0.4, 4.8) | 0.56 |
| Aortic aneurysm | 0.4 (0.1, 1.18) | 0.098 |
| Aortic dissection | 0.1 (0.01, 1.07) | 0.057 |
GCA=Giant cell arteritis; CI=confidence intervals
DISCUSSION
LV manifestations (large-artery stenosis, aortic aneurysm/dissection) were common in patients with GCA and increased over the observation period, likely related to increased use of imaging modalities. The incidence of aortic aneurysm/dissection increased 5 years after diagnosis of GCA and continued to increase throughout the follow-up. The overall survival of patients with GCA was similar to that of the general population. However, aortic aneurysm/dissection was associated with reduced survival in patients with GCA. Compared to the general population, patients with aortic aneurysm/dissection had an excess mortality attributed to cardiovascular and pulmonary causes.
We observed an increased incidence of LV manifestations in patients with GCA. However, the utilization of CT, MR, abdominal ultrasonography and echocardiography also increased for the cohort diagnosed with GCA after 1980 compared to those diagnosed in the earlier decades. This observation may account for the increased incidence of LV manifestations rather than a change in disease expression over time. Additionally, greater physician awareness of the extra-cranial manifestations of GCA over the study period may have led to increased utilization of imaging studies to evaluate for LV disease. While the numbers were small, surprisingly, the incidence of aortic dissection remained fairly stable in this cohort for different time-periods analyzed. Preexisting aortic aneurysm is a risk factor for aortic dissection. With the observed increased use of imaging modalities over the decades and the increased detection of aortic aneurysms, one might have expected aortic dissection to decrease over time. One possibility is that the detection of an aneurysm did not alter the management of the patient. Alternatively, the increased use of more sensitive imaging modalities (echocardiography, CT, MR) in patients diagnosed in the latter decades may have resulted in the detection of aneurysms at a smaller size. If aortic size is a predictor of dissection in GCA, the smaller size at detection may explain why no increase in aortic dissections were observed despite increased incidence of aortic aneurysms. It remains unclear whether patients with GCA differ from non-inflammatory aortic aneurysms where size and rate of growth appear to be major factors in risk of rupture.[24]
TIA or stroke, smoking (ever) and presence of a vascular bruit at diagnosis were the only factors associated with large-artery stenosis in this study, while coronary artery disease was associated with increased risk of aortic aneurysm/dissection. The only other population-based study evaluating predictors of aortic aneurysm/dissection in GCA did not evaluate CAD and its association with this manifestation.[25] The mechanism underlying the increased risk of aortic aneurysm/dissection in patients with GCA who have CAD is unclear. A common underlying pathogenic process such as atherosclerosis may be responsible. However, traditional cardiovascular risk factors such as hypertension, hyperlipidemia or smoking were not associated with increased risk of aortic aneurysm/dissection in this study. While the association we observed may be spurious or due to chance, the magnitude is large and warrants further study.
A better understanding of the timing of LV events is clinically relevant. A significant proportion of patients with newly diagnosed GCA have involvement of the aorta (45 to 65%) and its branches (29% to 74%) on imaging studies.[4–9] Patients in our study did not undergo systematic screening. However, we found that the incidence of LV events was high within the first year of diagnosis of GCA. This suggests disease may be present well before clinical detection. While the rate of large-artery stenosis did not significantly increase after 5 years, the rate of aortic aneurysms/dissections increased 5 years after diagnosis of GCA and continued to increase during the entire period of observation. A smaller cross-sectional imaging study of 54 patients with GCA (median duration 5.4 years), revealed evidence of aortic aneurysm or dilatation in 12 patients (22.5%); 5 of whom were candidates for surgical repair based on aneurysm size.[26] These findings highlight the importance of long-term surveillance in patients with GCA to monitor for aortic aneurysm formation.
Overall survival in our cohort of patients with GCA was similar to the general population, a finding that has been reported in previous studies.[10–16] While a few studies have reported increased mortality in patients with GCA,(27–30)] they usually included small numbers of patients with GCA. An analysis of cause-specific mortality found an excess of death attributable to the gastrointestinal system. While the numbers were small, the increased number of deaths due to mesenteric vascular disease in our cohort was unexpected. Mesenteric arteritis from GCA has not commonly been diagnosed, but may be under-recognized clinically.[31–34] Imaging studies, involvement of the mesenteric arteries has been described in 18% (cross-sectional study) and 22% (newly diagnosed) patients with GCA.[8,35] A previous study from this cohort did not find any increase in mortality in patients with GCA and LV manifestations with the exception of those who developed aortic dissection.[1] The analysis in the previous study compared patients in the different subgroups of LV involvement from the time of diagnosis of GCA.[1] However, as our data show, LV events are detected months to years after initial GCA diagnosis which was not accounted for by the previous analysis as patients in the different groups could not experience the outcome of death until after they developed LV manifestations. In our study, we used Cox regression models with time-dependent covariates which allowed patients with GCA to be modeled as unexposed to the risk factor (i.e. LV manifestations) until they developed the LV event. Contrary to the previous report, we found that patients with GCA and aortic manifestations had a 3.4 fold increased risk of death compared to patients with GCA without LV manifestations. Our study study also comprehensively evaluates survival and cause-specific mortality in patients with GCA and LV involvement. Survival compared to the general population was also decreased in patients with GCA and LV manifestations. Cause-specific mortality in these patients found increased deaths attributable to the cardiovascular and respiratory systems. Since aortic dissection is a dreaded complication of aortic aneurysms and is often a catastrophic event, the greater than expected number of deaths from cardiovascular causes is not entirely surprising. However, patients with aortic manifestations from GCA also had a greater than expected number of deaths from ischemic heart disease and non-ischemic cardiac disease. It is conceivable that the aortic inflammation in GCA and atherosclerosis share common pathobiologic pathways which may account for our observed association.[36,37] It is also possible that the underlying cause of death in patients with aortic aneurysms was dissection but was misdiagnosed as another event such as a coronary event, which is common in the elderly. While there were also a higher than expected number of deaths from respiratory causes, the reason for this observation is unclear and may be related to the small number of cases.
Since the cohort spans several decades during which time medical practice has changed, time-trends in mortality were evaluated in patients with LV manifestations. Overall, there were no differences in mortality between the two cohorts. One would expect that aggressive cardiovascular risk factor modification, changes in practice including the serial imaging of patients with aneurysms and surgical intervention might result in improved survival in patients with aortic aneurysms and dissections in recent decades but this was not supported by our data, although the total number of events was low.
The strengths of our study include the population-based incident cohort of patients with GCA. We had access to the entire medical record from all providers in Olmsted County which allowed more complete data collection. Since our primary outcome was death, vital status for each subject regardless of residency status was collected. We also had access to the death certificates for 98% of patients who died (data missing only in 3 patients). According to the 2000 US census data, 90.3% of the Olmsted County population is White. Since GCA predominantly affects individuals of Northern European descent, our population is representative of patients with this disease.
Limitations include the retrospective design. As is the case in current clinical practice, not all patients were systematically screened for development LV involvement which may result in misclassification of patients. Additionally, given this limitation, we were only able to evaluate complications of large-vessel disease including large-artery stenosis and aortic aneurysm/dissection. Using death certificates to determine the underlying cause of death has its limitations. Census data for the Minnesota population were available for the years 1979 through 2002 and was extrapolated for the missing years. A separate analysis using only the cohort diagnosed between 1979 and 2004 did not change our findings.
Our findings have important clinical implications in the care of patients with GCA. Since aortic aneurysm/dissection is associated with decreased survival, screening for aortic aneurysm formation is important. The incidence of aortic aneurysm/dissection increased 5 years after GCA diagnosis and continued to increase over the period of observation emphasizing the need for long-term surveillance in these patients. Patients with GCA and aortic manifestations had excess mortality from cardiovascular causes including ischemic heart disease in patients with aortic aneurysm/dissection. A better understanding of the mechanisms of aortic complications in GCA, as well as optimal imaging strategies for detecting them would provide useful information and may help guide future decisions in the care of these patients. Prospective studies of the cost-effectiveness of screening efforts and whether they translate into improved survival for patients with GCA are needed.
Supplementary Material
Acknowledgments
None.
Funding: This project was supported by NIH/NCRR CTSA Grant Number UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH. This study was made possible by the Rochester Epidemiology Project (Grant Number R01 AG034676 from the National Institute on Aging.
Dr. Kermani was supported by the Vasculitis Clinical Research Consortium (VCRC) which has received support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (U54AR057319), the National Center for Research Resources (U54 RR019497), and the Office of Rare Diseases Research. The VCRC is part of the Rare Diseases Clinical Research Network (RDCRN).
Footnotes
Competing interests: None declared.
Licence for Publication
The Corresponding Author has the right to grant on behalf of all authors and does grant on behalf of all authors, an exclusive licence (or non exclusive for government employees) on a worldwide basis to the BMJ Publishing Group Ltd to permit this article (if accepted) to be published in ARD and any other BMJPGL products and sublicences such use and exploit all subsidiary rights, as set out in our licence (http://group.bmj.com/products/journals/instructions-for-authors/licence-forms).
REFERENCES
- 1.Nuenninghoff DM, Hunder GG, Christianson TJ, et al. Mortality of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3532–3537. doi: 10.1002/art.11480. [DOI] [PubMed] [Google Scholar]
- 2.Evans JM, O'Fallon WM, Hunder GG. Increased incidence of aortic aneurysm and dissection in giant cell (temporal) arteritis. A population-based study. Ann Intern Med. 1995;122:502–507. doi: 10.7326/0003-4819-122-7-199504010-00004. [DOI] [PubMed] [Google Scholar]
- 3.Bongartz T, Matteson EL. Large-vessel involvement in giant cell arteritis. Curr Opin Rheumatol. 2006;18:10–17. doi: 10.1097/01.bor.0000197996.04709.4e. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt WA, Seifert A, Gromnica-Ihle E, et al. Ultrasound of proximal upper extremity arteries to increase the diagnostic yield in large-vessel giant cell arteritis. Rheumatology (Oxford) 2008;47:96–101. doi: 10.1093/rheumatology/kem322. [DOI] [PubMed] [Google Scholar]
- 5.Aschwanden M, Kesten F, Stern M, et al. Vascular involvement in patients with giant cell arteritis determined by duplex sonography of 2×11 arterial regions. Ann Rheum Dis. 2010;69:1356–1359. doi: 10.1136/ard.2009.122135. [DOI] [PubMed] [Google Scholar]
- 6.Blockmans D, de Ceuninck L, Vanderschueren S, et al. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis Rheum. 2006;55:131–137. doi: 10.1002/art.21699. [DOI] [PubMed] [Google Scholar]
- 7.Agard C, Barrier JH, Dupas B, et al. Aortic involvement in recent-onset giant cell (temporal) arteritis: a case-control prospective study using helical aortic computed tomodensitometric scan. Arthritis Rheum. 2008;59:670–676. doi: 10.1002/art.23577. [DOI] [PubMed] [Google Scholar]
- 8.Prieto-Gonzalez S, Arguis P, Garcia-Martinez A, et al. Large vessel involvement in biopsy-proven giant cell arteritis: prospective study in 40 newly diagnosed patients using CT angiography. Ann Rheum Dis. 2012;71:1170–1176. doi: 10.1136/annrheumdis-2011-200865. [DOI] [PubMed] [Google Scholar]
- 9.Ghinoi A, Pipitone N, Nicolini A, et al. Large-vessel involvement in recent-onset giant cell arteritis: a case-control colour-Doppler sonography study. Rheumatology (Oxford) 2012;51:730–734. doi: 10.1093/rheumatology/ker329. [DOI] [PubMed] [Google Scholar]
- 10.Bengtsson BA, Malmvall BE. Prognosis of giant cell arteritis including temporal arteritis and polymyalgia rheumatica. A follow-up study on ninety patients treated with corticosteroids. Acta Med Scand. 1981;209:337–345. doi: 10.1111/j.0954-6820.1981.tb11604.x. [DOI] [PubMed] [Google Scholar]
- 11.Andersson R, Malmvall BE, Bengtsson BA. Long-term survival in giant cell arteritis including temporal arteritis and polymyalgia rheumatica. A follow-up study of 90 patients treated with corticosteroids. Acta Med Scand. 1986;220:361–364. doi: 10.1111/j.0954-6820.1986.tb02778.x. [DOI] [PubMed] [Google Scholar]
- 12.Boesen P, Sorensen SF. Giant cell arteritis, and temporal arteritis, and polymyalgia rheumatica in a Danish county. A prospective investigation, 1982–1985. Arthritis Rheum. 1987;30:294–299. doi: 10.1002/art.1780300308. [DOI] [PubMed] [Google Scholar]
- 13.Nordborg E, Bengtsson BA. Death rates and causes of death in 284 consecutive patients with giant cell arteritis confirmed by biopsy. BMJ. 1989;299:549–550. doi: 10.1136/bmj.299.6698.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Matteson EL, Gold KN, Bloch DA, et al. Long-term survival of patients with giant cell arteritis in the American College of Rheumatology giant cell arteritis classification criteria cohort. Am J Med. 1996;100:193–196. doi: 10.1016/s0002-9343(97)89458-2. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Gay MA, Blanco R, Abraira V, et al. Giant cell arteritis in Lugo, Spain, is associated with low longterm mortality. J Rheumatol. 1997;24:2171–2176. [PubMed] [Google Scholar]
- 16.Ninan J, Nguyen AM, Cole A, et al. Mortality in Patients with Biopsy-proven Giant Cell Arteritis: A South Australian Population-based Study. J Rheumatol. 2011;38:2215–2217. doi: 10.3899/jrheum.101254. [DOI] [PubMed] [Google Scholar]
- 17.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–274. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 18.Kermani TA, Schafer VS, Crowson CS, et al. Increase in age at onset of giant cell arteritis: a population-based study. Ann Rheum Dis. 2010;69:780–781. doi: 10.1136/ard.2009.111005. [Letter] [DOI] [PubMed] [Google Scholar]
- 19.Salvarani C, Crowson CS, O'Fallon WM, et al. Reappraisal of the epidemiology of giant cell arteritis in Olmsted County, Minnesota, over a fifty-year period. Arthritis Rheum. 2004;51:264–268. doi: 10.1002/art.20227. [DOI] [PubMed] [Google Scholar]
- 20.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990;33:1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 21.Nuenninghoff DM, Hunder GG, Christianson TJ, et al. Incidence and predictors of large-artery complication (aortic aneurysm, aortic dissection, and/or large-artery stenosis) in patients with giant cell arteritis: a population-based study over 50 years. Arthritis Rheum. 2003;48:3522–3531. doi: 10.1002/art.11353. [DOI] [PubMed] [Google Scholar]
- 22.Gray R. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 23.Gerber Y, Jacobsen SJ, Frye RL, Weston SA, Killian JM, Roger VL. Secular trends in deaths from cardiovascular diseases: a 25-year community study. Circulation. 2006;113:2285–2292. doi: 10.1161/CIRCULATIONAHA.105.590463. [DOI] [PubMed] [Google Scholar]
- 24.Isselbacher EM. Thoracic and abdominal aortic aneurysms. Circulation. 2005;111:816–828. doi: 10.1161/01.CIR.0000154569.08857.7A. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Gay MA, Garcia-Porrua C, Pineiro A, et al. Aortic aneurysm and dissection in patients with biopsy-proven giant cell arteritis from northwestern Spain: a population-based study. Medicine. 2004;83:335–341. doi: 10.1097/01.md.0000145366.40805.f8. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Martinez A, Hernandez-Rodriguez J, Arguis P, et al. Development of aortic aneurysm/dilatation during the followup of patients with giant cell arteritis: a cross-sectional screening of fifty-four prospectively followed patients. Arthritis Rheum. 2008;59:422–430. doi: 10.1002/art.23315. [DOI] [PubMed] [Google Scholar]
- 27.Bisgard C, Sloth H, Keiding N, et al. Excess mortality in giant cell arteritis. J Intern Med. 1991;230:119–123. doi: 10.1111/j.1365-2796.1991.tb00418.x. [DOI] [PubMed] [Google Scholar]
- 28.Nesher G, Sonnenblick M, Friedlander Y. Analysis of steroid related complications and mortality in temporal arteritis: a 15-year survey of 43 patients. J Rheumatol. 1994;21:1283–1286. [PubMed] [Google Scholar]
- 29.Uddhammar A, Eriksson AL, Nystrom L, et al. Increased mortality due to cardiovascular disease in patients with giant cell arteritis in northern Sweden. J Rheumatol. 2002;29:737–742. [PubMed] [Google Scholar]
- 30.Crow RW, Katz BJ, Warner JE, et al. Giant cell arteritis and mortality. J Gerontol A Biol Sci Med Sci. 2009;64:365–369. doi: 10.1093/gerona/gln030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scola CJ, Li C, Upchurch KS. Mesenteric involvement in giant cell arteritis. An underrecognized complication? Analysis of a case series with clinicoanatomic correlation. Medicine (Baltimore) 2008;87:45–51. doi: 10.1097/MD.0b013e3181646118. [DOI] [PubMed] [Google Scholar]
- 32.Sujobert P, Fardet L, Marie I, et al. Mesenteric ischemia in giant cell arteritis: 6 cases and a systematic review. J Rheumatol. 2007;34:1727–1732. [PubMed] [Google Scholar]
- 33.Lorthioir A, Marie I, Tetart F, et al. Mesenteric artery involvement in giant cell arteritis: two cases and literature review] Rev Med Interne. 2008;29:1007–1012. doi: 10.1016/j.revmed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Azeez MA, Browne P, O'Connell P, et al. Giant cell arteritis involving the mesenteric arteries. J Rheumatol. 2009;36:2314–2315. doi: 10.3899/jrheum.090152. [DOI] [PubMed] [Google Scholar]
- 35.Grayson PC, Maksimowicz-McKinnon K, Clark TM, et al. Distribution of arterial lesions in Takayasu's arteritis and giant cell arteritis. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2011-200795. Published Online First: 10 February. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Martinez-Taboada VM, Bartolome MJ, Fernandez-Gonzalez MD, et al. Homocysteine levels in polymyalgia rheumatica and giant cell arteritis: influence of corticosteroid therapy. Rheumatology (Oxford) 2003;42:1055–1061. doi: 10.1093/rheumatology/keg293. [DOI] [PubMed] [Google Scholar]
- 37.Machado EB, Gabriel SE, Beard CM, et al. A population-based case-control study of temporal arteritis: evidence for an association between temporal arteritis and degenerative vascular disease? Int J Epidemiol. 1989;18:836–841. doi: 10.1093/ije/18.4.836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.