SUMMARY
Immunization with attenuated pre-erythrocytic malaria parasites can confer sterile protection against malaria in humans and rodents, and a single pre-erythrocytic antigen incorporated in a subunit vaccine has substantially reduced clinical Plasmodium falciparum malaria episodes in African infants during phase 2 trials. Building upon this success has been hindered by technical obstacles that limit research on pre-erythrocytic parasites, especially the liver stage (LS) parasites, and by an incomplete understanding of the immune mechanisms that confer protection in humans. Recent improvements in growing and isolating LS parasites have allowed progress in defining the transcriptome and proteome of the LS parasite, although more work remains to be done particularly for the early LS parasite of P. falciparum. Next generation pre-erythrocytic antigens can be assessed and prioritized based on immunization studies in animals, and on models of immunity such as attenuated parasite vaccines that confer sterile protection or naturally acquired LS-specific immune responses that correlate with protection in endemic areas. Although mechanisms of protection in humans remain poorly understood, the availability of a human malaria challenge model for early clinical testing of candidate vaccines is a valuable tool to confirm which immunogens should move forward to larger field trials.
Keywords: malaria, Plasmodium, pre-erythrocytic, vaccines
VACCINES AGAINST PRE-ERYTHROCYTIC PARASITES
The worldwide malaria burden would be reduced by vaccines that either prevent infection or prevent transmission. Vaccines that target the earliest stages of the malaria parasite in the human host have the potential to achieve both goals. The mosquito inoculates sporozoite forms into skin during its blood meal, and within an hour these have traversed vascular endothelium to enter the blood stream and passed through the circulation to the liver where they invade hepatocytes and initiate liver stage (LS) development. Because the sporozoite and liver stages precede the blood stage when disease and death occur, they are referred to as the pre-erythrocytic stages. The sporozoite is accessible to antibody while in the skin and bloodstream, and therefore much effort has focused on sporozoite surface antigens, most famously the major surface antigen called circumsporozoite protein (CSP). During LS development, parasite encoded proteins, including CSP, can be the target of CD4+ and CD8+ T-cell-mediated effector mechanisms that confer protection in animals [reviewed in (1)]. However, few LS antigens have been identified until recently, owing to the relative inaccessibility and paucity of the LS parasite in vivo, and our inability to grow Plasmodium falciparum LS parasites in vitro. Technical advances now allow the enrichment of sparse intrahepatocytic parasites from abundant host material (2), as well as improved in vitro LS culture (3), including the axenic (or host cell-free) growth and differentiation of LS parasites (4).
Proteins expressed by pre-erythrocytic parasites have strong evidence and appeal as vaccine antigens. First, the mosquito transmission event represents a bottleneck in the parasite lifecycle. Only a few dozen or hundred sporozoites are transmitted to the mammalian host (5), and not all of these reach the liver (6). The low parasite numbers increase the likelihood for complete elimination by vaccines, and reduce the likelihood that resistant mutants will emerge, in contrast to erythrocytic stage parasites that can rapidly multiply to number in the trillions in a single individual. Second, sterile immunity against LS parasites is achievable. Protective immunity was demonstrated in rodents in 1967 by Ruth Nussenzweig using radiation-attenuated sporozoites as vaccines (7), and shortly thereafter was achieved in humans (8). Scientists are now developing methods to manufacture irradiated sporozoites as a vaccine product (9), which at present requires that parasites be dissected from infected mosquitos. More recently, genetic attenuation has been used to modify rodent parasites that arrest during LS development and induce protection (10,11). Genetically-attenuated P. falciparum parasites are currently being prepared to test this vaccine concept in human trials (12,13).
Third, subunit pre-erythrocytic antigen vaccines have already elicited partial protection in human volunteers. A vaccine targeting CSP, expressed as a recombinant protein fused to hepatitis B surface antigen to form a viral-like particle and referred to as RTS,S, is already in late-stage clinical trials. In phase IIb trials in Mozambique, RTS,S formulated with the adjuvant AS02 had an adjusted vaccine efficacy against clinical malaria of 35.5% in infants over 6 months of follow-up (14), and of 35% in older toddlers sustained up to 18 months (15). In Tanzania, RTS,S/AS02 co-administered with standard infant vaccines did not interfere with antibody responses to the latter, and maintained efficacy against clinical malaria (16). RTS,S formulated with the adjuvant AS01E achieved vaccine efficacy of 53% over 4.5–10.5 months of follow-up among 5- to 17-month-old children in Kenya and Tanzania (17), exceeding the efficacy observed with RTS,S/AS02 in Mozambique.
Importantly, while the long-term antibody response against CSP in vaccinated children in Mozambique waned, protection against clinical malaria was evident for up to 21 months (15). RTS,S also appeared to reduce pneumonia hospitalizations in Tanzanian infants (16), and severe adverse events in East African infants and toddlers (17), compared to control vaccinees. Notably, by the end of follow-up in the Mozambique studies, nearly 80% of vaccinated children had experienced a parasitaemia (15). These trials suggest that RTS,S confers protection from clinical malaria and potentially other severe adverse events, but does this without completely preventing infection. The immune basis for RTS,S-induced protection has yet to be fully defined (17), although CD8 T cells do not appear to be involved (18). Further, the absence of sterile protection suggests significant scope to improve upon the efficacy of RTS,S.
The efficacy of RTS,S might be improved with more powerful adjuvants (17), or by the incorporation of additional protective antigens. In mice immunized with attenuated parasites, the CSP protein is an important but not exclusive target of protective immunity. For example, in CSP-transgenic mice that are tolerized to CSP antigen, protection afforded by irradiated P. yoelii sporozoites was reduced but not ablated (19). Mice immunized with irradiated P. berghei sporozoites were completely protected against transgenic P. berghei parasites expressing P. falciparum CSP, even in the absence of a functional response against the P. falciparum antigen (20). These elegant studies suggest that additional LS antigens can mediate protection, and offer models to delineate the relative contributions of CSP and novel antigens to protective immunity.
Another vaccine designed to induce protective immunity against pre-erythrocytic parasites, a composite antigen comprised of multiple epitopes fused to thrombospondin-related adhesion protein (ME-TRAP), has been tested in humans. Preliminary trials using a DNA-primed, viral-vectored boost immunization schedule in malaria naïve subjects produced a significantly delayed onset of blood stage parasitaemia (21). However, in Phase IIb trials in semi-immune adults, the vaccine was highly immunogenic but not protective in 372 Gambian men (22). Subsequently, 405 Kenyan children were immunized in a prime/boost system involving two viral vectors; these children generated moderate immune responses against the TRAP protein but also were not protected (23). Malaria exposure might have modulated the vaccine-induced immune response in the Gambian and Kenyan semi-immune populations. The studies emphasize that partially protective immunity achieved with a vaccine in malaria-naïve populations might not predict outcomes in malaria-experienced individuals.
MECHANISMS OF PRE-ERYTHROCYTIC IMMUNITY
While the intrahepatic immune response against P. falciparum parasites is extremely challenging to study, the parallel process in the mouse model has been relatively well characterized. Like wild-type parasites, irradiated sporozoites invade hepatocytes. However, shortly after infection their development arrests at the uninucleate trophozoite stage (24). The infected hepatocyte is a primary target of protective immunity in the murine irradiated sporozoite model (25), and antigens expressed during the early LS, therefore, represent potential targets of protective immunity. The extended presence of arrested parasites in the MHC-I-expressing hepatocyte may render parasite antigens more accessible to cell-mediated immune responses (26), or attenuation may disrupt parasite mechanisms for subverting host acquisition of protective LS immunity.
The irradiated sporozoite model of immunity has allowed the cells mediating protection in mice to be identified. Protection involves several mediators and can vary between models. Depletion studies show that CD8+ T cells play a central role in clearing LS infections in some animal models (27), and CSP-specific CD8+ T cells above a specific level correlate with protection in some models (28). Injection of IFN-γ alone can inhibit LS parasite growth (29), suggesting that this cytokine is a key effector molecule involved in protection. However, protection against irradiated parasites can also be achieved in the absence of CD8+ T cells (30). Additionally, CD8+ T cell-mediated protection is largely abolished when CD4+ T cells are depleted (31), demonstrating that CD8+ T cells are not sufficient to confer immunity in some models. The genetic background of both parasite and mouse impacts the nature of the protective response. For example, anti-IFN-γ antibodies ablate protection in the P. berghei system, but may have a limited effect in the P. yoelii system (32,33). In addition to cellular responses, antibody responses such as those against CSP can clear sporozoites before hepatocyte invasion and contribute to protection (34).
Although irradiated sporozoites confer full protection in humans, and RTS,S achieves partial protection, we nevertheless understand little about protective immunity induced by either vaccine strategy in humans. Irradiated sporozoite vaccines do induce cellular responses in humans (35), but CSP-specific CD8+ T cell responses are largely absent from individuals immunized with RTS,S (18), implying that this important effector of protection in mice may not be relevant to the type of protection induced by RTS,S in humans. In Gambian adults immunized with RTS,S, CSP peptide-specific IFN-γ-secreting T cells measured in a cultured ELISpot assay were significantly associated with vaccine-induced protection (36). By contrast, antibodies and proliferative responses to CSP antigens did not correlate with protection. Our limited ability to identify robust correlates of protection in humans may indicate that diverse mechanisms, or combinations of mechanisms, can confer resistance and these might vary between protected individuals. Without clear and consistent correlates of protection, the selection of antigens for next generation vaccines is problematic, and must rely on parallel lines of evidence and inconclusive criteria.
A NEW UNIVERSE OF LS ANTIGENS
High-throughput approaches to define LS gene and protein expression profiles have increased our understanding of parasite biology, and provided a more comprehensive list from which to select next-generation vaccine candidates. Since 2002, the genomes of six key Plasmodium species have been published. Genome data for three species infectious to humans (37–39) and three rodent model species (40,41) have been completed. For most of these species, data comparing the blood stage transcriptomes and proteomes are also available [reviewed in (42)]. Progress on expression profiles has been slower with LS parasites, which have been technically difficult to isolate in sufficient quantities for any of the Plasmodium species.
The pace of genome-scale LS studies is accelerating as parasite manipulation and genomic technologies improve. The development of axenic methods for cultivation of Plasmodium sporozoites into early LS-like parasites (4) allowed the partial definition of the P. yoelii 24 h LS transcriptome (43). Axenically grown P. yoelii parasites yielded expressed sequence tags (ESTs) representing 652 unique transcripts in the first study to detail at a genome-scale the transcriptional changes associated with the switch from sporozoite to LS. Many hallmarks of this transformation were detected; for example, the axenic parasite expresses A-type ribosomal RNAs (4), which are characteristic of development toward the blood stages. This suggests that transcriptional changes detected in this type of parasite are biologically relevant. Laser capture microdissection has also been used to isolate infected hepatocytes from host material (44). Plasmodium yoelii LS schizonts were micro-dissected from mouse liver, and the resulting ESTs were sequenced, revealing 623 P. yoelii genes expressed at 40 h of LS development.
Infection-associated transcriptional changes that occur in salivary gland sporozoites of P. falciparum after exposure to host cells have recently been profiled (45). Sporozoites co-cultured for 1 h with human hepatocytes in tissue culture increased their transcription of 532 genes, compared to nonexposed salivary gland sporozoites. Transcription of 21 genes was confirmed by quantitative PCR, and the expression of four encoded proteins was confirmed in sporozoites or infected hepatocytes. Two sporozoite proteins (PFD0425w and PF08_0005) localized in a pattern similar to CSP, suggesting display on the sporozoite surface. Antibodies against both proteins inhibited sporozoite invasion of hepatocytes, although to a lesser degree than anti-CSP antibodies. Because antibodies against PFD0425w can be detected in irradiated-sporozoite immunized humans (46), it will be valuable to assess whether antisera raised against PFD0425w and CSP in combination may be additive or synergistic for inhibiting sporozoite invasion. The other two proteins, PFL0065w and PFB0105c, were detected in the LS parasite, but not in the sporozoite. Both proteins were immunolocalized at the periphery of the early LS parasite, similar to the pattern seen with CSP. PFB0105c contains a putative PEXEL domain, and PFL0065w contains a predicted signal peptide, consistent with their localization at or near the PVM.
Using flow cytometry to sort GFP-expressing P. yoelii LS parasites, Tarun et al. (47) were able to obtain sufficient parasite material from infected rodent livers for DNA microarrays and for proteomics studies. Approximately 1100 genes were found to be differentially expressed at each of three mid- to late-LS timepoints, compared to blood stage parasites and to sporozoites. In parallel, 712 proteins of P. yoelii LS parasites were identified by mass spectrometry, including a so-called secretome of 93 proteins with putative signal peptides and 76 with predicted transmembrane domains. Secreted proteins will presumably be more accessible to the host proteasome, and it will be useful to assess whether these are more likely to elicit cellular immune responses or to confer protection as vaccine immunogens. The vaccine antigen CSP, for example, contains PEXEL/VTS motifs that mediate its export across the parasitophorous vacuole and into the hepatocyte cytoplasm (48).
Functional genomics studies of LS development have thus far profiled the transcriptome of P. falciparum at the hepatocyte invasion timepoint, and the transcriptome and proteome of the mid-to-late LS P. yoelii parasite. Expression profiles from early LS P. yoelii, and from early and late LS P. falciparum, remain to be completed. Because radiation-attenuated parasites arrest during early LS development (24), antigens expressed at this stage might be particularly valuable for subunit vaccine development. Proteomic analyses of P. falciparum in both the early and late LS await technical advances. Similar to the progress made with GFP-expressing P. yoelii-infected hepatocytes, methods for enrichment of P. falciparum LS parasites will be useful for expression profiling of these key vaccine targets.
PRIORITIZING NEXT GENERATION VACCINE ANTIGENS
In the absence of a single protective immune effector to guide the selection of pre-erythrocytic vaccine antigens, the candidates can be comparatively assessed in several models of immunity to determine which consistently display the features that might predict protective efficacy. Using CSP as a benchmark, an ideal candidate antigen would be present in the LS parasite, effective as an immunogen to protect mice, a target of immune effectors especially CD8+ T cells in rodents protected by attenuated parasite vaccines, and a target of immune effectors in humans protected by attenuated parasite vaccines or by naturally occurring exposure (Figure 1). The antigens that best meet these criteria could then be assessed as immunogens in human challenge studies, to confirm which should proceed to field testing and efficacy trials. Candidates that were synergistic with CSP immunogens for inducing protection in animals might be of particular interest, as a strategy to enhance the efficacy of RTS,S by incorporating additional antigens.
Figure 1.
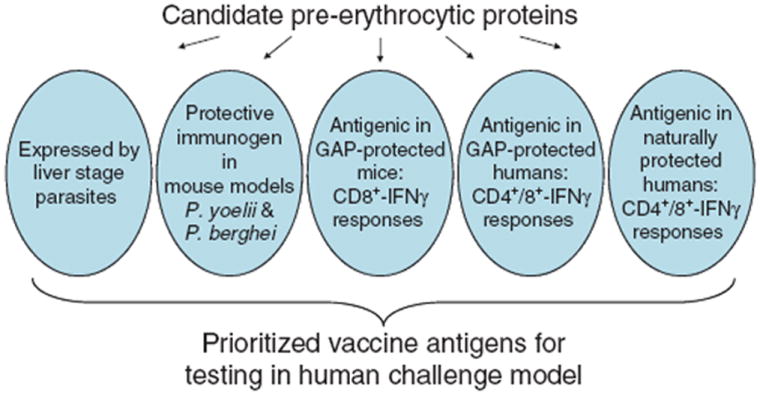
Potential criteria for selection of candidate antigens for pre-erythrocytic malaria vaccines. Hundreds of candidates have already been identified in functional genomics studies of pre-erythrocytic parasites, and future studies will likely expand this list, emphasizing the need for a systematic process of assessment and prioritization. Proteins expressed by liver stage parasites can be compared by several criteria that may be relevant to their potential role as human vaccines, such as their antigenicity in protected animals and humans, and their efficacy as vaccines in animal models of malaria. The candidates that meet most or all these criteria can be validated in human challenge trials, in which malaria-naïve individuals vaccinated with candidate immunogens receive sporozoite challenge by infected mosquito bite. Immunogens that prevent infection (i.e. appearance of blood stage parasitaemia) can be transitioned to field testing and efficacy trials in endemic areas. GAP, genetically attenuated parasite.
Mouse immunization studies: predicting vaccine efficacy in humans
DNA vaccinations have been used to screen panels of parasite genes as immunogens for protection in mouse models. In a survey of 19 P. yoelii genes from a sporozoite cDNA library, only CSP and the novel antigen PY01316 prepared as single-component DNA vaccines could protect Balb/c mice from subsequent sporozoite challenge (49). Of note, CSP elicited protective immunity when delivered by intramuscular inoculation but not by gene gun, whereas PY01316 elicited protective immunity when delivered by gene gun but not by intramuscular inoculation. Different pre-erythrocytic antigens might be targeted by different effector mechanisms involved in protection, and therefore formulations or routes of delivery required to confer protection may vary depending on the antigen.
Immunization studies can assess candidate immunogens for their efficacy against different parasite species and across different mouse genetic backgrounds. The partial protection afforded by CSP in clinical trials is mirrored in the partial protection of CSP as a DNA, protein, or viral vectored immunogen in multiple immunogenetic backgrounds of mice [reviewed in (1)]. In mouse systems, this makes CSP a useful benchmark for comparative studies, and allows immunogens to be combined with CSP to determine which may increase the degree of protection achieved with CSP alone. Notably, several antigens that conferred protection in mice failed to elicit protective immunity in humans when the P. falciparum orthologue was used, emphasizing that mouse studies are imperfect predictors for successful human vaccines, and should be interpreted with caution (21,50).
Attenuated sporozoite model: defining antigens associated with sterile immunity
The immune mechanisms conferring protection in mice after vaccination with attenuated parasites have been described in some detail (see above). In several of these models, IFN-γ-expressing CD8+ T cells play a key (28) but not always exclusive role in protection. CSP-specific antibodies, CD4+ T cells, and CD8+ T cells can each confer protection when passively transferred to naïve mice (34,51,52). Thus, pre-erythrocytic antigens can be comparatively assessed for reactivity with cellular and humoral responses in protected mice. Such studies can use radiation-attenuated sporozoites, or genetically attenuated sporozoites, as immunogens. Different genetically attenuated parasite vaccines were recently observed to confer different degrees and durations of protection (53), although other studies suggest similar levels of protection induced by radiation attenuated and different genetically attenuated parasite vaccines (54). A panel of whole parasite vaccines that varied in their efficacy might enable a more refined interrogation of protective immunity, and a better definition of the antigens specifically related to long-term protection.
Importantly, parasite attenuation is not required to induce protection. Live wild-type sporozoites can induce sterile protection directed against LS parasites when mice are inoculated and treated with chloroquine (55) or primaquine (56), a strategy termed infection-treatment vaccination. Infection-treatment vaccination with blood-stage parasites and chloroquine also confers protection, with immune responses directed to blood stage but also sporozoite and LS parasites (57). These models of protection that use wild-type parasites as vaccines might be useful for revealing novel mechanisms and targets of protection.
Our understanding of protective immunity in humans vaccinated with attenuated sporozoites is more rudimentary. CSP-specific antibody and cellular responses develop in irradiated sporozoite-immunized individuals (35), and their T cells proliferate in response to LSA-1 peptides (58). Based on knowledge gained from mouse models, LS antigens targeted by CD8+-IFN-γ responses would be of great interest as vaccine candidates. However, CSP-specific immunity confers protection in RTS,S-vaccinated individuals in the absence of detectable CD8+ T cell responses (18). CD4+ T cell IFN-γ responses to LS proteins and functional antibodies to sporozoite surface antigens that develop after vaccination with attenuated sporozoites can be considered criteria for antigen prioritization in addition to CD8+ T cell IFN-γ or polycytokine responses.
Naturally acquired immunity: assessing the effect of malaria on malaria vaccines
The ME-TRAP vaccination studies suggest that natural malaria exposure can subvert the development of partially protective responses after subunit vaccination. In naturally exposed Kenyans, memory T-cell IFN-γ responses against TRAP correlated with reduced malaria incidence (59), but these responses may be subverted during malaria episodes (60). Therefore, candidate antigens should be assessed by measuring antigen-specific responses in naturally exposed individuals and associating those responses with protection. Studies of the LS-specific protein LSA-1 have revealed the complexity of the immune response directed against LS parasites in naturally exposed humans [reviewed in (61)], and suggest that naturally acquired immunity to LS parasites is involved in protection. LSA-1-specific IFN-γ responses have been associated with an increase in time to next infection and a lower overall parasitaemia in children in Gabon (62). IL-10 responses to recombinant LSA-1 protein fragments were predictive of increased time to next infection and reduced parasite density and frequency in a treatment-reinfection study in adolescent and adult Kenyan males (63). Antibody responses to LSA-1 have been associated with a reduction in the risk of clinical malaria and a reduction in cumulative incidence of malaria in Kenyan children (64).
Collectively, these studies show that wild-type LS parasites are immunogenic in naturally exposed humans, and that immune responses to LS parasites correlate with protection in endemic populations including children. Similar studies should compare novel LS antigens to CSP and LSA-1 as targets of immune responses that correlate with protection. More evidence is needed to know whether pre-erythrocytic antigens identified by immunocorrelation studies in naturally exposed individuals are more likely confer protection when used as vaccines. Such studies may also give insights into which vaccine-specific immune responses occur naturally, and therefore will be boosted by exposure to the parasite after immunization.
CONCLUSION
In humans, complete protection against malaria can be induced by immunization with irradiated sporozoites, and partial protection from clinical disease occurs after vaccination with the CSP-based subunit vaccine RTS,S. Efforts are underway to develop processes needed to manufacture and vial irradiated sporozoites as a deliverable vaccine, and the initial trials using this product are imminent (9). Ultimately, a subunit vaccine would be preferable owing to ease of manufacture and delivery. The selection of novel pre-erythrocytic antigens for subunit vaccines is hindered because we do not understand protective immunity in humans. CSP is expressed on the surface of sporozoites where it is a target of antibody, and is subsequently carried into the hepatocyte by the LS parasite where it can be a target of CD4+ and CD8+ T cell responses. CSP can therefore serve as a model for studies of novel pre-erythrocytic antigens, and provides a useful benchmark for comparisons of immunogenicity and antigenicity. Genome-based studies have defined in part the universe of pre-erythrocytic proteins that can be considered for next generation vaccines; the transcriptome and proteome of early LS parasites remains to be determined. These novel LS proteins, along with sporozoite antigens, should be carefully assessed in our existing models of immunity –whole organism vaccination, subunit vaccination, and naturally acquired protection – to determine which antigens have the best combination of features that suggest likely efficacy as vaccines in humans. The availability of a human challenge model allows us to assess a limited number of immunogens in a highly controlled setting, before recommending those candidates that should proceed to larger field trials. Recent advances portend further progress in the development of pre-erythrocytic malaria vaccines, building on earlier successes such as attenuated sporozoite vaccines and RTS,S.
References
- 1.Doolan DL, Martinez-Alier N. Immune response to pre-erythrocytic stages of malaria parasites. Curr Mol Med. 2006;6:169–185. doi: 10.2174/156652406776055249. [DOI] [PubMed] [Google Scholar]
- 2.Tarun AS, Baer K, Dumpit RF, et al. Quantitative isolation and in vivo imaging of malaria parasite liver stages. Int J Parasitol. 2006;36:1283–1293. doi: 10.1016/j.ijpara.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Sattabongkot J, Yimamnuaychoke N, Leelaudomlipi S, et al. Establishment of a human hepatocyte line that supports in vitro development of the exo-erythrocytic stages of the malaria parasites Plasmodium falciparum and P. vivax. Am J Trop Med Hyg. 2006;74:708–715. [PubMed] [Google Scholar]
- 4.Kaiser K, Camargo N, Kappe SH. Transformation of sporozoites into early exoerythrocytic malaria parasites does not require host cells. J Exp Med. 2003;197:1045–1050. doi: 10.1084/jem.20022100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medica DL, Sinnis P. Quantitative dynamics of Plasmodium yoelii sporozoite transmission by infected anopheline mosquitoes. Infect Immun. 2005;73:4363–4369. doi: 10.1128/IAI.73.7.4363-4369.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yamauchi LM, Coppi A, Snounou G, Sinnis P. Plasmodium sporozoites trickle out of the injection site. Cell Microbiol. 2007;9:1215–1222. doi: 10.1111/j.1462-5822.2006.00861.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of plasmodium berghei. Nature. 1967;216:160–162. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 8.Clyde DF, Most H, McCarthy VC, Vanderberg JP. Immunization of man against sporozite-induced falciparum malaria. Am J Med Sci. 1973;266:169–177. doi: 10.1097/00000441-197309000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Luke TC, Hoffman SL. Rationale and plans for developing a non-replicating, metabolically active, radiation-attenuated Plasmodium falciparum sporozoite vaccine. J Exp Biol. 2003;206:3803–3808. doi: 10.1242/jeb.00644. [DOI] [PubMed] [Google Scholar]
- 10.Mueller AK, Camargo N, Kaiser K, et al. Plasmodium liver stage developmental arrest by depletion of a protein at the parasite-host interface. Proc Natl Acad Sci USA. 2005;102:3022–3027. doi: 10.1073/pnas.0408442102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mueller AK, Labaied M, Kappe SH, Matuschewski K. Genetically modified Plasmodium parasites as a protective experimental malaria vaccine. Nature. 2005;433:164–167. doi: 10.1038/nature03188. [DOI] [PubMed] [Google Scholar]
- 12.Mikolajczak SA, Aly AS, Kappe SH. Preerythrocytic malaria vaccine development. Curr Opin Infect Dis. 2007;20:461–466. doi: 10.1097/QCO.0b013e3282ef6172. [DOI] [PubMed] [Google Scholar]
- 13.van Schaijk BC, Janse CJ, van Gemert GJ, et al. Gene disruption of Plasmodium falciparum p52 results in attenuation of malaria liver stage development in cultured primary human hepatocytes. PLoS ONE. 2008;3:e3549. doi: 10.1371/journal.pone.0003549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aponte JJ, Aide P, Renom M, et al. Safety of the RTS,S/AS02D candidate malaria vaccine in infants living in a highly endemic area of Mozambique: a double blind randomised controlled phase I/IIb trial. Lancet. 2007;370:1543–1551. doi: 10.1016/S0140-6736(07)61542-6. [DOI] [PubMed] [Google Scholar]
- 15.Alonso PL, Sacarlal J, Aponte JJ, et al. Duration of protection with RTS,S/AS02A malaria vaccine in prevention of Plasmodium falciparum disease in Mozambican children: single-blind extended follow-up of a randomised controlled trial. Lancet. 2005;366:2012–2018. doi: 10.1016/S0140-6736(05)67669-6. [DOI] [PubMed] [Google Scholar]
- 16.Abdulla S, Oberholzer R, Juma O, et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359:2533–2544. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- 17.Bejon P, Lusingu J, Olotu A, et al. Efficacy of RTS,S/AS01E vaccine against malaria in children 5 to 17 months of age. N Engl J Med. 2008;359:2521–2532. doi: 10.1056/NEJMoa0807381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalvani A, Moris P, Voss G, et al. Potent induction of focused Th1-type cellular and humoral immune responses by RTS,S/SBAS2, a recombinant Plasmodium falciparum malaria vaccine. J Infect Dis. 1999;180:1656–1664. doi: 10.1086/315074. [DOI] [PubMed] [Google Scholar]
- 19.Kumar KA, Sano G, Boscardin S, et al. The circumsporozoite protein is an immunodominant protective antigen in irradiated sporozoites. Nature. 2006;444:937–940. doi: 10.1038/nature05361. [DOI] [PubMed] [Google Scholar]
- 20.Gruner AC, Mauduit M, Tewari R, et al. Sterile protection against malaria is independent of immune responses to the circumsporozoite protein. PLoS ONE. 2007;2:e1371. doi: 10.1371/journal.pone.0001371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McConkey SJ, Reece WH, Moorthy VS, et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nat Med. 2003;9:729–735. doi: 10.1038/nm881. [DOI] [PubMed] [Google Scholar]
- 22.Moorthy VS, Imoukhuede EB, Milligan P, et al. A randomised, double-blind, controlled vaccine efficacy trial of DNA/MVA ME-TRAP against malaria infection in Gambian adults. PLoS Med. 2004;1:e33. doi: 10.1371/journal.pmed.0010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bejon P, Mwacharo J, Kai O, et al. A phase 2b randomised trial of the candidate malaria vaccines FP9 ME-TRAP and MVA ME-TRAP among children in Kenya. PLoS Clin Trials. 2006;1:e29. doi: 10.1371/journal.pctr.0010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silvie O, Semblat JP, Franetich JF, Hannoun L, Eling W, Mazier D. Effects of irradiation on Plasmodium falciparum sporozoite hepatic development: implications for the design of pre-erythrocytic malaria vaccines. Parasite Immunol. 2002;24:221–223. doi: 10.1046/j.1365-3024.2002.00450.x. [DOI] [PubMed] [Google Scholar]
- 25.Renia L, Rodrigues MM, Nussenzweig V. Intrasplenic immunization with infected hepatocytes: a mouse model for studying protective immunity against malaria pre-erythrocytic stage. Immunology. 1994;82:164–168. [PMC free article] [PubMed] [Google Scholar]
- 26.Scheller LF, Azad AF. Maintenance of protective immunity against malaria by persistent hepatic parasites derived from irradiated sporozoites. Proc Natl Acad Sci USA. 1995;92:4066–4068. doi: 10.1073/pnas.92.9.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen DH, Tigelaar RE, Weinbaum FI. Immunity to sporozoite-induced malaria infeciton in mice. I. The effect of immunization of T and B cell-deficient mice. J Immunol. 1977;118:1322–1327. [PubMed] [Google Scholar]
- 28.Schmidt NW, Podyminogin RL, Butler NS, et al. Memory CD8 T cell responses exceeding a large but definable threshold provide long-term immunity to malaria. Proc Natl Acad Sci USA. 2008;105:14017–14022. doi: 10.1073/pnas.0805452105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferreira A, Schofield L, Enea V, et al. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986;232:881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- 30.Rodrigues M, Nussenzweig RS, Zavala F. The relative contribution of antibodies, CD4+ and CD8+ T cells to sporozoite-induced protection against malaria. Immunology. 1993;80:1–5. [PMC free article] [PubMed] [Google Scholar]
- 31.Carvalho LH, Sano G, Hafalla JC, Morrot A, Curotto de Lafaille MA, Zavala F. IL-4-secreting CD4+ T cells are crucial to the development of CD8+ T-cell responses against malaria liver stages. Nat Med. 2002;8:166–170. doi: 10.1038/nm0202-166. [DOI] [PubMed] [Google Scholar]
- 32.Rodrigues MM, Cordey AS, Arreaza G, et al. CD8+ cytolytic T cell clones derived against the Plasmodium yoelii circumsporozoite protein protect against malaria. Int Immunol. 1991;3:579–585. doi: 10.1093/intimm/3.6.579. [DOI] [PubMed] [Google Scholar]
- 33.Weiss WR, Berzofsky JA, Houghten RA, Sedegah M, Hollindale M, Hoffman SL. A T cell clone directed at the circumsporozoite protein which protects mice against both Plasmodium yoelii and Plasmodium berghei. J Immunol. 1992;149:2103–2109. [PubMed] [Google Scholar]
- 34.Charoenvit Y, Mellouk S, Cole C, et al. Monoclonal, but not polyclonal, antibodies protect against Plasmodium yoelii sporozoites. J Immunol. 1991;146:1020–1025. [PubMed] [Google Scholar]
- 35.Herrington D, Davis J, Nardin E, et al. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991;45:539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- 36.Reece WH, Pinder M, Gothard PK, et al. A CD4(+) T-cell immune response to a conserved epitope in the circumsporozoite protein correlates with protection from natural Plasmodium falciparum infection and disease. Nat Med. 2004;10:406–410. doi: 10.1038/nm1009. [DOI] [PubMed] [Google Scholar]
- 37.Carlton JM, Adams JH, Silva JC, et al. Comparative genomics of the neglected human malaria parasite Plasmodium vivax. Nature. 2008;455:757–763. doi: 10.1038/nature07327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gardner MJ, Hall N, Fung E, et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature. 2002;419:498–511. doi: 10.1038/nature01097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pain A, Bohme U, Berry AE, et al. The genome of the simian and human malaria parasite Plasmodium knowlesi. Nature. 2008;455:799–803. doi: 10.1038/nature07306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carlton JM, Angiuoli SV, Suh BB, et al. Genome sequence and comparative analysis of the model rodent malaria parasite Plasmodium yoelii yoelii. Nature. 2002;419:512–519. doi: 10.1038/nature01099. [DOI] [PubMed] [Google Scholar]
- 41.Hall N, Karras M, Raine JD, et al. A comprehensive survey of the Plasmodium life cycle by genomic, transcriptomic, and proteomic analyses. Science. 2005;307:82–86. doi: 10.1126/science.1103717. [DOI] [PubMed] [Google Scholar]
- 42.Winzeler EA. Malaria research in the post-genomic era. Nature. 2008;455:751–756. doi: 10.1038/nature07361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Brown S, Roos DS, Nussenzweig V, Bhanot P. Transcriptome of axenic liver stages of Plasmodium yoelii. Mol Biochem Parasitol. 2004;137:161–168. doi: 10.1016/j.molbiopara.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 44.Sacci JB, Jr, Ribeiro JM, Huang F, et al. Transcriptional analysis of in vivo Plasmodium yoelii liver stage gene expression. Mol Biochem Parasitol. 2005;142:177–183. doi: 10.1016/j.molbiopara.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 45.Siau A, Silvie O, Franetich JF, et al. Temperature shift and host cell contact up-regulate sporozoite expression of Plasmodium falciparum genes involved in hepatocyte infection. PLoS Pathog. 2008;4:e1000121. doi: 10.1371/journal.ppat.1000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doolan DL, Southwood S, Freilich DA, et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Natl Acad Sci USA. 2003;100:9952–9957. doi: 10.1073/pnas.1633254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tarun AS, Peng X, Dumpit RF, et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci USA. 2008;105:305–310. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Singh AP, Buscaglia CA, Wang Q, et al. Plasmodium circumsporozoite protein promotes the development of the liver stages of the parasite. Cell. 2007;131:492–504. doi: 10.1016/j.cell.2007.09.013. [DOI] [PubMed] [Google Scholar]
- 49.Haddad D, Bilcikova E, Witney AA, et al. Novel antigen identification method for discovery of protective malaria antigens by rapid testing of DNA vaccines encoding exons from the parasite genome. Infect Immun. 2004;72:1594–1602. doi: 10.1128/IAI.72.3.1594-1602.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Richards JS, Beeson JG. The future for blood-stage vaccines against malaria. Immunol Cell Biol. 2009;5:5. doi: 10.1038/icb.2009.27. [DOI] [PubMed] [Google Scholar]
- 51.Romero P, Maryanski JL, Corradin G, Nussenzweig RS, Nussenzweig V, Zavala F. Cloned cytotoxic T cells recognize an epitope in the circumsporozoite protein and protect against malaria. Nature. 1989;341:323–326. doi: 10.1038/341323a0. [DOI] [PubMed] [Google Scholar]
- 52.Tsuji M, Romero P, Nussenzweig RS, Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. J Exp Med. 1990;172:1353–1357. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tarun AS, Dumpit RF, Camargo N, et al. Protracted sterile protection with Plasmodium yoelii pre-erythrocytic genetically attenuated parasite malaria vaccines is independent of significant liver-stage persistence and is mediated by CD8+ T cells. J Infect Dis. 2007;196:608–616. doi: 10.1086/519742. [DOI] [PubMed] [Google Scholar]
- 54.Kumar KA, Baxter P, Tarun AS, Kappe SH, Nussenzweig V. Conserved protective mechanisms in radiation and genetically attenuated uis3(–) and uis4(–) Plasmodium sporozoites. PLoS ONE. 2009;4:e4480. doi: 10.1371/journal.pone.0004480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Belnoue E, Costa FT, Frankenberg T et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172:2487–2495. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 56.Putrianti ED, Silvie O, Kordes M, Borrmann S, Matuschewski K. Vaccine-like immunity against malaria by repeated causal-prophylactic treatment of liver-stage Plasmodium parasites. J Infect Dis. 2009;199:899–903. doi: 10.1086/597121. [DOI] [PubMed] [Google Scholar]
- 57.Belnoue E, Voza T, Costa FT, et al. Vaccination with live Plasmodium yoelii blood stage parasites under chloroquine cover induces cross-stage immunity against malaria liver stage. J Immunol. 2008;181:8552–8558. doi: 10.4049/jimmunol.181.12.8552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Krzych U, Lyon JA, Jareed T, et al. T lymphocytes from volunteers immunized with irradiated Plasmodium falciparum sporozoites recognize liver and blood stage malaria antigens. J Immunol. 1995;155:4072–4077. [PubMed] [Google Scholar]
- 59.Todryk SM, Bejon P, Mwangi T, et al. Correlation of memory T cell responses against TRAP with protection from clinical malaria, and CD4 CD25 high T cells with susceptibility in Kenyans. PLoS ONE. 2008;3:e2027. doi: 10.1371/journal.pone.0002027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bejon P, Mwacharo J, Kai O, et al. The induction and persistence of T cell IFN-gamma responses after vaccination or natural exposure is suppressed by Plasmodium falciparum. J Immunol. 2007;179:4193–4201. doi: 10.4049/jimmunol.179.6.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurtis JD, Hollingdale MR, Luty AJ, Lanar DE, Krzych U, Duffy PE. Pre-erythrocytic immunity to Plasmodium falciparum: the case for an LSA-1 vaccine. Trends Parasitol. 2001;17:219–223. doi: 10.1016/s0169-4758(00)01862-7. [DOI] [PubMed] [Google Scholar]
- 62.Luty AJ, Lell B, Schmidt-Ott R, et al. Interferon-gamma responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. J Infect Dis. 1999;179:980–988. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 63.Kurtis JD, Lanar DE, Opollo M, Duffy PE. Interleukin-10 responses to liver-stage antigen 1 predict human resistance to Plasmodium falciparum. Infect Immun. 1999;67:3424–3429. doi: 10.1128/iai.67.7.3424-3429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.John CC, Tande AJ, Moormann AM, et al. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis. 2008;197:519–526. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]


