Abstract
Epidemiological and clinical studies have indicated that low vitamin D activity is not only associated with an increased cancer risk and a more aggressive tumor growth, but also connected with an aggravated liver damage caused by chronic inflammation. Meanwhile, increasing evidence has demonstrated that 1,25(OH)2D3 (the most biologically active metabolite of vitamin D) can inhibit inflammatory response in some chronic inflammatory associated cancer, which is considered to have the anti-tumor potency. However, the interaction between 1,25(OH)2D3 and inflammation during hepatocellular carcinoma (HCC) initiation and progression is not yet clear. Here, we report an anti-tumorigenesis effect of 1,25(OH)2D3 via decreasing inflammatory cytokine secretion in HCC and hypothesize the possible underlying mechanism. Firstly, we show that the enhanced tumor growth is associated with elevated inflammatory cytokine IL-6 and TNF-α in 1α(OH)ase gene-knockout mice. Secondly, 1,25(OH)2D3 can inhibit vitamin D receptor (VDR) shRNA interfered tumor cell growth through decreasing inflammatory cytokine secretion in vitro and in vivo. Finally, using p27kip1 gene knock-out mouse model, we demonstrate that the effect of 1,25(OH)2D3 in inhibiting immune cell related inflammatory cytokine secretion, exerts in a p27kip1 gene dependent way. Collectively, 1,25(OH)2D3 inhibits HCC development through up-regulating the expression of p27kip1 in immune cell and reducing inflammatory cytokine production.
Keywords: HCC; chronic inflammation; 1,25(OH)2D3; 1α(OH)ase; gene knockout; IL-6; TNF-α; STAT3 signaling; p27kip1; co-culture
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for 90% of all primary liver malignancies, grows rapidly and is highly resistant to chemotherapy [1, 2]. The average survival time for HCC patients is less than 12 months after diagnosis. Only 10–20% of HCC cases are suitable for resection, with a 5-year recurrence-free survival of only 20–30% [3, 4]. Recent developments in new treatment modalities have led to an improved survival rate slightly [5]; however, current treatments are still not satisfactory, and thus, novel treatment strategies against HCC are in dire need.
Rapidly growing evidence reinforces the notion that tumors are promoted by inflammatory signals from the surrounding microenvironment [6,7]. Generally, in chronic inflammation, the inflammatory foci are dominated by infiltrated inflammatory cells which generate a great amount of growth factors, cytokines, reactive oxygen and nitrogen species that may cause DNA damage of normal cells [8]. A microenvironment comprehends the persistent inhabitation of activated inflammatory cells which may cause continued tissue damage and sustained cell proliferation, thus predisposes chronic inflammation to neoplasia [9]. The advancements in understanding the relationship between inflammation and hepatocarcinogenesis have highlighted that excessively and chronically produced pro-inflammatory cytokines contribute to HCC initiation and progression in virus hepatitis, non-alcoholic fatty liver disease (NAFLD) and chemical tumorigenesis of patients or mouse models [10, 11]. Previous studies have demonstrated that the tumor development in carcinogen-induced obesity mice is dependent on the elevated tumor-promoting cytokine IL-6 and TNF-α, which may augment the hepatic inflammatory response and the activation of oncogenic transcription factor STAT3, since inactivation of IL-6 or TNF-α inhibits the tumor promoting effects in these mice [12]. Meanwhile, a recent literature has also validated that, the reinforcement of STAT3 signaling in inflammatory cell, activated by inflammatory cytokine IL-6, accelerates HCC initiation and progression in vitro and in vivo [13]. Collectively, reducing the production of inflammatory cytokines, especially IL-6 and TNF-α, may serve as a new target for HCC therapy and prevention.
Epidemiological and clinical studies have indicated that the low circulating levels of vitamin D are associated with an increased risk of several types of cancer and a more aggressive tumor growth; while a high intake of 1,25(OH)2D3 reduces the risk of cancer [14,15]. Previous findings have already demonstrated that 1,25(OH)2D3 plays a major role in modulating calcium and skeletal homeostasis and exerts a significant influence on the growth and differentiation of a variety of tissues [16]. Likewise, existing evidence has also revealed that 1,25(OH)2D3 modulates the activity of various immune cells [17]. Therefore, except for reducing cell growth and inducing apoptosis, 1, 25(OH)2D3 has also the potency to inhibit inflammation, which is considered to exert the anti-tumor activity as well [18]. For instance, a recent literature has demonstrated that 1, 25(OH)2D3 interrupts the activating of Wnt signaling and the accelerating of cell proliferation by macrophage-derived IL-1β in colon cancer cells [19]. Furthermore, 1,25(OH)2D3 also decreases the production of pro-inflammatory cytokine IL-6 through inactivating the p38 stress-induced kinase, which is considered valuable for prostate cancer prevention [20]. Moreover, 1,25(OH)2D3 can inhibit ConA-induced mouse hepatitis [21], and a poor vitamin D status is considered to aggravate NAFLD [22]. Consequently, 1,25(OH)2D3 may have the potency to inhibit HCC development since both hepatitis and NAFLD are the major causes of HCC initiation. Finally, through up-regulating the cyclin dependent kinase inhibitor (CKI) p27kip1, 1,25(OH)2D3 inhibits the proliferation of many types of immune cells and thus reduces the production of inflammatory cytokines, such as IL-6 and TNF-α, which may also contribute to the prevention of tumor. [23].
Despite the multiple anti-tumor effects 1,25(OH)2D3 exerts an extraordinary high prevalence of vitamin D deficiency has been reported in patients with chronic liver disease [24, 25]. Furthermore, a recent clinical study has indicated the inverse correlation of vitamin D levels and liver dysfunction in several liver diseases [26]. Vitamin D deficiency of chronic liver disease patients is a consequence of impaired vitamin D synthesis and absorption, or vitamin D deficiency facilitates the pathogenesis of chronic liver diseases via decreasing the anti-inflammatory and anti-infectious effects, the fact that whether vitamin D deficiency is associated with HCC initiation and progression and the underlying mechanisms are still ambiguous. 1,25(OH)2D3 is a prohormone that can be metabolically converted from 25-hydroxyvitamin D3 by the enzyme 1α-hydroxylase [1α(OH)ase] to the active form of the vitamin, 1,25-dihydroxyvitamin D3 [27]. In order to investigate whether 1,25(OH)2D3 deficiency accelerates while exogenous 1,25(OH)2D3 supply inhibits HCC development in vivo, respectively, we use the carcinogen-induced or transplanted tumor bearing 1α(OH)ase and p27kip1 gene knockout (KO) mouse models, and validate the positive correlation between elevated inflammatory cytokines and enhanced HCC development.
MATERIALS AND METHODS
Cell Lines and Cell Culture
H22 (China Center for Type Culture Collection, Wuhan, China), and Hepa1–6 (from a C57BL mouse hepatoma; American Type Culture Collection, Rockville, MD, USA) were cultured in DMEM (Q&K Bio-Chemical Engineering Limited Company, Shanghai) supplemented with 10% heat-inactivated fetal bovine serum, 50 units/ml penicillin, and 50 units/ml streptomycin. For the experiments, 3×106 cells were plated in 75-cm2 tissue culture flasks and grown in a humidified atmosphere (37°C, 5% CO2). Treatments with different concentration (1 nM, 10 nM, 100 nM, 1000 nM) 1,25(OH)2D3 (Sigma-Aldrich, St. Louis, MO) or the vehicle were initiated the day after plating and the effects were observed 48 hours later.
Analyses of Proliferation and Apoptosis on HCC Cells
For proliferation analysis, we incubated HCC cells with 1,25(OH)2D3 for 48 hours and added BrdU (1μM) to the culture medium after 28 hours. For BrdU detection, the cells were incubated with an anti-BrdU antibody (1:100) overnight at 4°C. Secondary anti-rabbit antibody conjugated with FITC was used to label BrdU and allowed for the detection of proliferating cells. For apoptosis analysis, the phosphatidylserine (PS) exposure in HCC cells was detected with an annexin V–FITC/PI apoptosis detection kit (Beckman Coulter). HCC cell apoptosis was subsequently analyzed by flow cytometry (Epics XL Coulter).
Animals
1α(OH)ase KO Babl/c mice (1α(OH)ase−/−) were generated by homologous recombination in embryonic stem cells as previously described. We fed 1α(OH)ase−/− mice with a high-calcium diet (TD96348 Teklad, Madison, WI, USA) containing 2% calcium, 1.25% phosphorus, and 20% lactose for 3 months after weaning to rescue the abnormities and hypocalcemia of 1α(OH)ase−/− mice as previously described. p27 KO C57BL/J mice (p27−/−), first generated and described by Dr. Fero, were purchased from Jackson Laboratory (The Jackson Laboratory, Bar Harbor, USA. 1α(OH)ase+/+, p27+/+ and p27−/− mice fed with normal diet. Both 1α(OH)ase−/− and p27−/− mice were used along with corresponding age-matched WT (1α(OH)ase+/+ or p27+/+ mice). The use of animals in this study was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University.
Genotyping of Mice
Tail fragment genomic DNA was isolated by standard phenol–chloroform extraction and isopropanol precipitation. Mouse genotype was determined by PCR of tail DNA using the following primers: for p27 knockout allele (5′ primer: CTCTCTATCGCCTTCTTG, 3′ primer: TGGAACCCTGTGCCATCTCTAT); for p27 wild type allele (5′ primer: GATGGACGCCAGACAAGC, 3′ primer: ACGGGCTTA TGATTCTGAAAGTCG). For the wild-type 1α(OH)ase allele, the forward primer (5′-AGACTGCACTCCACT CTGAG-3′) and reverse primer (5′-GTT TCC TAC ACG GAT GTC TC-3′) were used. The neomycin gene was detected with the primers neo-F (5′-ACA ACA GAC AAT CGG CTG CTC-3′) and neo-R (5′-CCA TGG GTC ACG ACG AGA TC-3′). All PCR reactions were performed with 1 cycle of 95°C for 4 minutes and 35 cycles of 94°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds.
Chemical Hepatocarcinogenesis
Since the susceptibility to liver tumorigenesis in response to DEN in mouse is strain-dependent, and the pure C57BL/6 strain is highly resistant DEN for liver tumorigenesis, for the chemical tumorigenesis we used a DEN and PB two stage carcinogenesis protocol as described before (Sun et al., 2008). Briefly, male mice and their corresponding wild-type littermates were injected intraperitoneally with 100 mg/kg DEN (Sigma, St. Louis, MO). Four weeks after DEN injection, the mice received 0.07% phenol barbital (PB) (Sigma) in drinking water and randomly assigned to the treatment (0.1 μg/kg 1,25-(OH)2D3 per day), (5 mg/kg NSC 74859) or control group (propylene glycol) until their sacrifice at 9 month of age. After sacrifice, livers of mice were removed, and the tumor number and sizes were measured. Serum IL-6, and TNF-α were measured by ELISA (eBioscience). The p27−/− mice were used in addition to corresponding age-matched p27+/+ mice. The Institutional Animal Care and Use Committee of Nanjing Medical University approved the use of animals in this study.
Co-Culture of Immune Cells and HCC Cells
The mouse hepatocellular carcinoma cell line H22 or Hepa1–6 were grown in RPMI-1640 (Invitrogen) supplemented with 10% heat-inactivated fetal bovine serum. Monocytes were isolated by negative selection using Dynal Monocyte Negative Isolation Kit (Invitrogen) according to the manufacturer’s instructions. T cells were isolated with the MACS pan T cell Isolation kit II by negative selection in MACS LD depletion magnetic columns (MiltenyiBiotec, Bergisch Gladbach, Germany). All in vitro experiments were performed in Ultra Low Attachment Plates (Corning) to prevent monocytes & T lymphocytes activation by adhesion to the plastic plate. For monocytes & T lymphocytes culture, the immune cells were isolated and transferred to a vial of chilled RPMI-1640 medium (Invitrogen) and supplemented with 10% FBS, 5000 IU penicillin and streptomycin. Viable cells were identified by a trypan blue dye exclusion assay, counted using a hemocytometer and plated at 5×106 cells/well in a 24-well tissue culture plate. In co-culture experiments, freshly isolated splenocytes (5×106) were added to the inserts separated by 0.4μm membrane (Costar; Corning) from HCC cells.
Silencing of VDR by sh-RNA Plasmid
To silence the expression of VDR in H22 and Hepa1–6 cells, we used sh-RNA plasmid containing the sequence rat VDR, 5′-CCTGTCCCTTCAATGGAGATT-3′, or a scrambled plasmid as a negative control containing the sequence 5′-GGAATCTCATTCGATGCATAC-3′ (SA Bioscience, Frederick, Maryland, USA). HCCs at 80% confluence were transfected with 2 mg of sh-RNA plasmids using the Fugene 6 reagent (Roche Diagnostics) according to the manufacturer’s instructions.
3-(4, 5-Dimethylthiazol-2-yl)-2, 5-Diphenyltetrazolium Bromide (MTT) Assay
Cells (1×104) were seeded in 24-well microplates in complete culture medium in the absence or presence of 1,25-(OH)2D3 (100nM), anti-IL-6 antibody (10μm/ml) or NSC 74859 (100μM). After 72 h of culturing, the number of viable cells was measured by adding 100 μl/well of 2 mg/ml MTT solution. The medium was removed 2 h later and the formazan crystals were dissolved by adding 100 μl dimethyl-sulfoxide per well. The absorbance was read at 590 nm with an enzyme-linked immunosorbent assay reader. Each treatment point was performed with an n=6.
HCC Orthotopic Transplantation Model
To generate an orthotopic in vivo HCC model, 1α(OH)ase−/−, p27−/− and WT mice were used. After anesthetizing the mice, the liver was exposed following an upper middle incision, and approximately 1x106 HCC cells (H22 or Hepa1–6) suspended in 30 μL of PBS were implanted into the left lobe of the liver using a microsyringe. Twenty-four hours after inoculation, the animals were randomly assigned to either the treatment (0.1 μg/kg 1,25-(OH)2D3 per day) or control group (propylene glycol). Using vernier calipers, the tumor diameters (mm) were measured at 14 days after injection. Tumor volumes were calculated using the formula 1/6Πd3. For determining serum inflammatory factor levels, 0.5 ml of blood were taken from mice under general anesthesia at the end of the experiment by cardiac puncture. Serum IL-6 and TNF-α levels were measured by ELISA (Biomedical Technologies, Inc., Stoughton, MA, USA), following the manufacturer’s instructions.
Immunohistochemical Staining and TUNEL Assay
Phosphorylated STAT3 and proliferating cell nuclear antigen (PCNA) were detected by immunohistochemistry using the avidin-biotin-peroxidase complex technique with a mouse anti-p-STAT3(Y705) monoclonal antibody (Biomedical Technologies, Inc., Stoughton, MA, USA) and a mouse anti-PCNA monoclonal antibody (Medicorp Inc., Montreal, Canada). The in situ cell death detection assay kit was purchased from Roche and performed according to the manufacturer’s instructions. The percentage of apoptotic cells was calculated by counting the number of TUNEL-positive nuclei out of 500 total cells for each sample. At least three randomly chosen fields were assessed. The percentage of TUNEL-positive nuclei was determined in three independent experiments.
Statistical Analysis
Data from image analyses are presented as the mean ± SEM. Statistical comparisons were made using a two-way ANOVA, with P < 0.05 considered significant.
RESULTS
1,25(OH)2D3 Inhibits Cell Proliferation and Induces Apoptosis in HCC Cells
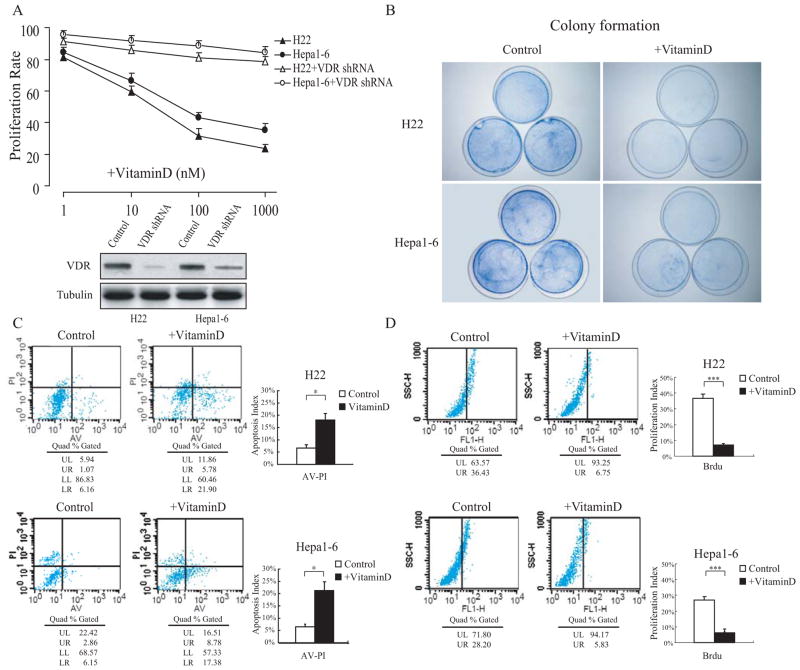
Prior to addressing the anti-tumor effects of 1,25(OH)2D3 in HCC-derived allografts, we first investigated its anti-proliferative and apoptosis-inductive effects in the mouse cell lines H22 and Hepa1–6. Both cell lines were either normally cultured or treated with different concentration of 1,25(OH)2D3 for 48 hours, after which cell growth status was investigated. Consistent with previous studies, cell growth in the 1,25(OH)2D3-treated group was inhibited in both cell lines with a dose dependant manner. In addition, the vitamin D receptor (VDR) RNAi in both H22 and Hepa1–6 cells effectually suppressed the proliferation inhibitory effect of 1,25(OH)2D3 (Fig. 1A). The colony formation experiment showed the dramatically proliferation inhibitory effect of 1,25(OH)2D3 with the dosage 100 nM (Fig. 1B). Analysis of BrdU incorporation revealed that the ratio of BrdU-positive cells decreased in the 1,25(OH)2D3-treated H22 and Hepa1–6 cells (Fig. 1C). Analyses of Annexin V and PI double-staining indicated that the ratio of apoptosis in 1,25(OH)2D3-treated H22 and Hepa1–6 cells evidently increased in comparison with the normally cultured group (Fig. 1D).
Fig. (1). 1,25(OH)2D3 inhibits cell proliferation and induces apoptosis in HCC cells.
The mouse-derived HCC cell lines H22 (Babl/c background) and Hepa1–6 (C57BL background) were different concentrations of 1,25(OH)2D3 the day after plating, and the effects were observed 48 hours later. (B) Colony formation was performed to assess cell proliferation in vitro. (C) H22 and Hepa1–6 cells were incorporated with 1μM BrdU to the culture medium 28 hours after 1,25(OH)2D3 or vehicle treatment, and cell proliferation analysis was performed by FACS. (D) Apoptosis analysis by Annexin V and PI double staining was performed by FACS. The results are representative of at least 3 independent experiments. *p < 0.05, ***p < 0.001.
1α(OH)ase Ablation Promotes Tumor Development, and Conversely, Supplying 1,25(OH)2D3 Inhibits Tumor Development
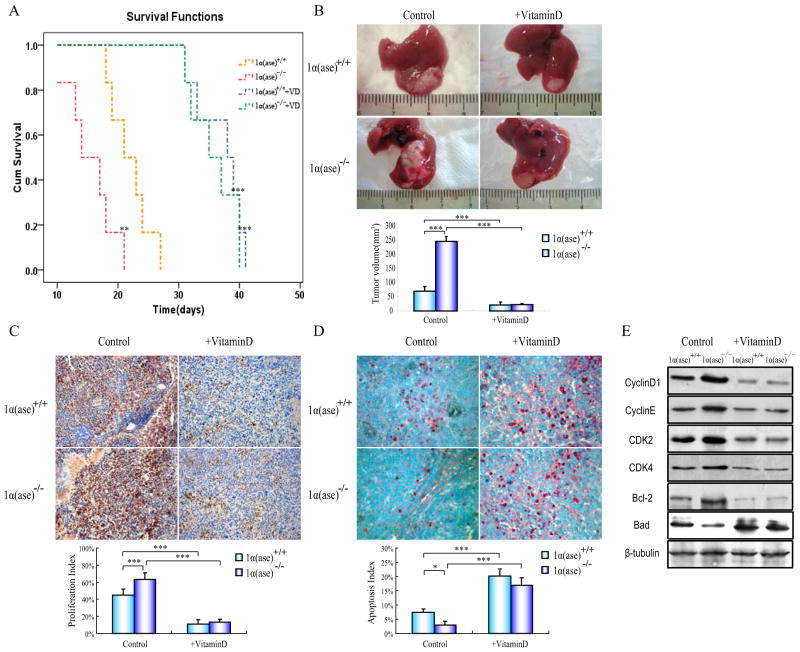
To further investigate the anti-tumor effects of 1,25(OH)2D3 in vivo, we performed an intervention survival study on both 1α(ase)+/+ (n=6) and 1α(ase)−/− (n=6) male mice that were orthotopically transplanted with HCC and monitored tumor volume biweekly. After operation, the average survival time of 1α(ase)−/− mice was shorter and the average tumor volume was larger in comparison with 1α(ase)+/+ mice. In contrast, the average survival time was extended and the average tumor volume was shrunk in both tumor bearing 1α(ase)+/+ and 1α(ase)−/− mice when supplied with 1,25(OH)2D3 (0.2 μg/kg per day). Of note, there was no difference in the survival and tumor volume between tumor bearing 1α(ase)+/+ and 1α(ase)−/− mice whice were supplied with 1,25(OH)2D3 (Fig. 2A and B). We next investigated the cell proliferation inhibition and apoptosis induction effects of 1,25(OH)2D3 by PCNA immunohistochemistry and TUNEL staining in tumor slices. In 1α(ase)−/− mice, the 1,25(OH)2D3 deficiency specifically increased proliferation and decreased apoptosis, relative to in 1α(ase)+/+ mice, whereas the 1,25(OH)2D3 supplementation significantly decreased the ratio of cell proliferation and increased the ratio of cell apoptosis in both 1α(ase)+/+ and 1α(ase)−/− mice (Fig. 2C and D). Immunoblotting results showed that 1,25(OH)2D3 deficiency raise the cell-cycle-related protein (Cyclin D, Cyclin E, Cdk2 and Cdk4) and anti-apoptotic protein Bcl-2 expression, and decrease the pro-apoptotic protein Bad in the tumor bearing 1α(ase)−/− mice. While the 1,25(OH) 2D3 supplementation inhibited the affection of 1,25(OH)2D3 deficiency in these cell-cycle-related and apoptotic related protein expression (Fig. 2E).
Fig. (2). 1α(OH)ase ablation promotes tumor development in allograft transplanted transplanted mice.
Babl/c background derived H22 cells (1x106) were implanted to the 1α(ase)+/+ or 1α(ase)−/− mice, and twenty-four hours after inoculation, the animals were randomly assigned to either the treatment intraperitoneal injection 0.1 μg/kg 1,25-(OH)2D3 or propylene glycol (control group) per day. (A) Overall survival time analysis of HCC transplanted 1α(ase)+/+ and 1α(ase)−/− mice. After H22 cell inoculation and 1,25-(OH)2D3 treatment, each mouse’s survival time was documented and made up to the survival curve. Compared with the PBS-injected 1α(OH)ase+/+ group (yellow dotted line): **p<0.01, ***p<0.001 (B) Gross appearances of representative livers with transplanted tumors in either normal or 1,25(OH)2D3-treated 1α(ase)+/+ and 1α(ase)−/− mice (n=6). (C) PCNA immunohistochemistry staining of representative tumor slices from either normal or 1,25(OH)2D3-treated 1α(ase)+/+ and 1α(ase)−/− mice (magnification 200x). (D) TUNEL staining of representative tumor slices from either 1,25(OH)2D3-untreated or -treated 1α(ase)+/+ and 1α(ase)−/− mice (magnification 400x). (E) Immunoblotting results of cell cycle and apoptosis-related proteins in transplanted tumors from either 1,25(OH)2D3-untreated or -treated 1α(ase)+/+ and 1α(ase)−/− mice. The results are representative of at least 3 independent samples. *p<0.05,***p<0.001.
1,25(OH)2D3 Deficiency Results in Enhanced Inflammatory Cytokine Production and STAT3 Signaling Activation in Orthotopic Transplantation Mice
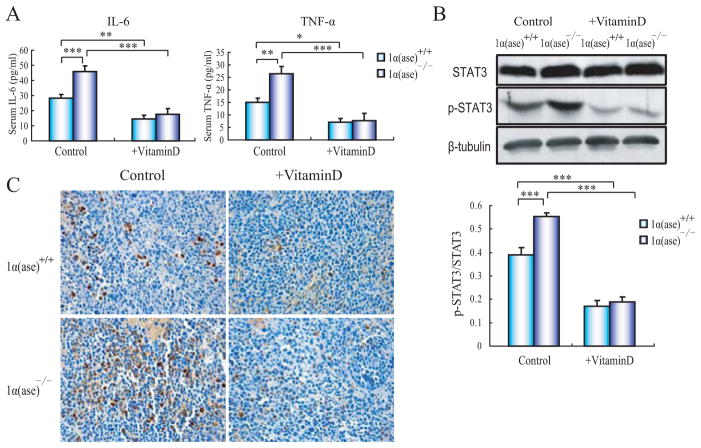
To further understand whether the 1,25(OH)2D3 deficiency influences the inflammatory response and thus affects the tumor growth in 1α(ase)−/− mice, we detected the serum inflammatory cytokines TNF-α and IL-6 levels in orthotopic transplantation 1α(ase)+/+ and 1α(ase)−/− mice. There were no apparent differences of serum TNF-α and IL-6 in normal 1α(ase)+/+ and 1α(ase)−/− mice (data not show). However, after orthotopic tumor transplantation, these two inflammatory cytokines’ levels were much higher in 1α(ase)−/− mice relative to their wild-type counterparts. While exogenous 1,25(OH)2D3 supplementation decreased these inflammatory cytokines’ levels in both 1α(ase)−/− and 1α(ase)+/+ mice (Fig. 3A). Since the tumor-promoting effect of IL-6 in HCC is mainly exerted via STAT3 signaling, we assessed whether STAT3 activation was affected by the ablation of 1α(ase) in mice following orthotopic tumor transplantation. Immunochemistry results showed that, p-STAT3-positive cells were obviously increased in 1α(ase)−/− tumor but dramatically decreased in both 1α(ase)−/− and 1α(ase)+/+ tumor slices after 1,25(OH)2D3 supply. Meanwhile, immunoblotting results of STAT3 and p-STAT3 validated our observation above (Fig. 3B and C).
Fig. (3). 1,25(OH)2D3 deficiency promotes enhanced inflammatory cytokine production and STAT3 phosphorylation.
(A) Serum biochemistry results for IL-6 and TNF-α levels in either tumor-transplanted normal or 1,25(OH)2D3-treated 1α(ase)+/+ and 1α(ase)−/− mice. (B) Immunoblotting results of STAT3 phosphorylation levels in transplanted tumors in either normal or 1,25(OH)2D3-treated 1α(ase)+/+ and 1α(ase)−/− mice. (C) p-STAT3 immunohistochemistry staining results of representative tumor slices in either normal or 1α(ase)+/+ and 1α(ase)−/− mice treated with or without 1,25(OH)2D3 (magnification x400). The results are representative of at least 3 independent samples. *p<0.05; **p<0.01; ***p<0.001.
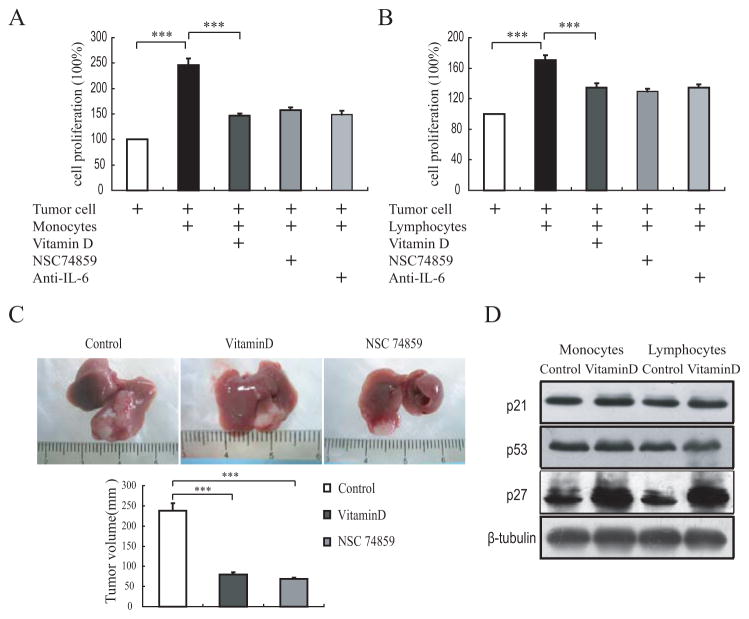
Since it was well documented that the vitamin D receptor (VDR) is existed in almost all immune cells, in order to understand whether 1,25(OH)2D3 will inhibit tumor growth via its anti-inflammation effect and elucidate the possible mechanism, we silenced VDR gene in tumor cell with VDR shRNA to rule out the direct anti-proliferation effect of vitamin D. After VDR gene silencing, 1,25(OH)2D3 treatment didn’t affect the cell proliferation (Fig. 1A). Then we conducted the immune cell and VDR gene silenced tumor cell co-culture experiment using transwell chambers and treated with either vitamin D or STAT3 signaling pathway inhibitors. Results showed that the tumor cell growth was evidently increased when co-cultured with monocytes or lymphocytes from wild-type Babl/c mice. Intriguingly, although VDR gene was silenced in these tumor cells (Babl/c strain derived), 1,25(OH)2D3 treatment can also inhibit tumor cell growth in the co-cultures. Furthermore, IL-6 antibody or STAT3 signaling inhibitor NSC74589 can also inhibit tumor cell growth when co-cultured with monocytes or lymphocytes (Fig. 4A and B). In order to demonstrate this effect of 1,25(OH)2D3 in vivo, we then transplanted the VDR gene silenced tumor cell to 1α(ase)−/− mice and treated with either 1,25(OH)2D3 or NSC74589. Consistent with the in vitro results, both 1,25(OH)2D3 and NSC74589 inhibited the transplanted tumor growth (Fig. 4C). Considering the well established cell cycle arrest effect of vitamin D, finally we detected cell cycle inhibitor p21, p27 and p53 in monocytes or lymphocytes treated with or without vitamin D. Results showed that after vitamin D treatment, only p27 protein was increased significantly in both monocytes and lymphocytes (Fig. 4D). These findings implicated that the elevated inflammatory cytokines of 1,25(OH)2D3 deficient mice facilitates the progression of orthotopic transplanted HCC via activation of the STAT3 signaling pathway.
Fig. (4). 1,25(OH)2D3 treatment inhibits VDR gene silenced tumor cell proliferation through IL-6-STAT3 signaling pathway during which co-cultured with immune cell or transplanted to 1α(ase)−/− mice.
The proliferation of H22 cell alone or after co-cultured with (A) monocytes or (B) lymphocytes, and treated with 1,25(OH)2D3, IL-6 anti-body or NSC 74859, respectively. (C) Gross appearances of representative livers with VDR gene silenced tumor cell transplantation in vehicle, NSC74589 or 1,25(OH)2D3 treated 1α(ase)−/− mice (n=6). (D) Immunoblotting results of p21, p27 and p53 protein levels of monocytes or lymphocytes from WT mice when co-cultured with tumor cells and treated with or without 1,25(OH)2D3. The results are representative of at least 3 independent samples. *p<0.05; **p<0.01; ***p<0.001.
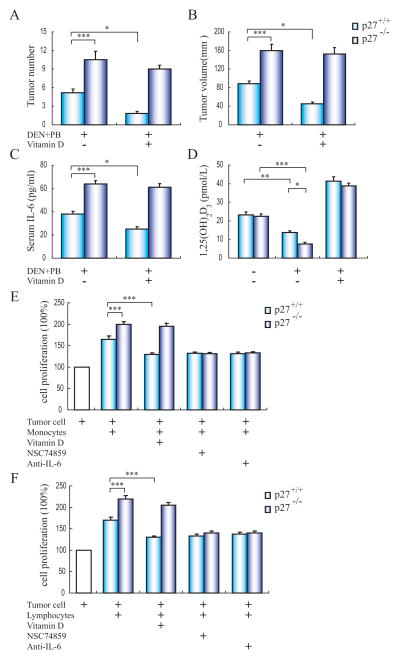
Lack of p27kip1 Suppresses the Anti-Tumor Effect of 1,25(OH)2D3
Since previous studies have demonstrated that p27kip1 deficiency promotes carcinogen induced HCC initiation and progression in mice, and p27kip1 gene knock-out mice spontaneously developed thymic hyperplasia with an increased T lymphocyte population. To decipher whether p27kip1 deficiency suppresses the anti-inflammation and anti-tumor effects of 1,25(OH)2D3, we examined the tumor development, serum inflammatory cytokines and vitamin D level in carcinogen induced p27+/+ or p27−/− mice, supplemented with or without 1,25(OH)2D3. Results showed that in consistent with previous studies, the tumor number and tumor volume were increased in p27−/− mice after 9 months chemical tumorigenesis. However, 1,25(OH)2D3 treatment inhibited the tumor development in p27+/+ mice, but not in p27−/− mice (Fig. 5A and B). Likewise, serum IL-6 level was increased p27−/− mice after chemical tumorgenesis, and 1,25(OH)2D3 only decreased serum IL-6 in p27+/+ mice (Fig. 5C). In addition, although serum 1,25(OH)2D3 level was decreased in both p27+/+ and p27−/− mice followed chemical tumorigenesis, the 1,25(OH)2D3 level was much lower in p27−/− mice than that of in p27+/+ mice (Fig. 5D). Then we used the VDR silenced tumor cell Hepa1–6 (C57BL strain derived) co-cultured with immune cell either from p27+/+ and p27−/− mice. Results showed that the proliferation of tumor cell was increased when co-cultured with p27−/− mice derived monocytes or lymphocytes, and IL-6 antibody and STAT3 signaling inhibitor NSC74589 inhibited the tumor cell proliferation when co-cultured with both p27+/+ and p27−/− mice derived immune cell, while 1,25(OH)2D3 only suppressed the tumor cell proliferation when co-cultured with p27+/+ derived immune cell (Fig. 5E and F).
Fig. (5). 1,25(OH)2D3 treatment inhibits chemical hepatocarcinogenesis through decreasing inflammatory cytokine IL-6 and through a p27kip1 gene dependent way.
Male p27−/− mice (n=8) and their corresponding wild-type p27+/+ littermates (n=8) at postnatal day 15 were treated with DEN+PB for six months, after which the 1,25(OH)2D3 or vehicle were treated every other day after. (A) The number and (B) size of tumors on the liver surface were counted. (C) Serum biochemistry results for IL-6 and (D) 1,25(OH)2D3 levels in p27+/+ or p27−/− mice with vehicle or 1,25(OH)2D3 for nine months. (E) The proliferation of VDR gene silenced Hepa1–6 cell alone or after co-cultured with monocytes or (F) lymphocytes from p27+/+ or p27−/− mice, and treated with 1,25(OH)2D3, IL-6 anti-body or NSC 74859, respectively. The results are representative of at least 3 independent samples. *p<0.05; **p<0.01; ***p<0.001.
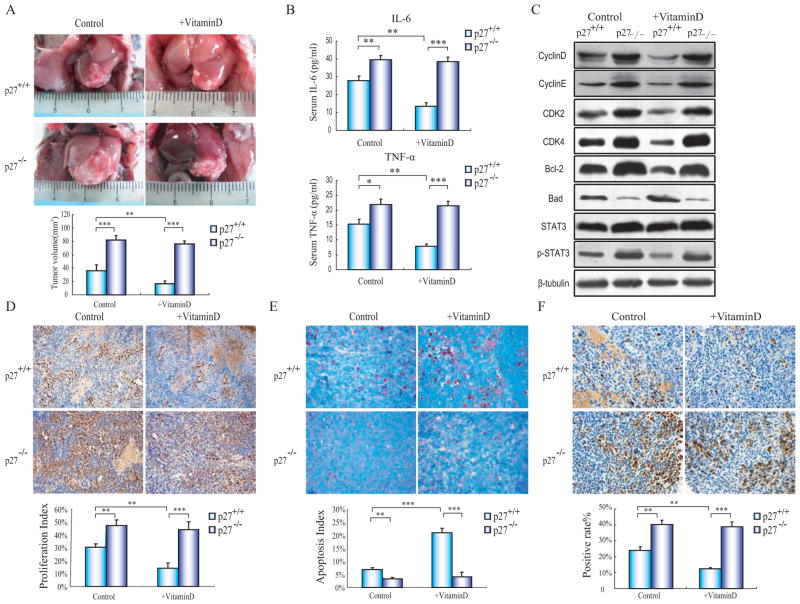
Finally, we transplanted these VDR silenced Hepa1–6 cells to p27+/+ and p27−/− mice (C56BL/J strain) and examined the tumor growth and the serum inflammatory cytokine, as well as the activation of STAT3 signaling. In consistent with prior results, the tumor growth, serum inflammatory cytokines, tumor cell proliferation, cell cycle related protein, and phosphorylation of STAT3 were increased while apoptosis was decreased in p27−/− orthotopic transplanted tumors. Nevertheless, 1,25(OH)2D3 supplement only effected these elements above in p27+/+ orthotopic transplanted tumors (Fig. 6A–E).
Fig. (6).
1,25(OH)2D3 inhibits VDR gene silenced tumor cell growth in wild-type mice, but not in p27kip1 gene knock-out mice. C57BL background derived Hepa1–6 cells were pre-treated with VDR shRNA and implanted to the p27+/+ or p27−/− mice, addition with 1,25(OH)2D3 or PBS intraperitoneal injection every day. (A) Gross appearances of representative livers with transplanted tumors in either normal or 1,25(OH)2D3-treated p27+/+ and p27−/− mice (n=6). (B) Serum IL-6 and TNF-α level. (C) Immunoblotting results of cell cycle, apoptosis-related, and STAT3 phosphorylation proteins in transplanted tumors from either 1,25(OH)2D3-untreated or -treated p27+/+ and p27−/− mice. (D) PCNA immunohistochemistry of representative tumor slices (magnification 200x). (E) TUNEL staining of representative tumor slices (magnification 400x). (F) p-STAT3 immunohistochemistry of representative tumor slices from either vehicle or 1,25(OH)2D3-treated p27+/+ and p27−/− mice (magnification 400x). The results are representative of at least 3 independent samples. *p<0.05, **p<0.005, ***p<0.001.
DISCUSSION
Nowadays, epidemiological studies indicate that reduced concentration of serum vitamin D is associated with an increased risk of many types of cancers [14, 15]. Although previous studies have indicated that 1,25(OH)2D3 inhibits the proliferation of human liver cancer cell line HepG2 in a dose dependent manner [28], the usage of nude mice and lack of a 1,25(OH)2D3 deficient animal model limits these research into the detailed mechanisms behind these observations. Since the 1α(ase)−/− mice has a significantly reduced survival time thus not available for chemical hepatocarcinogenesis, in this study, we conduct the HCC homotransplantation to 1α(ase) KO mice and their WT littermates. Despite the 1α(ase)−/− mice develop hypocalcemia, hypophosphatemia, retarded growth, and skeletal abnormalities characteristic of rickets, when fed with a high calcium after weaning, the serum calcium and phosphorus levels, growth, development, and skeletal phenotype are normalized [29]. Therefore, the usage of the 1α(OH)ase KO tumor bearing mouse enable us the first to demonstrate that the lack of 1,25(OH)2D3 accelerates HCC development in vivo.
Rapidly growing evidence reinforces the notion that excessively and chronically produced pro-inflammatory cytokines contribute to HCC initiation and progression [30]. The excessive production of pro-inflammatory cytokine IL-6 and TNF-α, generated by macrophages and lymphocytes in the inflammatory tumor microenvironment, lead tumor cells to exert anti-apoptotic and pro-angiogenic effects [31]. Meanwhile, clinical data has indicated that the inflammatory conditions of liver correlates with high circulating IL-6 levels, which is further elevated in patients who develop HCC [32]. In addition, a recent literature has demonstrated that the enhanced tumor formation and progression in carcinogen-induced obesity mice is attribute to the elevated tumor-promoting cytokines IL-6 and TNF-3, which further accelerates the proliferation of tumor cell through activating STAT3 signaling [33].
Considerable evidence suggests that the capability to inhibit inflammatory response makes 1,25(OH)2D3 has the potency to exert the anti-tumor activity as well [18]. For example, a resent literature has demonstrated that 1,25(OH)2D3 interrupts the activating of Wnt signaling and the accelerating of cell proliferation by macrophage-derived IL-1β in colon cancer cells [19]. Furthermore, 1,25(OH)2D3 also decreases the production of pro-inflammatory cytokine IL-6 through inactivating the p38 stress-induced kinase, which is considered valuable for prostate cancer prevention [20]. Moreover, 1,25(OH)2D3 can inhibit ConA-induced mouse hepatitis [21], and a poor vitamin D status is considered to aggravate NAFLD [22]. Consequently, 1,25(OH)2D3 has the potency to inhibit HCC development since both hepatitis and NAFLD are the major causes of HCC initiation. Paralleled with those findings above, our results show that the lack of 1,25(OH)2D3 in 1α(OH)ase ablation mice promotes tumor growth and serum inflammatory cytokines secretion, and conversely, 1,25(OH)2D3 supplement diminishes the secretion of serum inflammatory cytokine and the tumor growth concurrently. In addition, the growth inhibitory effect of 1,25(OH)2D3 in the VDR gene silenced tumor cells expels the direct cell cycle inhibitory effect of 1,25(OH)2D3, further indicating the positive correlation between elevated inflammatory cytokines and enhanced tumor development. The elevated production of inflammatory cytokine IL-6 and sequential activation of IL-6/STAT3 signaling will affect the target gene and thus enhancing cell cycle progression which is considered to be an important factor for HCC progression. And also, the elevated TNF-α can also promote HCC progression through inducing the expression of genes encoding NF-κB–dependent anti-apoptotic molecules, such as Bcl-2.
It has been generally accepted that an inflammatory microenvironment comprehending many innate immune cells and a variety of mediators such as cytokines and chemokines, will persistently activate the proliferation of the surrounding stroma cells such as hepatic stellate cells (HSCs) thus cause the tissue remodeling and liver fibrosis, and eventually predisposes chronic inflammation to neoplasia. Although a recent study has demonstrated that treatment with 1,25(OH)2D3 significantly reduces extracellular matrix deposition and lowers the fibrotic score in TAA-induced liver fibrosis through inhibiting the proliferation of HSCs [34]. On the other hand, recent studies have also demonstrated the elevated inflammatory cytokine and consequently activated STAT3 signaling in monocytes can also accelerate liver cancer progression [13]. Meanwhile, clinical data have validated that some chronic liver disease patients developed HCC are without liver fibrosis. Therefore, our findings further indicate that the direct tumor-promoting effect of inflammation in HCC initiation and progression.
Among the various types of cell-cycle regulators, the p27Kip1 protein is initially identified due to its ability to bind and inhibit cyclin/cdk2 complexes, thus leading to an arrest in the G1-phase of the cell cycle [35]. Previous studies have documented that loss of p27Kip1 enhances tumor progression in chronic hepatocyte injury-induced liver tumorigenesis and promotes carcinogen-induced mouse liver tumorigenesis [36, 37]. Furthermore, our recent studies have indicated that p27kip1 inactivation promotes DEN-PB induced liver hepatocarcinogenesis through enhancing inflammatory cytokine IL-6 and TNF-α secretion and STAT3 signaling activation in vivo. And we also demonstrate that p27 gene silencing in splenocytes will promote the proliferation of the co-cultured tumor cells [38]. Moreover, previous report has demonstrated that 1,25(OH)2D3 inhibits cell proliferation in many types of immune cells and subsequently diminishes the production of inflammatory cytokines through up-regulating p27kip1 [23]. In this study, we found that 1,25(OH)2D3 supplement only inhibits tumor development and decreases inflammatory cytokine level in p27kip1 WT mice, indicating the anti-inflammation effect of 1,25(OH)2D3 exerts in a p27kip1 dependent way.
CONCLUSION
Although 1,25(OH)2D3 has the potency to inhibit inflammatory response in several liver diseases, the fact that whether 1,25(OH)2D3 can inhibit HCC initiation and progression is still unclear. Our current study provides a novel finding that through up-regulating the cyclin dependent kinase inhibitor p27kip1, 1,25(OH)2D3 reduces the secretion of inflammatory cytokines and consequently inhibits the activation of STAT3 signaling, which eventually suppresses HCC development in tumor bearing mice. First, 1α(OH)ase ablation promotes tumor development and enhances inflammatory response, and conversely, supplying 1,25(OH)2D3 inhibits tumor development and diminishes inflammatory response. Second, the elevated serum inflammatory cytokine IL-6 and activated STAT3 signaling exists in the tumor bearing 1α(OH)ase−/− mice, while 1,25(OH)2D3 supplement decreases serum IL-6 concentration and inactivates STAT3 signaling. Third, 1,25(OH)2D3 and STAT3 signaling inhibitor NSC 74859 restrain the VDR gene silenced tumor cell proliferation through suppressing the inflammatory response and diminishing the secretion of inflammatory cytokines from immunocytes in vitro and in vivo. And finally, 1,25(OH)2D3 can not inhibit tumor initiation and progression in p27kip1 gene KO mice. In aggregate, although the precise mechanism of 1,25(OH)2D3 in regulating inflammation remains ambiguous, further studies on the anti-tumor effects of 1,25(OH)2D3 during hepatocarcinogenesis will provide better insights into the relationship between inflammation and cancer. In addition, the decreased vitamin D level in patients with chronic liver disease might be a consequence of impaired vitamin D synthesis and absorption, which in turn facilitates HCC initiation and progression. Concurrently, the use of 1,25(OH)2D3 may provide us an effective preventive or therapeutic strategies for HCC.
Acknowledgments
This study was supported by a grant from National Natural Science Foundation of China (No.81301981), The New Drug Target Discovery Project (Grant No. 08143073) of the First Affiliated Hospital, Xi’an Jiaotong University, China. The Fundamental Research Funds for the Central Universities (08143014) and project grants from the NCRR (P20 RR020151) and the NIGMS (P20 GM103505 and P30 GM103332-01) from the NIH. The contents of this report are solely the responsibility of the authors and do not necessarily reflect the official views of the Xi’an Jiaotong University, NSFC, the NIH, National Center for Research Resources (NCRR), or National Institute of General Medical Sciences (NIGMS).
ABBREVIATIONS
- HCC
Hepatocellular carcinoma
- 1,25(OH)2D3
1,25-dihydroxyvitamin D3
- NAFLD
Non-alcoholic fatty liver disease
- KO
Knockout
- CDK
Cyclin-dependent kinase
- TNF-α
Tumor necrosis factor-α
- IL-6
Interleukin-6
- DEN
Diethylnitrosamine
- 1α(OH)ase
1α-hydroxylase
- BrdU
5-bromo-2′-deoxy-uridine
- PB
Phenobarbital
Footnotes
CONFLICT OF INTEREST
The author(s) confirm that this article content has no conflicts of interest.
References
- 1.Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–1917. doi: 10.1016/S0140-6736(03)14964-1. [DOI] [PubMed] [Google Scholar]
- 2.McGlynn KA, London WT. The global epidemiology of hepatocellular carcinoma: present and future. Clin Liver Dis. 2011;15:223–243. doi: 10.1016/j.cld.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez PM, Villanueva A, Llovet JM. Systematic review: evidence-based management of hepatocellular carcinoma--an updated analysis of randomized controlled trials. Aliment Pharmacol Ther. 2006;23:1535–1547. doi: 10.1111/j.1365-2036.2006.02932.x. [DOI] [PubMed] [Google Scholar]
- 4.Portolani N, Coniglio A, Ghidoni S, Giovanelli M, Benetti A, Tiberio GA, Giulini SM. Early and late recurrence after liver resection for hepatocellular carcinoma: prognostic and therapeutic implications. Ann Surg. 2006;243:229–235. doi: 10.1097/01.sla.0000197706.21803.a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolligs FT, Hoffmann RT, Winkel M, Bruns CJ, Herrmann K, Jakobs TF, Lamerz R, Trumm C, Zech CJ, Wilkowski R, Graeb C. Diagnosis and multimodal therapy for hepatocellular carcinoma. Z Gastroenterol. 2011;48:274–288. doi: 10.1055/s-0028-1109901. [DOI] [PubMed] [Google Scholar]
- 6.Lin WW, Karin MA. Cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest. 2007;117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 10.Tilg H, Wilmer A, Vogel W, Herold M, Nolchen B, Judmaier G, Huber C. Serum levels of cytokines in chronic liver diseases. Gastroenterology. 1992;103:264–274. doi: 10.1016/0016-5085(92)91122-k. [DOI] [PubMed] [Google Scholar]
- 11.Prieto J. Inflammation, HCC and sex: IL-6 in the centre of the triangle. J Hepatol. 2008;48:380–381. doi: 10.1016/j.jhep.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 12.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2009;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu WY, Li J, Wu ZS, Zhang CL, Meng XL. STAT3 activation in monocytes accelerates liver cancer progression. BMC Cancer. 2011;11:506. doi: 10.1186/1471-2407-11-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giovannucci E. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol. 2009;19:84–88. doi: 10.1016/j.annepidem.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Deeb KK, Trump DL, Johnson CS. Vitamin D signalling pathways in cancer: potential for anticancer therapeutics. Nat Rev Cancer. 2007;7:684–700. doi: 10.1038/nrc2196. [DOI] [PubMed] [Google Scholar]
- 16.Gocek E, Studzinski GP. Vitamin D and differentiation in cancer. Crit Rev Clin Lab Sci. 2009;46:190–209. doi: 10.1080/10408360902982128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhalla AK, Amento EP, Clemens TL, Holick MF, Krane SM. Specific high-affinity receptors for 1,25-dihydroxyvitamin D3 in human peripheral blood mononuclear cells: presence in monocytes and induction in T lymphocytes following activation. J Clin Endocrinol Metab. 1983;57:1308–1310. doi: 10.1210/jcem-57-6-1308. [DOI] [PubMed] [Google Scholar]
- 18.Vanoirbeek E, Krishnan A, Eelen G, Verlinden L, Bouillon R, Feldman D, Verstuyf A. The anti-cancer and anti-inflammatory actions of 1,25(OH)(2)D(3) Best Pract Res Clin Endocrinol Metab. 2011;25:593–604. doi: 10.1016/j.beem.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah S, Islam MN, Dakshanamurthy S, Rizvi I, Rao M, Herrell R, Zinser G, Valrance M, Aranda A, Moras D, Norman A, Welsh J, Byers SW. The molecular basis of vitamin D receptor and beta-catenin crossregulation. Mol Cell. 2006;21:799–809. doi: 10.1016/j.molcel.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Nonn L, Peng L, Feldman D, Peehl DM. Inhibition of p38 by vitamin D reduces interleukin-6 production in normal prostate cells via mitogen-activated protein kinase phosphatase 5: implications for prostate cancer prevention by vitamin D. Cancer Res. 2006;66:4516–4524. doi: 10.1158/0008-5472.CAN-05-3796. [DOI] [PubMed] [Google Scholar]
- 21.Hu XD, Jiang SL, Liu CH, Hu YY, Liu C, Sun MY, Chen GF, Liu P. Preventive effects of 1,25-(OH)2VD3 against ConA-induced mouse hepatitis through promoting vitamin D receptor gene expression. Acta Pharmacol Sin. 2010;31:703–708. doi: 10.1038/aps.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Drocourt L, Ourlin JC, Pascussi JM, Maurel P, Vilarem MJ. Expression of CYP3A4, CYP2B6, and CYP2C9 is regulated by the vitamin D receptor pathway in primary human hepatocytes. J Biol Chem. 2002;277:25125–25132. doi: 10.1074/jbc.M201323200. [DOI] [PubMed] [Google Scholar]
- 23.Yang ES, Burnstein; KL. Vitamin D inhibits G1 to S progression in LNCaP prostate cancer cells through p27Kip1 stabilization and Cdk2 mislocalization to the cytoplasm. J Biol Chem. 2003;278:46862–46868. doi: 10.1074/jbc.M306340200. [DOI] [PubMed] [Google Scholar]
- 24.Petta S, Camma C, Scazzone C, Tripodo C, Di Marco V, Bono A, Cabibi D, Licata G, Porcasi R, Marchesini G, Craxi A. Low vitamin D serum level is related to severe fibrosis and low responsiveness to interferon-based therapy in genotype 1 chronic hepatitis C. Hepatology. 2010;51:1158–1167. doi: 10.1002/hep.23489. [DOI] [PubMed] [Google Scholar]
- 25.Pappa HM, Bern E, Kamin D, Grand RJ. Vitamin D status in gastrointestinal and liver disease. Curr Opin Gastroenterol. 2008;24:176–183. doi: 10.1097/MOG.0b013e3282f4d2f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malham M, Jorgensen SP, Ott P, Agnholt J, Vilstrup H, Borre M, Dahlerup JF. Vitamin D deficiency in cirrhosis relates to liver dysfunction rather than aetiology. World J Gastroenterol. 2011;17:922–925. doi: 10.3748/wjg.v17.i7.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLuca HF. The kidney as an endocrine organ for the production of 1, 25-dihydroxyvitamin D3, a calcium-mobilizing hormone. N Engl J Med. 1973;289:359–365. doi: 10.1056/NEJM197308162890710. [DOI] [PubMed] [Google Scholar]
- 28.Pourgholami MH, Akhter J, Lu Y, Morris DL. In vitro and in vivo inhibition of liver cancer cells by 1,25-dihydroxyvitamin D3. Cancer Lett. 2000;151:97–102. doi: 10.1016/s0304-3835(99)00416-4. [DOI] [PubMed] [Google Scholar]
- 29.Panda DK, Miao D, Bolivar I, Li J, Huo D, Hendy GN, Goltzman D. Inactivation of the 25-hydroxyvitamin D 1alpha-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcium and vitamin D on skeletal and mineral homeostasis. J Biol Chem. 2004;279:16754–16766. doi: 10.1074/jbc.M310271200. [DOI] [PubMed] [Google Scholar]
- 30.Ben-Baruch A. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin Cancer Biol. 2006;16:38–52. doi: 10.1016/j.semcancer.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 31.Khan QJ, Reddy PS, Kimler BF, Sharma P, Baxa SE, O’Dea AP, Klemp JR, Fabian CJ. Effect of vitamin D supplementation on serum 25-hydroxy vitamin D levels, joint pain, and fatigue in women starting adjuvant letrozole treatment for breast cancer. Breast Cancer Res Treat. 2010;119:111–118. doi: 10.1007/s10549-009-0495-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smyth MJ, Cretney E, Kershaw MH, Hayakawa Y. Cytokines in cancer immunity and immunotherapy. Immunol Rev. 2004;202:275–293. doi: 10.1111/j.0105-2896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 33.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abramovitch S, Dahan-Bachar L, Sharvit E, Weisman Y, Ben Tov A, Brazowski E, Reif S. Vitamin D inhibits proliferation and profibrotic marker expression in hepatic stellate cells and decreases thioacetamide-induced liver fibrosis in rats. Gut. 2011;60:1728–1737. doi: 10.1136/gut.2010.234666. [DOI] [PubMed] [Google Scholar]
- 35.Kossatz U, Malek NP. p27: tumor suppressor and oncogene …? Cell Res. 2007;17:832–833. doi: 10.1038/cr.2007.86. [DOI] [PubMed] [Google Scholar]
- 36.Sun D, Ren H, Oertel M, Sellers RS, Zhu L. Loss of p27Kip1 enhances tumor progression in chronic hepatocyte injury-induced liver tumorigenesis with widely ranging effects on Cdk2 or Cdc2 activation. Carcinogenesis. 2007;28:1859–1866. doi: 10.1093/carcin/bgm079. [DOI] [PubMed] [Google Scholar]
- 37.Sun D, Ren H, Oertel M, Sellers RS, Shafritz DA, Zhu L. Inactivation of p27Kip1 promotes chemical mouse liver tumorigenesis in the resistant strain C57BL/6J. Mol Carcinog. 2008;47:47–55. doi: 10.1002/mc.20360. [DOI] [PubMed] [Google Scholar]
- 38.Guo J, Ma Q, Zhou X, Shan T, Fan P, Miao D. Inactivation of p27kip1 promotes carcinogens induced liver hepatocarcinogenesis through enhancing inflammatory cytokine secretion and STAT3 signaling activation. J Cell Physiol. 2013 Mar 4; doi: 10.1002/jcp.24357. [DOI] [PubMed] [Google Scholar]