Abstract
Rodents rely heavily on odor detection, discrimination, and memory to locate food, find mates, care for pups, and avoid predators. Estrogens have been shown to increase memory retention in rodents performing spatial memory and object placement tasks. Here we evaluate the extent to which 17β-estradiol modulates memory formation and duration in the olfactory system. Adult CD-1 mice were gonadectomized (GDx) and given either systemic 17β-estradiol replacement, local 17β-estradiol in the main olfactory bulb, or no replacement. Before performing the behavioral task the mice were given saline or PHTPP (an estrogen receptor β (ER-β) antagonist) via bilateral infusion into the main olfactory bulb. As the beta-type estrogen receptor (ER-β) is more abundant than the alpha-type estrogen receptor in the murine main olfactory bulb, the current study focuses on 17β-estradiol and its interactions with ERβ. Habituation, a simple non-associative learning task in which an animal is exposed to the same odor over successive presentations, was used to evaluate the animals' ability to detect odors and form an olfactory memory. To evaluate memory duration, we added a final trial of inter-trial interval time (30 or 60 minutes) in which we presented the habituated odor. Neither surgical nor drug manipulation affected the ability of mice to detect or habituate to an odor. After habituation, GDx 17β-estradiol treated mice retained memory of an odor for 30 minutes while non-estradiol treated, 17β-estradiol + ERβ antagonist (PHTPP), and untreated male mice did not remember an odor 30 minutes post habituation. The results show that both systemic and local bulbar infusions of 17β-estradiol enhance odor memory duration in mice.
Keywords: Olfactory bulb, estrogen, olfactory memory, habituation, neuromodulation
Introduction
Rodents rely heavily on odor detection, discrimination, and memory to locate food, find mates, care for pups, and avoid predators (Sanchez-Andrade and Kendrick 2011; Pompili et al. 2010). These functions are known to be modulated by classical neuromodulators such as acetylcholine (ACh), noradrenaline (NE), and serotonin (Fletcher and Chen 2010), as well as by hormonal inputs (Tong et al. 2011; Martin et al. 2009; Doty and Cameron 2009). While the effects of classical neuromodulators such as ACh and NE on non-social odor processing have been studied (Escanilla et al. 2010; Chaudhury et al. 2010), effects of steroid hormone inputs usually focus on social interactions (Kelliher 2007), sexual behavior (Koyama 2004), or stress (Fujita et al. 2010).
Along with its well-known effect on the development of secondary sex characteristics, reproductive behavior (Keller et al. 2009), and neuronal circuitry (reviewed in Maggi et al. 2004; see also Woolley 2007), 17β-estradiol (E2) has also been shown to increase memory retention. E2 improved performance in rodents performing hippocampus based spatial memory and object placement tasks (Frye et al. 2007). In slices of hippocampus, beta-type estrogen receptor (ER-β) activation (with β-receptor agonist WAY 200070) increased dendritic branching and the density of spines, and increased expression of AMPA receptor subunit Glu1 and postsynaptic scaffold proteins found on glutamatergic synapses (Liu et al. 2008). There is a growing body of evidence that indicates acute increases in spine density and dendrite outgrowth, increased intrinsic excitability, and increased long term potentiation in the hippocampus are all initiated by estrogens. These hippocampal changes are correlated to performance improvements in spatial memory tasks in rats and mice (reviewed in Woolley 2007; see also Luine and Dohanich 2008). Physiological and behavioral processes, both social and cognitive, and modulated by estrogens in male rodents also. For example, Activation of ER-β increased social aggression in male and female mice (Clipperton et al. 2010), castrated male rats regained mounting behavior when provided with an acute dose of E2 (Cross and Roselli 1999) and absence of aromatase, the enzyme responsible for the conversion of testosterone to 17β-estradiol, impaired coital behavior (mounting, intromission, and ejaculation) in male mice, in conjunction with decreased olfactory investigation of estrous females (Bakker et al. 2002). In addition to modulation of social behaviors, effects on cognitive behaviors have been shown in male mice/ Among others, the absence of aromatase impaired spatial memory in males and females subject to a Y-maze spatial reference test (Martin et al. 2003), in a different study, blocking aromatase improved spatial memory in male rats (Moradpour et al. 2006). This existing evidence of robust E2 effects in both sexes lead to the inclusion of males in the current study.
In the present study, we test how 17β-estradiol (E2) affects olfactory memory via a non-associative olfactory habituation and memory test. We find that 17β-estradiol modulates the duration of olfactory memory via local mechanisms in the olfactory bulb: male and female mice with either systemic E2 replacement or acute local E2 infusions into olfactory bulbs remembered an odorant for a longer delay than mice with no E2 treatment.
Materials and Methods
Subjects
A total of 26 female and 21 male CD-1 mice (Charles River Laboratories International, Wilmington, MA, USA), aged 7 wks at the beginning of the study, served as subjects. Eleven female mice were used for systemic 17β-estradiol behavioral tests (Experiments 1) and 15 were used in cannulation studies (Experiment 2&3). Fourteen male mice were used for systemic 17β-estradiol behavioral tests (Experiment 1) and 7 were used in cannulation studies (Experiment 3). Mice were housed singly in standard laboratory cages and kept on a reversed 12:12 hr light:dark cycle. Food and water were provided ad libitum. All experiments were carried out under protocols approved by the Cornell University Institutional Animal Care and Use Committee and in accordance with NIH guidelines.
Experimental Groups
Table 1 shows the breakdown of experimental groups and treatments at each phase of the study. The design focuses on the beta type ER because it is more abundant in the MOB than both ER-α and the plasma-bound ER known as GPR30 (Shughrue et al. 1997; Shughrue and Merchenthaler 2001; Mitra et al. 2003; Hazell et al. 2009).
Table 1.
Details of group break, downexperimental treatments and summary of results. Gonadectomy (GDx) was performed 7 days prior to infusions and behavioral trials. Systemic E2 treatment was provided via subcutaneous slow-release pellet inserted at time of GDx, or locally via bilateral infusion into the main olfactory bulbs.
| Experimental Groups | |||||
|---|---|---|---|---|---|
| Experiment 1 | |||||
| Experimental Group | 1A | 1B | 1C | 1D | 1E |
| Sex | Female | Female | Male | Male | Male |
| Surgical Tx | GDx | GDx | GDx | GDx | Sham |
| Systemic Drug | E2 | None | E2 | None | None |
| Local Bulbar Infusion | None | None | None | None | None |
| Odor memory duration | > 30 min | < 30 min | > 30 min | < 30 min | < 30 min |
| Experiment 2 | |||
|---|---|---|---|
| Experimental Group | 2A | 2B | |
| Sex | Female | Female | |
| Surgical Tx | GDx + Cannulation | GDx + Cannulation | |
| Systemic Drug | E2 | None | |
| Local Bulbar Infusion | Saline | PHTPP | Saline |
| Odor memory duration | > 30 min | < 30 min | < 30 min |
| Experiment 3 | ||||||
|---|---|---|---|---|---|---|
| Experimental Group | 3A | 3B | ||||
| Sex | Female | Male | ||||
| Surgical Tx | GDx + Cannulation | GDx + Cannulation | ||||
| Systemic Drug | None | None | ||||
| Local Bulbar Infusion | Saline | E2 | PHTPP+E2 | Saline | E2 | PHTPP+E2 |
| Odor memory duration | < 30 min | > 30 min | < 30 min | < 30 min | > 30 min | < 30 min |
Experiment 1
In Experiment 1, female mice were gonadectomized (GDx) and separated into one of two experimental groups. Experimental group 1A was given systemic 17β-estradiol replacement via subcutaneous time-release pellet (Innovative Research of America, Sarasota, FL, USA) at time of GDx. Experimental group 1B was given no E2 replacement at time of GDx. Male mice were also gonadectomized and separated into two groups: Experimental group 1C consisted of GDx male mice given systemic 17β-estradiol replacement via subcutaneous time-release pellet, Experimental group 1D consisted of GDx males given no E2 replacement.
Experiment 2
In order to ascertain if any effect of 17β-estradiol was due to local modulation in the main olfactory bulb, we conducted a second experiment using mice with infusion cannulae implanted into their olfactory bulbs. This experiment tested whether the effect seen in Experiment 1 could be blocked by infusing the ERβ antagonist 4-[2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a]pyrimidin-3-yl]phenol (PHTPP) directly into the olfactory bulbs. ERβ antagonists were chosen because β receptors are highly expressed in the OB as compared to other ERs (Shughrue et al. 1997; Shughrue and Merchenthaler 2001; Mitra et al. 2003; Hazell et al. 2009). Experimental groups 2A and 2B were subjected to bilateral cannulation of the olfactory bulbs at time of GDx and given either systemic 17β-estradiol replacement via subcutaneous time-release pellet (group 2A) or given no E2 replacement (group 2B). During behavioral testing animals were bilaterally infused with either saline or PHTPP.
Experiment 3
In Experiment 3 we tested if acute E2 in the olfactory bulb was sufficient to observe the effects seen with systemic E2 replacement. Mice in Experiment 3 had bilateral olfactory bulb cannulae implanted at the time of GDx. and were tested either with saline, E2 or E2+antagonist infusions. The cannulated mice in phase 3 were given either saline, β-estradiol-3-sodium-sulfate (E3S), or a combination of E3S + PHTPP via bilateral infusion through cannulae on experimental days.
Drug Delivery
All pharmaceutical drugs in this study were delivered to the mice either systemically or via infusion cannulae. Systemic delivery in Experiments 1 and 2 was achieved using subcutaneous implant of 0.1 mg 17β-Estradiol-cypionate in pellet form (21 day release; Innovative Research of America, Sarasota FL). This pellet concentration and release schedule has been shown to mimic serum estrogen levels in mice (Gao and Dluzen 2001).
For local drug infusions via cannulation (Experiments 2&3), mice were briefly anesthetized with 2% isoflurane gas in oxygen. In Experiment 2, one of two solutions was infused bilaterally into the olfactory bulbs: 0.9% sterile saline vehicle or 120 μM PHTPP in vehicle. In Experiment 3, one of three solutions was infused bilaterally into the main olfactory bulbs: 0.9% sterile saline vehicle or 0.25 mM E3S (Sigma Aldrich Corporation, St. Louis, MO, USA) in vehicle (saline) or a mixture of 0.25 mM E3S + 100 μM PHTPP in vehicle. Drug concentrations were chosen because of previous work done in brain slices of rats and mice (Kelly et al. 1977) and evidence that circulating 17α- and 17β-estradiol levels in the brain are higher than peripheral serum concentrations (Toran-Allerand et al. 2005; Woolley 2007). For infusion studies E3S was used because it is soluble in saline, our control vehicle, whereas 17β-estradiol is highly soluble only in oil. A total volume of 2 μL of each solution was administered into each olfactory bulb at a rate of 0.2 μL per minute. Infusion cannulae were left in place for 5 min after the infusion to allow for complete diffusion into the olfactory bulbs. This lab has previously shown 2 μL to be a sufficient volume to allow for perfusion throughout the olfactory bulbs (Guerin et al. 2008; after Doucette et al. 2007). Figure 1B depicts mouse olfactory bulbs after bilateral infusion of 2μL methylene blue dye (1mg/mL).
Figure 1.
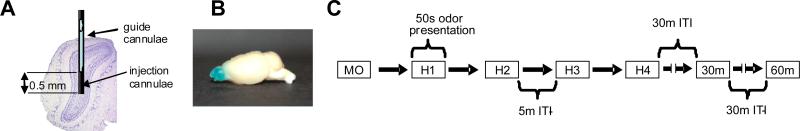
A. Placement of infusion cannulae in the olfactory bulb. Guide cannulae are placed at about 0.5 mm above the center of the olfactory bulb in such a manner that injection cannulae, protruding 0.5 mm beyond the guide cannulae target the approximate center of the olfactory bulb. B. Sagittal view of CD-1 mouse olfactory bulbs after bilateral infusion of 2μL methylene blue dye (1mg/mL). Perfusion throughout the bulbs is evident with very little spread beyond the bulbs into the rest of the brain. C. Schematic depiction of the habituation and memory behavioral trial experimental setup. MO is the mineral oil blank presented prior to the habituation presentations (H1-H4). In the cannulation studies, the behavioral trials were preceded by a 10 minute infusion and a 30 minute wait period. Mice are first presented with mineral oil only (MO), followed by four presentations of the habituation odor (H1-H4) at 5 minute ITIs. The habituation odor is presented again 30 or 60 minutes after the last habituation trial.
Cannulation Surgery
Mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (12 mg/kg) (Sigma Aldrich Corporation, St. Louis, MO, USA) given via intraperitoneal injection and placed in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA, USA). Anesthesia was confirmed by reduced respiratory rate, lack of response to gentle foot pinch, and lack of blink reflex. Double guide cannulae (26-gauge; Plastics One Incorporated, Roanoke, VA, USA) were implanted into both olfactory bulbs for drug infusions. Cannulae placement coordinates relative to bregma were +5.0 mm anteroposterior, 0.75 mm mediolateral, and 1.5 mm dorsoventral. Implants were secured in place with screws and dental cement, with dummy cannulae placed inside the guides to protect against blockage and infection (Figure 1A shows a schematic picture of cannula locatin).
Gonadectomy Surgery
Gonadectomy (GDx) was performed directly following cannulation surgery; mice not subject to cannulation underwent GDx as a single surgical procedure. Briefly, mice were anesthetized with a mixture of ketamine (80 mg/kg) and xylazine (12 mg/kg) (Sigma Aldrich Corporation, St. Louis, MO, USA) given via intraperitoneal injection. A 1 cm square area on the dorsal (females) or ventral (males) surface of the animal was shaved and swabbed with 32% Novalsan (chlorhexidine diacetate; Fort Dodge Animal Health, Fort Dodge, IA, USA) and 2% Lidocaine hydrochloride (topical pain relief; Hi-Tech Pharmacal, Amityville, NY, USA). In females, a single ¾ cm incision was made on the dorsal surface approximately 2.5 cm above the base of the tail. The skin was separated from the underlying muscle. The ovaries were then pulled through the incision with blunt forceps and removed. After removing the ovary and oviduct, the ovarian arteries are ligated to prevent excessive bleeding. In males, a single ¾ cm incision was made on the ventral surface approximately 0.25 cm above the base of the tail. The skin was separated from the underlying tissue and the testes were located. The testes were then pulled through the incision with blunt forceps and removed. The inguinal canal was sutured with Vicryl 3-0 absorbable sutures (Ethicon Endo-Surgery, Inc. Somerville, NJ, USA). The skin incision was then closed with VetBond Tissue Adhesive (3M Animal Care, St. Paul, MN, USA). Mice were allowed to recover from surgery for a minimum of seven days before they were subjected to behavioral testing
Behavioral Testing
Mice were tested between 1000 h and 1600 h each day, corresponding to the first half of their dark cycle (active period), though they were tested in a lighted room. To minimize non-test odors impinging upon the test environment, mice were tested in their home cages on which micro-isolator cage lids, equipped with filters, replaced the standard cage tops.
A standard habituation paradigm was used to assess odor detection, memory formation, and odor memory duration. Briefly, mice were presented with 1 drop (60 μL) of odor applied to filter paper, placed in a tea ball, and then placed in the corner of the cage. Each odor presentation lasted 50 seconds, after which the tea ball was removed from the cage. After the presentation of a blank (mineral oil) tea ball, each odor was presented 4 times, with a 5 minute inter-trial interval between each presentation. The amount of time a mouse spent actively investigating the odor (sniffing with its nose <1 cm from the tea ball) was recorded with a stopwatch. Eleven different odors were used across three experiments (Table 2); odorants were chosen based on previous experiments in our lab. All odors were diluted in mineral oil to 0.01 Pa partial vapor pressure. Odors were presented in a pseudo-randomized order such that no animal was presented with the same odor more than once per treatment.
Table 2.
List of odors used. Odors (μL) were diluted in mineral oil (mL) to a concentration of 0.01 Pa vapor partial pressure. This concentration has been previously shown to be easily detected by mice. Each odor was presented to an individual mouse only once per experimental treatment.
| Odor | Quality or Synonym | Formula | vol/vol dilution |
|---|---|---|---|
| Butyl Acetate | Fruity, Diffusive | C6H12O2 | 0.109μL/50mL |
| Butyl Propionate | Propionic acid n-butylester | C7H14O2 | 0.302μL/50mL |
| Butyl Butyrate | Butyl butanoate | C8H16O2 | 0.826μL/50mL |
| Butyle Penatanoate | Butyle valerate | C=H18O= | 0.286μL/50mL |
| Butyl Hexanoate | n-Caproic acid n-Butyl ester | C10H=O2 | 0.813μL/50mL |
| n-Amyl Acetate | Banana | C7H14O2 | 0.361μL/50mL |
| Hexanal | Hexyl aldehyde | C6H12O | 0.111μL/50mL |
| Propanoic Acid | Propionic acid | C3H6O2 | 0.166μL/50mL |
| Butanoic Acid | Butyric acid | C4H8O2 | 0.636μL/50mL |
| Pentanoic Acid | Valeric acid | C5H10O= | 0.225μL/50mL |
| Octanoic Acid | Caprylic acid | C8H16O2 | 6.871μL/50mL |
In this habituation paradigm, a progressive reduction in investigation time over successive trials indicates that the animal remembers its prior experience with the odor and no longer investigates as though it were a novel stimulus. Animals that investigate the mineral oil blank and the first odor presentation a similar amount have shown positive detection of the test odor. Animals that exhibit a reduction in investigation times over the course of four presentations of the same odor are demonstrating recognition of a previously experienced odor, generally termed habituation, and regarded here as evidence of odor memory formation.
Memory duration was evaluated by presenting the habituated odor at longer time points following the end of the last habituation trial. The habituated odor was presented 30 and 60 minutes after the final habituation presentation (Figure 1B). Investigation times that did not significantly differ from that of the last habituation trial indicate that the mouse treated the odor as a non-novel stimulus (i.e. remembered the odor to which it was habituated). The 30 minute post habituation time point was established with a small pilot study, which tested mice at 15, 30, 60, and 75 minutes post habituation. The pilot indicated a non-treatment memory duration between 15 and 30 minutes, in agreement with results from a previous study in our lab (McNamara et al. 2008).
Statistical analysis
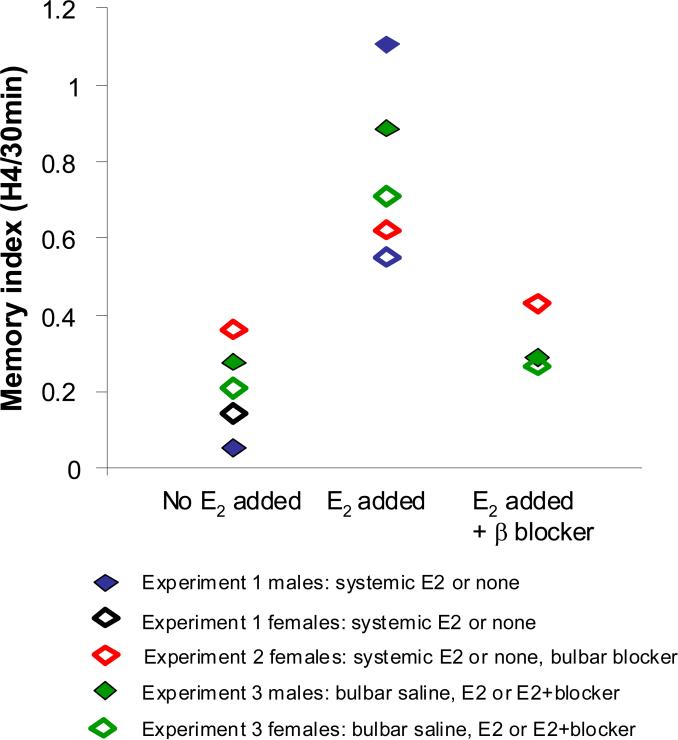
All data were recorded as investigation time in seconds. Data were plotted using Microsoft Excel (Microsoft Corporation, Bellevue, WA, USA) and are presented as mean ± standard error. Statistical analyses were performed with SPSS 20 (IBM Corporation, Somers, NY, USA). Standard repeated measures analyses of variance (ANOVA) with trials as within-subjects factors and treatment group as between-subjects factors were performed on all data sets, followed by post hoc testing using Tukey's LSD criterion (α = 0.05) to assess the differences between individual trials. Habituation was determined as a significant decrease in investigation time between the first and last habituation trial (H1 compared to H4); memory of an odor after the delay was determined as no significant increase between investigation during the test trial and the last habituation trial (H4 compared to30m and H4 compared to 60m). For the summary shown in Figure 5, the relative investigation times to the last habituation trial (H4) as compared to the test trial at 30 minute (30 min) was calculated (“memory index”). This ratio approaches 1.0 if mice investigate similarly during both trials, indicating that they remember the last habituation trial but approaches 0.0 if mice investigate longer during the test trial indicating that the habituation odor had been forgotten.
Figure 5. Summary of experimental results.
The graph shows the ratio between investigation during the last habituation trial and the 30 minute test trial. This ratio is an indicator for how well the odor is remembered after 30 minutes. The graph shows the average degree of odor memory for each treatment group at the 30 minute delay for mice with E2 (systemic or local), without E2, or with E2 and local blockers.
Results
Overall response levels
Neither surgical nor drug treatment affected the ability of mice in any group to detect or habituate to an odor presented dissolved in mineral oil to a standard vapor pressure of 0.01 Pa. All groups showed significant reductions in investigation time over four successive presentations of the same odor, indicating successful acquisition of odor memory for the presented habituation odor (H1-H4).
Experiment 1: Effect of long term E2 treatment on olfactory memory duration
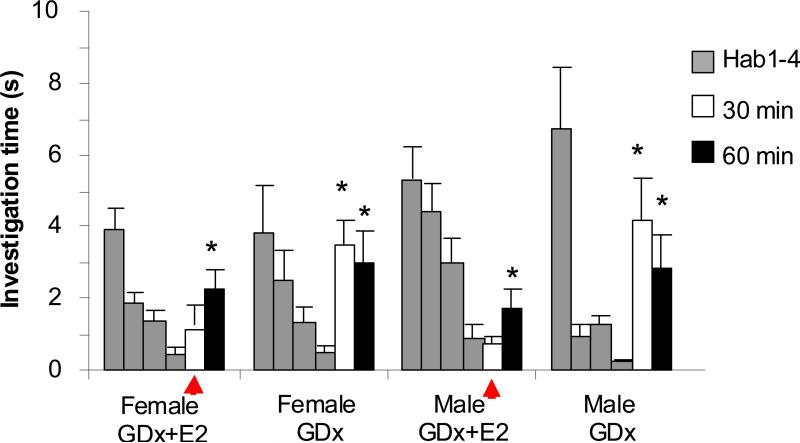
In this experiment, female and male mice were gonadectomized and provided systemic E2 replacement or no E2 replacement (Table 1). All mice were subjected to the habituation task described above. Statistical analysis on investigation times during habituation and later test trials revealed significant main effects of trial number (Ftrial(3,165)=25, p<0.001) indicating that mice behaved differently during different trials. There was also a significant interaction between trial and treatment group (FTrial*Tx(7,165)=3.3, p=0.003), indicating that treatment affected the response pattern. All treatment groups responded significantly less on the last habituation trial when compared to the first (p < 0.001), suggesting that habituation memory formation occurred. Post habituation, female mice receiving systemic E2 replacement (group 1A) retained the odor memory for at least 30 minutes, as indicated by a non-significant difference in investigation time between H4 and the 30 minute presentation (p=0.203, n=8; fig 2), but not as long as 60 minutes, as indicated by a significant increase in investigation time at that delay (p<0.02, n=8). Non-estradiol treated females (group 1B) did not retain the memory of an odor 30 minutes post habituation, as indicated by a significant increase in investigation time at that delay (p<0.005, n=10). Male mice receiving systemic E2 replacement (group 1C) retained the odor memory for at least 30 minutes, as indicated by a non-significant difference in investigation time between H4 and the 30 minute presentation (p=0.732, n=6), but not as long as 60 minutes (p<0.02, n=8). Non-estradiol treated males (group 1D) did not retain the memory of an odor 30 minutes post habituation (p<0.005, n=8). These results indicate that systemic E2 treatment increases odor memory duration in gonadectomized female and male mice (Figure 2).
Figure 2. Experiment 1 tested the effect of systemic E2 replacement in female and male mice.
Treatment groups consisted of non-cannulated females and males given either no E2 replacement at time of GDx or systemic E2 replacement. Graphs show average (+/- standard deviation) investigation times in response to odorized teaballs. Investigation time was significantly reduced between H1 and H4 for all groups (p<0.001). A significant difference between H4 and 30m trial indicates that the odor was treated as a novel odor rather than a familiar odor. A non-significant difference between the H4 and 30m trials indicates the odor was treated as a familiar odor and the animal retained the memory of that odor for at least 30 minutes. None of the groups exhibit memory duration of 60 minutes, as indicated by the significant difference between the H4 and 60m presentation investigation times. Significant differences between 30 or 60 minute test trials are indicated by *. The red arrows highlight 30 minute trials for treatment groups that did remember the odor at 30 minutes.
Experiment 2
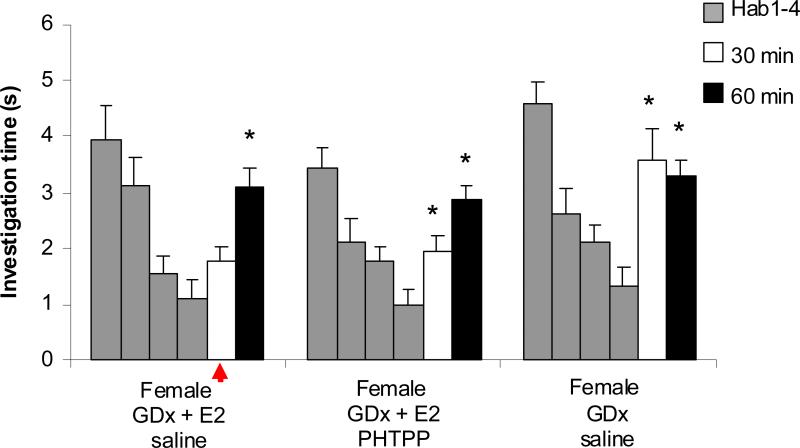
In order to ascertain if the effect of estradiol seen in experiment 1 was due to local modulation in the main olfactory bulb, we tested whether the effect could be reversed by infusing an E2 antagonist (PHTPP) directly into the bulb of gonadectomized female mice with or without systemic E2 replacement. In this experiment, we found a significant main effect of Trial (Ftrial(3,111)=39, p<0.001) with a significant interaction between trial and treatment group (FTrial*Tx(3,111)=2.7, p=0.036). All treatment groups showed significant habituation (p < 0.001) suggesting intact memory formation. Mice provided with no E2 replacement and infused with saline (group 2B) performed as in Experiment 1, with no odor memory retention at 30 minutes as indicated by the increase in investigation time between H4 and the 30 minute presentation (p<0.001). In contrast, mice provided with systemic E2 replacement and subject to bilateral infusion of physiological grade saline retained memory of the habituation odor for at least 30 minutes (p=0.162), but not 60 minutes (p<0.001; Figure 3). Administration of PHTPP, an ER-β antagonist, directly into the olfactory bulbs blocked the effect of systemic 17β-estradiol replacement. Mice given the antagonist prior to behavioral tests show an odor memory duration of less than 30 minutes (p<0.02), similar to those with no systemic E2 replacement. The enhanced odor memory duration induced by systemic E2 replacement is at least counteracted by an ER-β antagonist infused directly into the MOB, indicating that the modulation of odor memory duration is occurring, in part, in the olfactory bulb itself.
Figure 3. Experiment 2 investigated whether effects of systemic E2 replacement could be blocked by local bulbar blockade of E2 b receptors.
Treatment groups consisted of cannulated GDx females given either no E2 replacement at time of GDx or systemic E2 replacement. Each animal also received a local infusion of saline or PHTPP (ER-β antagonist) before behavioral testing. Investigation time was significantly reduced between H1 and H4 for all groups (p<0.001). This indicates that surgical and drug treatments did not impair the animals' ability to detect an odor or form an habituation to the odor. A significant difference between H4 and 30m trial in the non-estradiol group indicates that the odor was treated as a novel odor rather than a familiar odor. No significant difference between the H4 and 30m odor presentation indicates the odor was familiar rather than novel. * indicates a significant difference between a 30 or 60 minute test trial and the last habituation trial. Red arrows highlight the fact the female mice with E2 replacement remembered the odor at 30 minutes.
Experiment 3
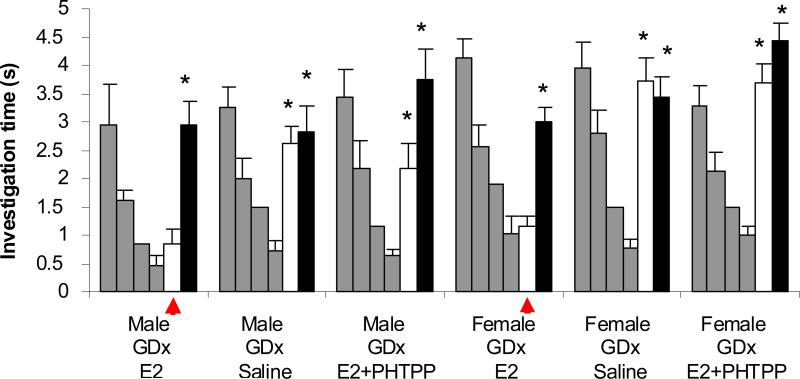
Experiment 3 tested the role of acute estradiol effects restricted to the olfactory bulb on the observed modulation of memory duration. In this experiment all mice were cannulated and subject to GDx. No systemic E2 replacement was provided and all mice performed the behavioral assay under each of 3 drug conditions (saline, E2, E2+β-antagonist), administered in random order and with at least 24 hours between behavioral runs. As in Experiments 1 and 2, a significant main effect of trial (Ftrial(4,151)=82, p<0.001) and significant interactions between trial number and treatment group (FTrial*Tx(8,151)=7.7, p<0.001) were found. All treatment groups exhibited significant habituation (p < 0.001). Mice without E2 replacement were unable to retain memory of an habituated odor for 30 minutes (p<0.001; Figure 4). Mice infused with E2 exhibited memory retention of at least 30 minutes (p=0.583) but not 60 minutes (p<0.001). When infused with E2 and PHTPP together, the animals performed similar to the non-estradiol saline infused group, showing a lack of odor memory retention at 30 minutes post habituation (p<0.001). Without systemic E2 available, odor memory duration was enhanced via direct infusion of E2 into the olfactory bulbs. This effect is reversed when E2 application occurs simultaneously with ER-β antagonist infusion (Figure 4).
Figure 4. Experiment 3: Local bulbar modulation.
Treatment groups consisted of cannulated males and females given no systemic E2 replacement at time of GDx and receiving bilateral infusions of either saline (Non-Estradiol), E2 (Estradiol), or E2+PHTPP (ERβ antagonist; Estradiol+PHTPP) approximately 40 minutes before behavioral trials. Investigation time significantly reduced between H1 and H4 indicates that surgical and drug treatments did not impair the animals' ability to detect and odor or form an habituation to the odor. A significant difference between H4 and 30m trial indicates that the odor was treated as a novel odor rather than a familiar odor. * indicates a significant difference between a 30 or 60 minute test trial and the last habituation trial. Red arrows highlight the fact the female mice with E2 replacement remembered the odor at 30 minutes.
Summary
Experiments 1 and 2 show that systemic estrogen exerts a memory enhancing effect upon odor processing in gonadectomoized female and male mice. This odor memory enhancement increases the performance of E2 mice beyond the performance of non-E2 treated and non-GDx mice in an odor habituation memory duration task. The enhancement of memory by systemic E2 replacement can be blocked by blocking olfactory bulb ER-β receptors. Experiment 3 shows that systemic (circulating) E2 is not necessary for olfactory memory enhancement as long as local infusion of E2 occurs. Local acute E2 infusion is sufficient to induce an olfactory memory enhancement. Both systemic and locally induced memory enhancement can be blocked with local application of an ER-β antagonist. The overall results for all treatment groups are summarized in Table 1; Figure 5 shows the summary of all results: the presence of E2, whether systemic or local to the olfactory bulb, extends the duration of olfactory habituation memory and this effect can be locally blocked by blocking ER-β receptors locally in the olfactory bulbs.
The overall results for all treatment groups are summarized in Figure 5, which graphs the memory index for each group. The graph indicates a clear trend of groups with E2 treatment showing ratios closer to 1. Ratios of groups without E2 treatment hover near or below an index of 0.4.
Discussion
Using an olfactory habituation paradigm, we find that the duration of an odor memory is modulated by 17β-estradiol in the main olfactory bulb. In the present experiments, systemic 17β-estradiol, as well as local administration directly into the olfactory bulb, enhanced memory duration in male and female mice. Mice provided with systemic E2 replacement at time of gonadectomy, or with local bulbar infusions, show enhanced odor memory duration compared to males and females not treated with replacement 17-β estradiol or local infusions. The effect of systemic 17-β estradiol could be blocked by blocking bulbar receptors, and could be mimicked by local infusions into the bulb, suggesting that the site of action in our behavioral paradigm is the olfactory bulb. Interestingly, while we originally included male mice in the study as a control group, we found that both systemic and bulbar 17-b modulated odor memory similarly to the effects seen in female mice. Effects of ER-b receptor modulation in male mice were also seen in at least one other study (Sanchez-Andrade and Kendrick, 2011); this study used a knockout mouse model to manipulate estrogen effects. Circulating aromatase in male mice has been described in several brain areas (Roselli et al. 1984) and can therefore not be excluded in the male mouse OB.
Among all experimental groups, only the E2 treatment groups exhibited enhanced memory duration (positive odor memory at 30 minutes post habituation) during the behavioral tests, independent of the method of E2 delivery (Table 1; Figure 5). No group retained memory of the odor for 60 minutes post habituation. In mice that received systemic E2 replacement, local bulbar infusion of an E2 antagonist prior to testing abolished the enhancement in memory seen in the non-antagonist group (Figure 3), strongly suggesting that the location of action was in the MOB. This result was confirmed by experiment 3, in which animals received E2 replacement or E2+ER-β antagonist locally in the OB (Figure 4). Though systemic E2 replacement elicited a robust behavioral response (memory duration enhancement) which could be modulated by local bulbar blockade, we cannot necessarily assume that receptors the MOB are solely responsible for the observed modulation of olfactory memory duration. Our data indicate, however, that acute local administration of E2 into the olfactory bulb is sufficient to produce enhanced olfactory memory duration. The MOB is the site of a primary step in odor processing, and modulation occurring therein can directly affect the behavioral output of the animal in a variety of behavioral paradigms (see Mandairon and Linster 2009 for review). Previous experiments have shown that bulbar NMDA receptors are crucial to the formation of olfactory habituation memory (McNamara et al., 2008) and that behavioral habituation is reflected directly in adaptation of mitral cell responses in the OB (Chaudhury et at 2010). The present experiments further support the notion that habituation memory is at least partially supported by bulbar networks (Wilson and Linster, 2008).
Our present findings are complementary to the myriad of documented E2 effects on the CNS (reviewed in Maggi et al. 2004; see also Woolley 2007). E2 has been shown to increase memory retention in rodents performing hippocampus based spatial memory and object placement tasks (Frye et al. 2007). In a food motivated task utilizing a radial arm maze, proestrus females performed better than females at lower plasma E2 stages of the estrus cycle (Pompili et al. 2010). Our findings indicate that the memory enhancing function of E2 observed in spatial memory also extends to olfactory memory.
The current study focuses particularly on the beta type ER because it is more abundant in the MOB than both ER-α and the plasma-bound ER known as GPR30 (Shughrue et al. 1997; Shughrue and Merchenthaler 2001; Mitra et al. 2003; Hazell et al. 2009). GPR30 is found in the mitral cells of the main olfactory bulb as well as in the glomerular layer, but not the external plexiform or granule cell layers (Hazell et al. 2009). In contrast, ER-β is found in the glomerular and granule cell layers, as well as in the fibers of the external plexiform layer. ER-α is only very weakly detected in mouse olfactory bulb, and is localized to the granule cell layer (Mitra et al. 2003). Some attempt has been made to determine if activation of a single class of ER is responsible for the majority of learning enhancement seen in E2 studies, but results are largely contradictory (Gibbs 1999; Hammond et al. 2009; Liu et al. 2008). While the current study does not specifically address the mechanism by which E2 enhances olfactory memory, our results show that antagonizing ER-β in the MOB blocks the memory enhancing effects of E2, indicating that the beta receptor is of primary importance to estradiol modulation in the bulb.
Traditional genomic effects of E2 modulation occur on the timescale of hours to days because they rely upon the transcription of protein products instigated by activation of an ER on the nuclear membrane (reviewed in Prossnitz and Maggiolini 2009). Heikkinen et al. (2002) found that E2 treatment would enhance learning in a radial arm maze spatial memory task if treatment was consistent (ongoing rather than punctate) and long term (more than 7 days), indicating that the modulation taking place relies primarily on genomic effects. The present study, in contrast, shows marked robust enhancement of olfactory memory in both chronic (systemic E2 replacement) and acute (local E2 infusions) treatment paradigms, with E2 having a robust effect on the olfactory bulb after only 40 minutes. The results presented here support a growing body of research indicating that hormonal modulation, and 17β-estradiol modulation in particular, initiates both genomic and rapid responses (Srivastava et al. 2011). The acute activational effects described in this study could be due to the binding of E2 to the recently identified ER known as GPR30. This receptor is found on the cell surface as well as in the cytoplasm, where it has access to E2 after it crosses the plasma membrane (Prossnitz and Maggioloni 2009). Further experiments need to be performed to clarify the effects of GPR30 in the MOB.
Both systemic 17β-estradiol treatment and local bulbar infusions enhance odor memory duration in mice in our hands. These two treatments may modulate the olfactory bulb via different mechanisms with respect to different cellular receptors, yet result in similar functional effects.
Acknowledgments
This work was supported by NIH grants R01DC009948 (CL), and T32 GM007469 (SD).
References
- Bakker J, Honda S, Harada N, Balthazart J. Sexual partner preference requires a functional aromatase (Cyp 19) gene in male mice. Hormones and Behavior. 2002;42:158–171. doi: 10.1006/hbeh.2002.1805. [DOI] [PubMed] [Google Scholar]
- Chaudhury D, Manella L, Arellanos A, Escanilla O, Cleland TA, Linster C. Olfactory bulb habituation to odor stimuli. Behavioral Neuroscience. 2010;124(4):490–499. doi: 10.1037/a0020293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clipperton AE, Cragg CL, Wood AJ, Pfaff D, Choleris E. Agonistic behavior in males and females: Effects of an estrogen receptor beta agonist in gonadectomized and gonadally intact mice. Psychoneuroendocrinology. 2010;35:1008–1022. doi: 10.1016/j.psyneuen.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross E, Roselli CE. 17β-Estradiol rapidly facilitates chemoinvestigation and mounting in castrated male rats. American Journal of Physiology - Regulatory, Integrative and Comparative Physiology. 1999;276:R1346–R1350. doi: 10.1152/ajpregu.1999.276.5.R1346. [DOI] [PubMed] [Google Scholar]
- Doty RL, Cameron EL. Sex differences and reproductive hormone influences on human odor perception. Physiology & Behavior. 2009;97(2):213–228. doi: 10.1016/j.physbeh.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doucette W, Midler J, Restrepo D. Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learning and Memory. 2007;14:539–547. doi: 10.1101/lm.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escanilla O, Arrellano A, Karnow A, Ennis M, Linster C. Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory bulb. European journal of Neuroscience. 2010;32:458–468. doi: 10.1111/j.1460-9568.2010.07297.x. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Chen WR. Neural correlates of olfactory learning: critical role of centrifugal modulation. Learning and Memory. 2010;17:561–570. doi: 10.1101/lm.941510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiology of Learning and Memory. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita S, Ueki S, Miyoshi M, Watanabe T. “Green odor” inhalation by stressed rat dams reduces behavioral and neuroendocrine signs of prenatal stress in the offspring. Hormones and Behavior. 2010;58:264–272. doi: 10.1016/j.yhbeh.2010.03.007. [DOI] [PubMed] [Google Scholar]
- Gai X, Dluzen DE. Tamoxifen abolishes estrogen's neuroprotective effect upon methamphetamine neurotoxicity of the bigrostriatal dopaminergic system. Neuroscience. 2001;103(2):385–394. doi: 10.1016/s0306-4522(01)00014-8. [DOI] [PubMed] [Google Scholar]
- Gibbs RB. Estrogen Replacement Enhances Acquisition of a Spatial Memory Task and Reduces Deficits Associated with Hippocampal Muscarinic Receptor Inhibition. Hormones and Behavior. 1999;36:222–233. doi: 10.1006/hbeh.1999.1541. [DOI] [PubMed] [Google Scholar]
- Hammond R, Mauk R, Ninaci D, Nelson D, Gibbs RB. Chronic treatment with estrogen receptor agonists restores acquisition of a spatial learning task in young ovariectomized rats. Hormones and Behavior. 2009;56:309–314. doi: 10.1016/j.yhbeh.2009.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell GGJ, Yao ST, Roper JA, Prossnitz ER, O'Carroll A, Lolait SJ. Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. Journal of Endocrinology. 2009;202:223–236. doi: 10.1677/JOE-09-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkinen T, Puoliva J, Liu L, Rissanen A, Tanila H. Effects of Ovariectomy and Estrogen Treatment on Learning and Hippocampal Neurotransmitters in Mice. Hormones and Behavior. 2002;41:22–32. doi: 10.1006/hbeh.2001.1738. [DOI] [PubMed] [Google Scholar]
- Keller M, Baum MJ, Brock O, Brennan PA, Bakker J. The main and the accessory olfactory systems interact in the control of mate recognition and sexual behavior. Behavioural Brain Research. 2009;200:268–276. doi: 10.1016/j.bbr.2009.01.020. [DOI] [PubMed] [Google Scholar]
- Kelliher KR. The combined role of the main olfactory and vomeronasal systems in social communication in mammals. Hormones and Behavior. 2007;52(5):561–570. doi: 10.1016/j.yhbeh.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MJ, Moss RL, Dudley CA. The Effects of Microelectrophoretically Applied Estrogen, Cortisol and Acetylcholine on Medial Preoptic-Septal Unit Activity throughout the Estrous Cycle of the Female Rat. Experimental Brain Research. 1977;30:53–64. doi: 10.1007/BF00237858. [DOI] [PubMed] [Google Scholar]
- Koyama S. Primer effects by conspecific odors in house mice: a new perspective in the study of primer effects on reproductive activities. Hormones and Behavior. 2004;46:303–310. doi: 10.1016/j.yhbeh.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, Grauer S, Zhang G, Kelley C, Pulito V, Sung A, Mervis RF, Navarra R, Hirst WD, Reinhart PH, Marquis KL, Moss SJ, Pangalos MN, Brandon NJ. Activation of estrogen receptor-b regulates hippocampal synaptic plasticity and improves memory. Nature Neuroscience. 2008;11(3):334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- Luine V, Dohanich G. Sex differences in cognitive function in rodents. In: Becker JB, Berkley KJ, Geary N, Hampson E, Herman JP, Young EA, editors. Sex differences in the brain. Oxford University Press; New York: 2008. pp. 227–251. [Google Scholar]
- McNamara AM, Magdison PD, Linster C, Wilson DA, Cleland TA. Distinct neural mechanisms mediate olfactory memory formation at different timescales. Learn. Mem. 2008;15(3):117–25. doi: 10.1101/lm.785608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi A, Ciana P, Belcredito S, Vegeto E. Estrogens in the Nervous System: Mechanisms and Nonreproductive Functions. Annu. Rev. Physiol. 2004;66:291–313. doi: 10.1146/annurev.physiol.66.032802.154945. [DOI] [PubMed] [Google Scholar]
- Mandairon N, Linster C. Odor perception and olfactory bulb plasticity in adult mammals. Journal of Neurophysiology. 2009;101:2204–2209. doi: 10.1152/jn.00076.2009. [DOI] [PubMed] [Google Scholar]
- Martin B, Maudsley S, White CM, Egan JM. Hormones in the naso-oropharynx: Endocrine modulation of taste and smell. Trends in Endocrinology and Metabolism. 2009;20(4):163–170. doi: 10.1016/j.tem.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SW, Hoskin E, Yudkovitz J, Pear L, Wilkinson HA, Hayashi S, Pfaff DW, Ogawa S, Rohrer SP, Schaeffer JM, McEwen BS, Alves SE. Immunolocalization of Estrogen Receptor β in the Mouse Brain: Comparison with Estrogen Receptor α. Endocrinology. 2003;144(5):2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- Pompili A, Tomaz C, Arnone B, Tavares MC, Gasbarri A. Working and reference memory across the estrous cycle of rat: A long-term study in gonadally intact females. Behavioural Brain Research. 2010;213:10–18. doi: 10.1016/j.bbr.2010.04.018. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Maggiolini M. Mechanisms of estrogen signaling and gene expression via GPR30. Molecular and Cellular Endocrinology. 2009;308:32–38. doi: 10.1016/j.mce.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roselli CE, Ellinwood WE, Resko JA. Regulation of brain aromatase activity in rats. Endocrinology. 1984;114:192. doi: 10.1210/endo-114-1-192. [DOI] [PubMed] [Google Scholar]
- Sánchez-Andrade G, Kendrick KM. Roles of α- and β-estrogen receptors in mouse social recognition memory: Effects of gender and the estrous cycle. Hormones and Behavior. 2011;59:114–122. doi: 10.1016/j.yhbeh.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. Comparative Distribution of Estrogen Receptor-a and -b mRNA in the Rat Central Nervous System. Journal of Comparative Neurology. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Merchenthaler I. Distribution of Estrogen Receptor β Immunoreactivity in the Rat Central Nervous System. Journal of Comparative Neurology. 2001;436:64–81. [PubMed] [Google Scholar]
- Srivastava DP, Waters EM, Mermelstein PG, Kramar EA, Shors TJ, Liu F. Rapid estrogen signaling in the brain: implications for the fine-tuning of neuronal circuitry. Journal of Neuroscience. 2011;31(45):16056–16063. doi: 10.1523/JNEUROSCI.4097-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Mannea E, Pascaline A, Pfluger PT, Yi C, Castaneda TR, Davis HW, Ren X, Pixley S, Benoit S, Julliard K, Woods SC, Horvath TL, Sleeman MM, D'Alessio D, Obici S, Frank R, Tschöp MH. Ghrelin Enhances Olfactory Sensitivity and Exploratory Sniffing in Rodents and Humans. Journal of Neuroscience. 2011;31(15):5841–5846. doi: 10.1523/JNEUROSCI.5680-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17α-Estradiol: a brain-active estrogen? Endocrinology. 2005;146(9):3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- Wilson DA, Linster C. Neurobiology of a simple memory. J Neurophysiol. 2008;100(1):2–7. doi: 10.1152/jn.90479.2008. [DOI] [PubMed] [Google Scholar]
- Woolley CS. Acute Effects of Estrogen on Neuronal Physiology. Annu. Rev. Pharmacol. Toxicol. 2007;47:657–80. doi: 10.1146/annurev.pharmtox.47.120505.105219. [DOI] [PubMed] [Google Scholar]