Abstract
In this study, we examined the morphology of the basal ganglia and thalamus in bipolar disorder (BP), schizophrenia-spectrum disorders (SCZ-S), and healthy controls (HC) with particular interest in differences related to the absence or presence of psychosis. Volumetric and shape analyses of the basal ganglia and thalamus were performed in 33 BP individuals [12 without history of psychotic features (NPBP) and 21 with history of psychotic features (PBP)], 32 SCZ-S individuals [28 with SCZ and 4 with schizoaffective disorder], and 27 HC using FreeSurfer-initiated large deformation diffeomorphic metric mapping. Significant volume differences were found in the caudate and globus pallidus, with volumes smallest in the NPBP group. Shape abnormalities showing inward deformation of superior regions of the caudate were observed in BP (and especially in NPBP) compared with HC. Shape differences were also found in the globus pallidus and putamen when comparing the BP and SCZ-S groups. No significant differences were seen in the nucleus accumbens and thalamus. In summary, structural abnormalities in the caudate and globus pallidus are present in BP and SCZ-S. Differences were more apparent in the NPBP subgroup. The findings herein highlight the potential importance of separately examining BP subgroups in neuroimaging studies.
Keywords: Structural magnetic resonance imaging, Morphometry, Basal ganglia, Thalamus
1. Introduction
Differentiating bipolar disorder (BP) and schizophrenia (SCZ) is challenging, particularly with their overlap in symptomatology such as psychosis. Neuroimaging studies, including those that directly compare BP and SCZ, indicate both overlapping and disparate abnormalities between BP and SCZ; however, identification of biomarkers that clearly differentiate between the two disorders remains elusive due to complicating factors such as varying medication exposure and clinical heterogeneity (McIntosh et al., 2008a, 2008b; Arnone et al., 2009; Hamilton et al., 2009; Ellison-Wright and Bullmore, 2010; Hall et al., 2010; Ongür et al., 2010; Rimol et al., 2010; Brown et al., 2011; Sui et al., 2011).
Studying brain structure in non-psychotic and psychotic subgroups of BP individuals may mitigate the challenges related to clinical heterogeneity in BP, and could elucidate underlying mechanisms of psychosis when these BP subgroups are compared with SCZ. However, studies comparing psychotic and non-psychotic BP subgroups with SCZ are scarce (Strasser et al., 2005). Yet, preliminary evidence does support the potential for such approaches to enhance our understanding of psychosis. Recent studies suggest unique and shared abnormalities among affective psychosis and schizophrenia-spectrum disorders (SCZ-S) (de Castro-Manglano et al., 2011; Ivleva et al., 2012). Differences in brain structure and function, as well as dopamine receptor density and ventricular volume, have been found between non-psychotic and psychotic affective disorders (Pearlson et al., 1995; Wang and Ketter, 2000; Strasser et al., 2005; Garrett et al., 2011; Busatto, 2013).
Potential key regions involved in psychosis include the basal ganglia and thalamus. Both these regions have been increasingly implicated in emotional and cognitive processing, particularly via cortico-basal ganglia and cortico-thalamic circuits, and appear to play important roles in executive functions that are commonly impaired in psychotic conditions (Byne et al., 2009; Haber and Calzavara, 2009; Marchand and Yurgelun-Todd, 2010). Moreover, the basal ganglia and thalamus are rich in dopaminergic innervation (Byne et al., 2009), which is significant in light of the evidence implicating the critical role of dopamine in psychosis (Howes and Kapur, 2009). Structural abnormalities in the basal ganglia and thalamus have been observed in psychosis (Brandt and Bonelli, 2008; Byne et al., 2009; Smith et al., 2011). Volume abnormalities in the basal ganglia and thalamus have been more consistently found in SCZ than BP, when compared with controls (Byne et al., 2009; Ellison-Wright and Bullmore, 2010; Marchand and Yurgelun-Todd, 2010; Radenbach et al., 2010). Most studies have found increased basal ganglia and decreased thalamic volumes in SCZ, whereas findings have been quite variable in BP. The inconsistencies in BP may possibly relate to clinical heterogeneity within samples (e.g., inclusion of BP individuals with and without psychotic features).
Shape analysis of specific brain regions could provide more refined discrimination of structural differences between groups and localize structural abnormalities within a brain region, thus complementing volumetric analyses (Csernansky et al., 1998; Csernansky et al., 2002; Csernansky et al., 2004). Shape abnormalities in the basal ganglia and thalamus have been found in BP and SCZ, although such studies are limited and have largely focused on SCZ (Harms et al., 2007; Mamah et al., 2007; Coscia et al., 2009; Smith et al., 2011; Ong et al., 2012). We are not aware of any morphometric neuroimaging studies directly comparing BP and SCZ in the basal ganglia and thalamus.
In this study, we examined the volume and shape of basal ganglia structures and the thalamus in BP, SCZ-S, and healthy controls (HC) using FreeSurfer-initiated large deformation diffeomorphic metric mapping (FS+LDDMM), a fully automated brain-segmentation methodology (Khan et al., 2008). We hypothesized that structural abnormalities in these regions would vary across clinical subgroups as follows: (1) for basal ganglia volumes, SCZ-S > bipolar disorder with psychotic features (PBP) > bipolar disorder without psychotic features (NPBP) and healthy controls (HC); (2) for thalamic volume, HC and NPBP > PBP > SCZ-S; and (3) shape abnormalities in the basal ganglia and thalamus would be most prominent in SCZ-S and intermediate in PBP.
2. Methods
2.1. Participants
Written informed consent was obtained from all participants in accordance with the institutional review boards at Washington University School of Medicine and Northwestern University Feinberg School of Medicine.
Participants included 33 adults with BP [12 without history of psychotic features (NPBP) and 21 with history of psychotic features (PBP)], 32 adults with SCZ-S [28 with SCZ and 4 with schizoaffective disorder (SAD)], and 27 healthy controls (HC). Participants were recruited at two sites, Washington University (St. Louis, MO) [WU] and Northwestern University (Chicago, IL) [NU], through advertisements in the community and mental health centers/clinics, and through research participant registries. The BP participants were recruited at WU. The SCZ-S and HC participants were selected to match in age, gender, and race with the BP participants from a larger study sample recruited at WU and NU. For the SCZ-S group, 18 participants were recruited at WU, and 14 were recruited at NU. For the HC group, eight participants were recruited at WU, and 19 were recruited at NU. For each participant at his or her respective sites, DSM-IV Axis I diagnoses were determined through consensus between a research psychiatrist and trained research clinicians using the Structured Clinical Interview for DSM-IV Axis I Diagnoses (SCID) and an independent psychiatric evaluation by a research psychiatrist. Individuals were excluded if they had neurological disorders, unstable medical disorders, head injury with loss of consciousness, or contraindication to magnetic resonance imaging (e.g., metal implant or claustrophobia). Additionally, to minimize clinical heterogeneity within the BP group, only participants with a history of euphoric mania (versus mania characterized by primarily irritable mood) were included in the study.
Demographic and clinical characteristics of the participants are detailed in Table 1. The SCID was used to identify participants with lifetime substance use disorders for alcohol, cannabis, cocaine, stimulants, hallucinogens, sedatives, and opioids. Substance use disorders were defined as meeting lifetime DSM-IV-TR criteria for abuse or dependence. Participants did not have a history of substance dependence within the past 6 months, except for one BP participant with cannabis dependence. Results were unchanged when volume analyses were performed excluding the BP participant with cannabis dependence in the past 6 months. Table 1 lists comorbid substance use disorders for each group. With respect to other Axis I comorbid disorders, in the BP group, four participants had current generalized anxiety disorder, one had current agoraphobia without panic disorder, one had lifetime but not current panic disorder with agoraphobia, three had current social phobia, one had current obsessive-compulsive disorder (OCD), one had lifetime but not current OCD, three had current post-traumatic stress disorder (PTSD), three had current social phobia, one had lifetime but not current social phobia, three had current specific phobia, three had lifetime but not current anorexia nervosa (AN), and two had lifetime but not current bulimia nervosa; in the SCZ-S group, three participants had lifetime but not current major depressive disorder, one had lifetime but not current depressive disorder-not otherwise specified, one had current social phobia, two had current PTSD, six had lifetime but not current PTSD, one had current specific phobia, and one had lifetime but not current AN; in the HC group, one participant had lifetime but not current PTSD. Current comorbidity was defined as having symptoms that met DSM-IV criteria within the past month.
Table 1. Participant demographic profiles and characteristics.
| Control (n=27) | SCZ-S (n=32) | BP (n=33) | NPBP (n=12) | PBP (n=21) | F/χa | pa | F/χb | pb | |
|---|---|---|---|---|---|---|---|---|---|
| Mean age* | 25.5 (4.3) | 25.8 (4.1) | 25.5 (3.9) | 27.0 (3.8) | 24.6 (3.7) | 0.1 | 0.9 | 1.0 | 0.4 |
| Gender | 0.2 | 0.9 | 2.6 | 0.5 | |||||
| Female | 14 (51.9) | 17 (53.1) | 16 (48.5) | 8 (66.7) | 8 (38.1) | ||||
| Male | 13 (48.1) | 15 (46.9) | 17 (51.5) | 4 (33.3) | 13 (61.9) | ||||
| Race | 3.0 | 0.5 | 6.7 | 0.4 | |||||
| Black | 6 (22.2) | 11 (34.4) | 9 (27.3) | 1 (8.3) | 8 (38.1) | ||||
| Caucasian | 15 (55.6) | 17 (53.1) | 21 (63.6) | 10 (83.3) | 11 (52.4) | ||||
| Other | 6 (22.2) | 4 (12.5) | 3 (9.1) | 1 (8.3) | 2 (9.5) | ||||
| Handedness | 0.2 | 0.9 | 4.4 | 0.2 | |||||
| Right | 26 (96.3) | 30 (93.8) | 31 (93.9) | 10 (83.3) | 21 (100) | ||||
| Left | 1 (3.7) | 2 (6.3) | 2 (6.1) | 2 (16.7) | 0 (0) | ||||
| Lifetime substanc use | |||||||||
| Alcohol | 0 (0) | 10 (31.2) | 18 (54.6) | 5 (41.7) | 13 (61.9) | 20.9 | <.0001 | 22.3 | <.0001 |
| Cannabis | 0 (0) | 16 (50.0) | 12 (36.4) | 2 (16.7) | 10 (47.6) | 18.1 | 0.0001 | 21.6 | <.0001 |
| Cocaine | 0 (0) | 5 (15.6) | 3 (9.1) | 1 (8.33) | 2 (9.5) | 4.5 | 0.1 | 4.5 | 0.2 |
| Stimulant | 0 (0) | 4 (12.5) | 0 (0) | 0 (0) | 0 (0) | 7.8 | 0.01 | 7.8 | 0.05 |
| Sedative | 0 (0) | 0 (0.0) | 2 (6.1) | 0 (0) | 2 (9.5) | 3.6 | 0.2 | 6.9 | 0.07 |
| Hallucinogen | 0 (0) | 1 (3.1) | 3 (9.1) | 0 (0) | 3 (14.3) | 3.1 | 0.2 | 6.6 | 0.07 |
| Opiate | 0 (0) | 0 (0) | 3 (9.1) | 0 (0) | 3 (14.3) | 5.5 | 0.06 | 10.5 | 0.01 |
| Antipsychotic use past 2 years** | |||||||||
| FGA only | N/A | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.02* | 0.9* | 0.6* | 0.7* |
| SGA only | N/A | 26 (81.2) | 22 (66.7) | 5 (41.7) | 15 (71.4) | ||||
| Both FGA + SGA | N/A | 3 (9.4) | 2 (6.1) | 0 (0.0) | 2 (9.5) |
SCZ: Schizophrenia-spectrum disorders; BP: Bipolar disorder; NPBP: Non-psychotic bipolar disorder; PBP: Psychotic bipolar disorder Values are given as number of participants (percentages), unless stated otherwise.
F statistic, chi-square statistic or p value for 3-group analysis comparing control, SCZ-S, and BP
F statistic, chi-square statistic or p value for 4-group analysis comparing control, SCZ-S, NPBP, and PBP
Mean age is expressed in years with standard deviation in parentheses.
For antipsychotic use, controls were excluded from the analysis
Total psychotic symptoms equaled the number of symptoms rated as “3” on the SCID Module B “Psychotic and Associated Symptoms” for the following: delusions of reference, persecutory delusions, grandiose delusions, delusion of external control (including thought insertion and thought withdrawal), thought broadcasting, auditory hallucinations, and visual hallucinations. Antipsychotic use in the 2 years preceding the time of scan were assessed using retrospective self-reports.
2.2. Magnetic resonance imaging (MRI) and surface mapping
Magnetic resonance (MR) scans were obtained using a Siemens 3-Tesla Tim-TRIO scanner for each participant at his or her respective site using the same acquisition protocol at each of the two sites. Quality-assurance studies, which were conducted using standardized and traveling human phantoms, strongly supported the compatibility between scanners at the two sites. MPRAGE scans were collected from two traveling human phantoms at both sites, with each pair of scans being within 1 month of each other. For subject 1, the stretch factors between the two scanners were 1.0031, 1.0003 and 0.9953 in the x, y and z directions, respectively, with an overall scaling of 0.9987 (or 0.13% relative volume change). For subject 2, the stretch factors between the two scanners were 1.0035, 1.0006 and 0.9948 in the x, y and z directions, respectively, with an overall scaling of 0.9989 (or 0.11% relative volume change).
Two-to-four structural images were acquired for each participant using a 3D T1-weighted sagittal magnetization-prepared radiofrequency rapid gradient- echo (MPRAGE) sequence (repetition time=2400 ms, echo time=3.16 ms, flip angle=8 degrees, voxel resolution=1 mm3). Scans for each participant were aligned with the first scan and averaged to create a low-noise image (Buckner et al., 2004).
Surface mapping for all participants was performed at NU. Surfaces of the caudate, putamen, globus pallidus, nucleus accumbens, and thalamus were automatically generated using FS+LDDMM. For full details, please refer to Khan et al. (Khan et al., 2008). In brief, this method combines the probabilistic voxel-based classification method of FreeSurfer (Desikan et al., 2006) and the deformable template-based method of large deformation diffeomorphic metric mapping (LDDMM) (Beg et al., 2005). The initial subcortical segmentations for the caudate, putamen, globus pallidus, nucleus accumbens, and thalamus were obtained with FreeSurfer version 5.1.0, followed by image registration with LDDMM that produced smooth transformations for each region of interest (ROI). The template was a healthy participant used previously (Wang et al., 2008), obtained from the same source as the other participants but not included in the data analysis. Each ROI volume was calculated as the enclosed volume of the mapped surface. Cortical gray matter volume was obtained directly from the FreeSurfer pipeline output.
2.3. Statistical analysis
Analyses were performed to compare within diagnostic groups (BP, SCZ-S, and HC) and clinical subgroups (NPBP, PBP, SCZ-S, and HC).
Multivariate analysis of variance (MANOVA) was initially performed for combined volumes of the basal ganglia structures (i.e., caudate, putamen, nucleus accumbens and globus pallidus) and thalamus with group as an independent factor and covariates of age, gender, and cortical gray matter volume to examine omnibus effects and to address concerns for multiple comparisons. MANOVA was then followed by repeated measures analyses of variance (ANOVA) for volumes of individual structures with group and hemisphere as fixed effects and covariates of age, gender, and cortical gray-matter volume. Exploratory analyses were also performed to examine the effects of typical (first generation) antipsychotic use and total psychotic symptoms on the volumes of individual structures. The effects of typical antipsychotic use were examined using repeated measures analyses as described above with an additional covariate of typical antipsychotic use. The effects of total psychotic symptoms were explored using Pearson correlation analyses in the BP and SCZ-S groups. All analyses were performed using the Statistical Analysis Software (SAS) version 9.3 (SAS Institute, Cary, NC, USA). P values ≤ 0.05 were considered significant.
To visualize potential regional shape changes, surface displacement maps were first generated by computing the surface-normal component of the displacement of each surface vertex relative to the overall average for every participant. An analysis of covariance (ANCOVA) was then performed at each vertex with group as an independent factor and covariates of age, gender, and cortical gray matter volume. Last, contrasts for pairwise group comparison (i.e., SCZ-S vs. HC, etc) were performed within this model, providing least square means of these surface vertex displacements for each group. For each pairwise group comparison, significant differences of these least square means between the two selected groups were displayed as a color map. Significance was corrected for multiple comparisons across surface vertices using a false discovery rate (FDR) threshold of 5% (i.e., q=0.05).
3. Results
3.1. Demographic and clinical characteristics
There were no significant differences in age, gender, race, or handedness between the participant groups. There were no significant differences in the frequency of antipsychotic use between the patient groups (BP and SCZ-S; and NPBP, PBP, and SCZ-S). There were significant differences in alcohol, cannabis, and opiate use disorders between the participant groups (Table 1).
3.2. Overall effects of diagnostic group and clinical subgroup
There was a significant effect of diagnostic group [λ=0.68, F(20,154)=1.64, p=0.05] and a trend-level effect of clinical subgroup [λ=0.59, F(30, 224)=1.47, p=0.06] on combined volumes.
3.3. Caudate volume and shape
A trend-level effect of diagnostic group (F(2,86)=2.63, p=0.08) on caudate volume was observed, while a significant effect of clinical subgroup [F(3,85)=2.91, p=0.04] was found (Table 2). As the effect of diagnostic group was only trend level, post hoc comparisons of the diagnostic groups were exploratory. Volumes of the left caudate were significantly smaller in the BP group than in the SCZ-S and HC groups (p<0.05). A trend-level difference was seen in the right caudate between the BP and SCZ-S groups (p=0.08), with smaller volumes in the BP group. Post hoc comparisons of the clinical subgroups showed significantly smaller bilateral caudate volumes in the NPBP group than in the SCZ-S and HC groups (p<0.02). Differences in bilateral caudate volumes between the NPBP and PBP groups were of trend-level significance (p<0.09), with smaller volumes in the NPBP group. (Fig. 1)
Table 2. Absolute mean volumes (mm3) for basal ganglia structures and the thalamus.
| Control (n=27) | SCZ-S (n=32) | BP (n=33) | F(2,86) | p | ||
|---|---|---|---|---|---|---|
| Caudate | 2.6 | 0.08 | ||||
| Left caudate | 3906 (473) | 3786 (520) | 3722 (470) | |||
| Right caudate | 3743 (481) | 3626 (489) | 3600 (455) | |||
| Putamen | 1.1 | 0.3 | ||||
| Left putamen | 4923 (564) | 4932 (562) | 4900 (596) | |||
| Right putamen | 5269 (614) | 5223 (610) | 5202 (692) | |||
| Globus pallidus | 5.3 | 0.007* | ||||
| Left globus pallidus | 1494 (148) | 1546 (198) | 1448 (162) | |||
| Right globus pallidus | 1654 (177) | 1687 (229) | 1636 (206) | |||
| Nucleus accumbens | 0.2 | 0.8 | ||||
| Left nucleus accumbens | 514 (72) | 497 (74) | 512 (65) | |||
| Right nucleus accumbens | 467 (38) | 444 (73) | 462 (77) | |||
| Thalamus | 1.0 | 0.4 | ||||
| Right thalamus | 7484 (626) | 7203 (654) | 7299 (758) | |||
| Left thalamus | 7523 (639) | 7329 (716) | 7378 (725) | |||
|
| ||||||
| Control (n=27) | SCZ-S (n=32) | NPBP (n=12) | PBP (n=21) | F(3,85) | p | |
|
| ||||||
| Caudate | 2.9 | 0.04* | ||||
| Left caudate | 3906 (473) | 3786 (520) | 3498 (397) | 3851 (468) | ||
| Right caudate | 3744 (481) | 3626 (489) | 3363 (408) | 3736 (432) | ||
| Putamen | 1.7 | 0.2 | ||||
| Left putamen | 4923 (564) | 4932 (562) | 4634 (625) | 5053 (535) | ||
| Right putamen | 5269 (614) | 5223 (610) | 4883 (535) | 5384 (599) | ||
| Globus pallidus | 5.9 | 0.001* | ||||
| Left globus pallidus | 1494 (148) | 1546 (198) | 1356 (204) | 1501 (105) | ||
| Right globus pallidus | 1654 (177) | 1687 (229) | 1500 (201) | 1713 (168) | ||
| Nucleus accumbens | 0.1 | 1.0 | ||||
| Left nucleus accumbens | 514 (72) | 497 (74) | 500 (77) | 519 (58) | ||
| Right nucleus accumbens | 467 (38) | 444 (73) | 442 (89) | 474 (70) | ||
| Thalamus | 0.9 | 0.4 | ||||
| Right thalamus | 7484 (626) | 7203 (654) | 7096 (886) | 7414 (670) | ||
| Left thalamus | 7523 (639) | 7329 (716) | 7139 (912) | 7523 (574) | ||
SCZ-S: Schizophrenia-spectrum disorders; BP: Bipolar disorder; NPBP: Non-psychotic bipolar disorder; PBP: Psychotic bipolar disorder
Mean volumes are expressed in mm3 with standard deviations in parentheses. F and p values for the effects of diagnostic group and clinical subgroup are shown.
denotes significant p values.
Fig. 1.
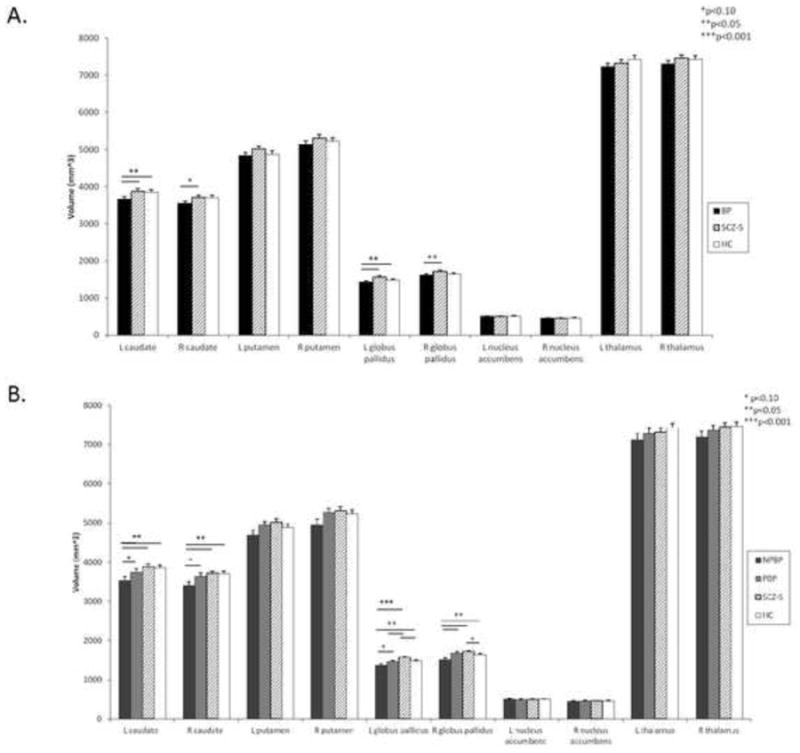
LS means volume with standard error bars for (A) diagnostic group and (B) clinical subgroup analyses. Diagnostic groups consist of bipolar disorder (BP), schizophrenia-spectrum disorders (SCZ-S), and healthy controls (HC). Clinical subgroups consist of nonpsychotic bipolar disorder (NPBP), psychotic bipolar disorder (PBP), SCZ-S, and HC.
Significant differences in caudate shape were found when comparing diagnostic groups and clinical subgroups, and some differences remained significant after FDR correction (Fig. 2). Specifically, in a comparison with the HC group, a region of inward deformation was observed in the superior surface of the left caudate in the BP group (Fig. 2). When the NPBP group was compared with the HC group, a larger area of inward deformation was observed bilaterally in the superior caudate, but particularly on the left. No significant shape differences were observed between the BP and SCZ-S groups, and the NPBP and PBP groups.
Fig. 2.
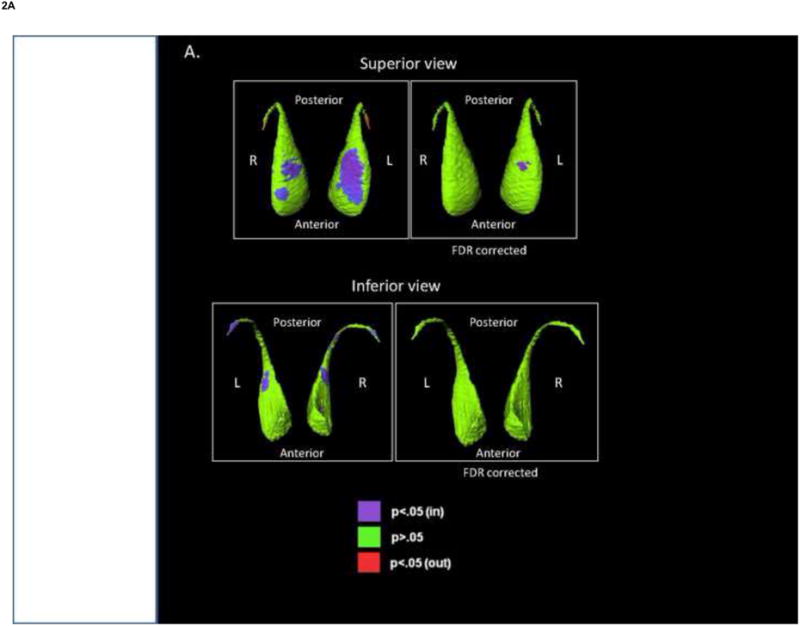
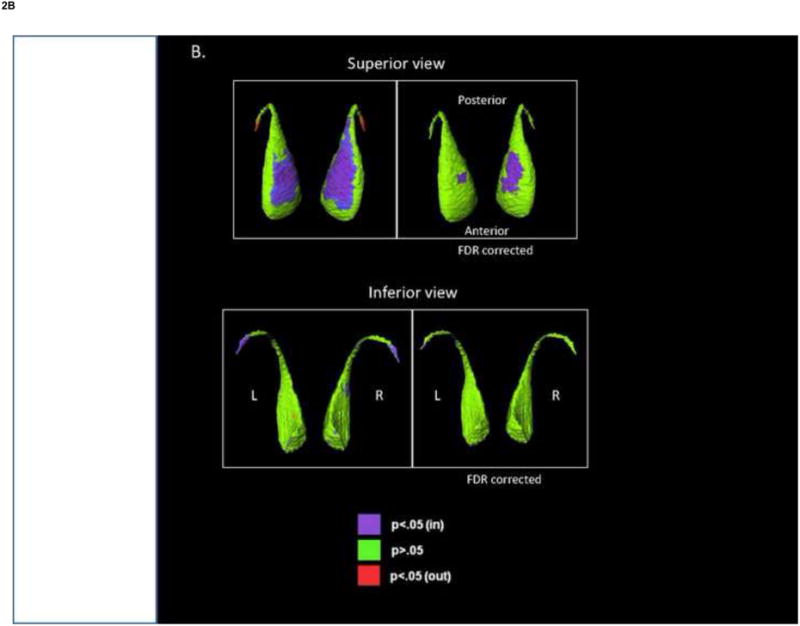
Shape analysis of the caudate. The figures show displacement maps generated from mean caudate surfaces in (A) bipolar disorder (BP) group, relative to healthy controls (HC), and (B) nonpsychotic bipolar disorder group (NPBP), relative to HC. Red shading denotes significant regions of outward deformity before FDR correction. Purple shading denotes significant regions of inward deformity after FDR correction (q=0.05).
3.4. Putamen volume and shape
There were no significant effects on putamen volume by diagnostic group or clinical subgroup (Table 2).
There were no significant differences in putamen shape when comparing the BP and SCZ-S groups with the HC group, or among the clinical subgroups. Significant differences in putamen shape were observed when comparing the BP and SCZ-S groups, after FDR correction. Regions of outward deformation were largely in the posterio-inferior tip in the SCZ-S group, compared with the BP group (Fig. 3A).
Fig. 3.
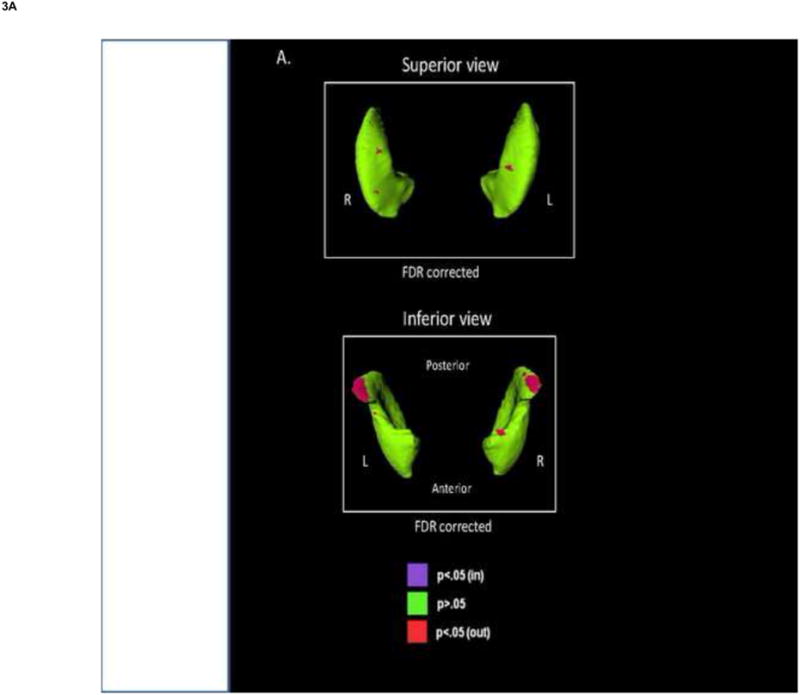
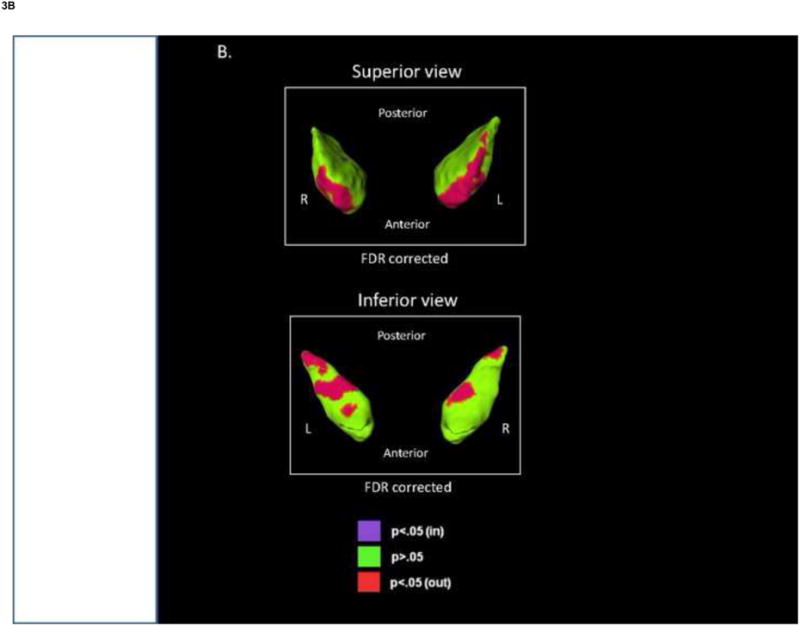
Shape analysis of the globus pallidus and putamen. The figures show the displacement maps generated from (A) the mean putamen surface and (B) the mean globus pallidus surface in the schizophrenia spectrum disorders (SCZ-S) group relative to the bipolar disorder (BP) group. Red shading denotes significant regions of outward deformity in SCZ-S relative to BP after FDR correction (q=0.05).
3.5. Globus pallidus volume and shape
The effects of diagnostic group [F(2,86)=5.3, p=0.007] and clinical subgroup [F(3,85)=5.9, p=0.001] on globus pallidus volume were significant (Table 2). Post hoc comparisons of the diagnostic groups showed significantly smaller bilateral globus pallidi volumes in the BP group compared with the SCZ-S and HC groups (p<0.03), while such comparisons of the clinical subgroups found significantly smaller bilateral globus pallidi volumes in the NPBP group compared with the SCZ-S and HC groups (p<0.04). The NPBP group had significantly smaller volumes in the right globus pallidus compared with the PBP group (p=0.008). Differences between the NPBP and PBP groups were of trend-level significance in the left globus pallidus (p=0.06), with smaller volumes in the NPBP group. The SCZ-S group had significantly larger left globus pallidus volumes compared with the PBP and HC groups (p<0.02). (Fig. 1)
No significant shape differences were observed in comparisons of the BP and SCZ-S groups with the HC group or among the clinical subgroups. However, several regions of outward deformation in the globus pallidus were observed in the SCZ-S group, compared with the BP group, anterio-superiorly and intermittently along the inferior surface, after FDR correction (Fig. 3B).
3.6. Nucleus accumbens volume and shape
No significant effects of diagnostic or clinical group on nucleus accumbens volume (Table 2) or shape were observed.
3.7. Thalamus volume and shape
There were no significant effects of diagnostic group or clinical subgroup on thalamus volume (Table 2) or shape.
3.8. Antipsychotic use and total psychotic symptoms
Findings were similar to those as described above when analyses also included typical antipsychotic use as a covariate. There were no significant effects of typical antipsychotic use in either the diagnostic or clinical subgroup analyses.
Across the BP and SCZ-S participants, significant positive correlations between total psychotic symptoms and left (r=0.30, p=0.01) and right globus pallidus volume (r=0.25, p=0.05) were found. There were no significant correlations observed between total psychotic symptoms and right or left volumes of the caudate, putamen, nucleus accumbens or thalamus.
4. Discussion
In this study, we found significant group differences in the caudate and globus pallidus, particularly in the clinical subgroup comparisons. Our results suggest that the caudate and globus pallidus are smallest in the NPBP group compared with the PBP, SCZ-S, and HC groups. Volume differences were complemented by differences found in shape analyses. Inward deformation of the superior caudate was found in BP when compared to HC, with more prominent deformation in NPBP. Outward deformations were found in the SCZ-S compared to BP in superior and inferior regions of the globus pallidus in SCZ-S, as well as in the posterio-inferior region of the putamen. No significant volume or shape differences were found in the nucleus accumbens and thalamus.
One obvious potential explanation for our results is that the structural differences in the caudate and globus pallidus found herein may be attributed to antipsychotic effects on the brain. Typical antipsychotics, which have primarily D2-type dopaminergic receptor antagonistic effects, are associated with enlargement of basal ganglia structures (Corson et al., 1999; Boonstra et al., 2011); however, the effects of atypical antipsychotics on these structures are less clear. In general, atypical antipsychotics do not appear to affect basal ganglia structures, although increases, decreases, and no change in caudate volume have been associated with atypical antipsychotic use (Tauscher-Wisniewski et al., 2002; Boonstra et al., 2011). The majority of our study participants were exposed to only atypical antipsychotics during the 2 years before scanning (Table 1). The NPBP group had a lower frequency of atypical antipsychotic exposure and no exposure to typical antipsychotics compared with the PBP and SCZ-S groups, although antipsychotic exposure in the 2 years before scanning was not found to be significantly different among the patient groups (Table 1). Furthermore, the NPBP group was observed to have significantly smaller caudate and globus pallidus volumes even when compared with the HC group, which did not have antipsychotic exposure. It appears less likely that our findings were primarily driven by antipsychotic effects; however, antipsychotic exposure that may have occurred earlier than the 2 years preceding scanning was not evaluated in this study. Further characterization of antipsychotic exposure is needed in future studies to better understand the relationship between antipsychotics and basal ganglia structure.
Although antipsychotic effects may have, at least in part, contributed to our findings, there is evidence that supports an association between basal ganglia structure and psychosis itself. Increased basal ganglia volumes, particularly in the caudate, have been associated with behavioral abnormalities and cognitive deficits, and have also been found in neurodevelopmental disorders such as autism and velocardiofacial syndrome, both of which have demonstrated increased risk for psychosis (Kates et al., 2004; Hollander et al., 2005; Voelbel et al., 2006; Toal et al., 2009; Hallahan et al., 2011). Abnormalities in prefrontal-basal ganglia functional connectivity have been observed in SCZ and schizoaffective disorder (Yoon et al., 2013). Earlier work by Mamah et al. found shape abnormalities in the basal ganglia, including the caudate and globus pallidus, with most pronounced changes in SCZ participants and similar changes of lesser magnitude in their unaffected siblings, compared with controls (Mamah et al., 2008). These findings suggest potential genetic influences related to a predisposition for psychosis on the basal ganglia. Furthermore, Gur et al. demonstrated a positive association between psychotic symptom severity and basal ganglia volumes in previously treated and neuroleptic-naïve patients with SCZ (Gur et al., 1998). While they found a positive correlation between negative symptom severity and caudate volumes and between positive symptom severity and globus pallidus volumes in previously treated patients, they also observed a positive correlation between positive symptom severity and caudate volumes in neuroleptic-naïve patients. These correlations persisted even after accounting for antipsychotic dose, duration of treatment, age, age at onset, and duration of illness. A later study of neuroleptic-naïve SCZ patients found a negative correlation between global symptom severity and globus pallidus volume but did not find a significant correlation between severity of specific psychotic symptom domains (i.e., positive, negative, and disorganized) and globus pallidus volume (Spinks et al., 2005). Other basal ganglia structures, including the caudate, were not examined in the study. In this present study, a positive correlation was found between total psychotic symptoms and globus pallidus volume. However, our characterization of clinical symptoms was limited to only positive symptoms. More in-depth assessment of clinical symptoms would include examining positive, negative, and disorganization symptoms, correlation between psychotic symptoms and mood episodes, and the duration of active psychotic symptoms, and such an examination should be included in future studies.
While we found larger caudate and globus pallidus volumes in the psychotic subgroups (PBP and SCZ-S), interestingly, we observed the smallest volumes in the nonpsychotic patient subgroup (NPBP), even when compared with the HC group. Smaller caudate volumes have been shown in unipolar depression (Parashos et al., 1998; Kim et al., 2008; Matsuo et al., 2008; Butters et al., 2009; Koolschijn et al., 2009; Pizzagalli et al., 2009; Bora et al., 2012), suggesting an association between caudate volumes and depressive symptoms. The smaller caudate volumes in the NPBP group may relate to a particular vulnerability to depression in this group. Unfortunately, details regarding mood symptoms among the study participants were limited in this study.
Alterations in the basal ganglia may also relate to interconnections with other structures such as the anterior cingulate (AC) and dorsolateral prefrontal cortex (DLPFC). Studies have implicated significant connections between the caudate and AC and DLPFC (Beckmann et al., 2009; Verstynen et al., 2012), and reductions in caudate volumes have been shown following anterior cingulotomy in humans (Rauch et al., 2000). Additionally, a correlation between dopamine D1 receptor density in the caudate and DLPFC-parietal functional connectivity during a working memory task has also been demonstrated (Rieckmann et al., 2011). Differences in dopamine receptor density in the caudate between psychotic groups (PBP and SCZ) and nonpsychotic groups (NPBP and controls) have been previously reported (Pearlson et al., 1995). Interestingly, the regions of significant shape differences that were found in this study between BP and HC, and NPBP and HC receive substantial projections from the DLPFC (Verstynen et al., 2012). Future studies examining the caudate and interconnected regions, such as the AC and DLFPC, may provide further insight into the role of the caudate in BP and SCZ.
The specific involvement of the globus pallidus and putamen in emotion and cognition are not clearly understood. Hence, potential explanations for the volume and shape differences observed in these regions are less apparent. However, the association of lesions in the globus pallidus with depression and apathy, as well as diminished reward effects of drugs of abuse, suggest roles in reward and motivation (Strub, 1989; Levy and Dubois, 2006; Miller et al., 2006; Singh et al., 2011). The globus pallidus is connected with structures in the reward circuitry of the brain, such as the habenula, nucleus accumbens, and the ventral tegmental area (Miller et al., 2006; Hong and Hikosaka, 2008), but its specific roles in this circuitry need to be further elucidated.
Findings may have been limited by the small sample sizes in the NPBP and PBP groups, and future studies with larger sample sizes are warranted to draw more definitive conclusions. Nevertheless, differences in findings between the two classification approaches in this study, diagnostic groups versus clinical subgroups, indicate the need for careful consideration in determining acceptable within-group heterogeneity in studies. They also highlight the potential importance of classification of bipolar disorder based on psychotic history. Such an approach may reduce inconsistencies in findings among studies. Results of our study may also have been influenced by recruitment of diagnostic groups from different sites. However, similar protocols for recruitment and clinical characterization were used in obtaining clinical data across both sites, and the identical scanners and protocols were used in obtaining the MRI data. Quality-assurance studies indicated compatibility between the scanners at the two sites. Thus, the effect of this on our results would be expected to be minimal. High reliability across scanners of the same model and field strength has been previously described for automated segmentation of subcortical regions (Nugent et al., 2013). Additionally, comorbid disorders, which were present in all groups to varying extents, could also contribute to alterations in basal ganglia and thalamus morphology. In particular, attention-deficit hyperactivity disorder (ADHD) and BP have been shown to have independent effects on basal ganglia structure with differential alterations in children and adolescents with ADHD only, BP only, and comorbid ADHD and BP (Liu et al., 2011). Unfortunately, comorbid ADHD was not assessed in our study sample.
In summary, we found structural abnormalities in the caudate and globus pallidus in BP and SCZ-S patients, with findings more prominent in the NPBP group. The observed group differences between may be, in part, attributed to disparity in antipsychotic exposure, although this was not overtly evident in the present study. Future studies in larger samples, and with more extensive medication histories, may provide greater insight into the etiology of observed group differences. Additionally, our findings suggest that examining BP subgroups may be important and useful in understanding the underlying mechanisms of BP and SCZ.
Highlights.
Basal ganglia and thalamic morphology in bipolar disorder and schizophrenia.
Particular interest in the differences related to the presence of psychosis.
Significant differences were found in the caudate and globus pallidus.
Differences were more apparent in non-psychotic bipolar disorder.
It may be important to separately examine clinical subgroups of bipolar disorder.
Acknowledgments
Support for the preparation of this article was provided by National Institute of Mental Health (NIMH) R01 MH056584 (JGC), NIMH grant K08 MH085948 (DM), and NIMH R01 MH084803 (LW). The authors have no financial conflicts of interest with regard to the work presented in this article.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM. Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. British Journal of Psychiatry. 2009;195:194–201. doi: 10.1192/bjp.bp.108.059717. [DOI] [PubMed] [Google Scholar]
- Beckmann M, Johansen-Berg H, Rushworth MFS. Connectivity-based parcellation of human cingulate cortex and its relation to functional specialization. Journal of Neuroscience. 2009;29:1175–1190. doi: 10.1523/JNEUROSCI.3328-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg MF, Miller MI, Trouve A, Younes L. Computing large deformation metric mappings via geodesic flows of diffeomorphisms. International Journal of Computer Vision. 2005;61:139–157. [Google Scholar]
- Boonstra G, van Haren NEM, Schnack HG, Cahn W, Burger H, Boersma M, de Kroon B, Grobbee DE, Hulshoff Pol HE, Kahn RS. Brain volume changes after withdrawal of atypical antipsychotics in patients with first-episode schizophrenia. Journal of Clinical Psychopharmacology. 2011;31:146–153. doi: 10.1097/JCP.0b013e31820e3f58. [DOI] [PubMed] [Google Scholar]
- Bora E, Harrison BJ, Davey CG, Yücel M, Pantelis C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychological Medicine. 2012;42:671–681. doi: 10.1017/S0033291711001668. [DOI] [PubMed] [Google Scholar]
- Brandt GN, Bonelli RM. Structural neuroimaging of the basal ganglia in schizophrenic patients: a review. Wiener Medizinische Wochenschrift. 2008;158:84–90. doi: 10.1007/s10354-007-0478-7. [DOI] [PubMed] [Google Scholar]
- Brown GG, Lee JS, Strigo IA, Caligiuri MP, Meloy MJ, Lohr J. Voxel-based morphometry of patients with schizophrenia or bipolar I disorder: a matched control study. Psychiatry Research. 2011;194:149–156. doi: 10.1016/j.pscychresns.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Busatto GF. Structural and functional neuroimaging studies in major depressive disorder with psychotic features: a critical review. Schizophrenia Bulletin. 2013;39:776–786. doi: 10.1093/schbul/sbt054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butters MA, Aizenstein HJ, Hayashi KM, Meltzer CC, Seaman J, Reynolds CF, Toga AW, Thompson PM, Becker JT. Three-dimensional surface mapping of the caudate nucleus in late-life depression. American Journal of Geriatric Psychiatry. 2009;17:4–12. doi: 10.1097/JGP.0b013e31816ff72b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byne W, Hazlett EA, Buchsbaum MS, Kemether E. The thalamus and schizophrenia: current status of research. Acta Neuropathologica. 2009;117:347–368. doi: 10.1007/s00401-008-0404-0. [DOI] [PubMed] [Google Scholar]
- Corson PW, Nopoulos P, Miller DD, Arndt S, Andreason NC. Change in basal ganglia volume over 2 years in patients with schizophrenia: typical versus atypical neuroleptics. American Journal of Psychiatry. 1999;156:1200–1204. doi: 10.1176/ajp.156.8.1200. [DOI] [PubMed] [Google Scholar]
- Coscia DM, Narr KL, Robinson DG, Hamilton LS, Sevy S, Burdick KE, Gunduz-Bruce H, McCormack J, Bilder RM, Szeszko PR. Volumetric and shape analysis of the thalamus in first-episode schizophrenia. Human Brain Mapping. 2009;30:1236–45. doi: 10.1002/hbm.20595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Joshi S, Wang L, Haller JW, Gado M, Miller JP, Grenander U, Miller MI. Hippocampal morphometry in schizophrenia by high dimensional brain mapping. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:11406–11411. doi: 10.1073/pnas.95.19.11406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csernansky JG, Schindler MK, Splinter NR, Wang L, Gado M, Selemon LD, Rastogi-Cruz D, Posener JA, Thompson PA, Miller MI. Abnormalities of thalamic volume and shape in schizophrenia. American Journal of Psychiatry. 2004;161:896–902. doi: 10.1176/appi.ajp.161.5.896. [DOI] [PubMed] [Google Scholar]
- Csernansky JG, Wang L, Jones D, Rastogi-Cruz D, Posener JA, Heydebrand G, Miller P, Miller MI. Hippocampal deformities in schizophrenia characterized by high dimensional brain mapping. American Journal of Psychiatry. 2002;159:2000–2006. doi: 10.1176/appi.ajp.159.12.2000. [DOI] [PubMed] [Google Scholar]
- De Castro-Manglano P, Mechelli A, Soutullo C, Landecho I, Gimenez-Amaya JM, Ortuño F, McGuire P. Structural brain abnormalities in first-episode psychosis: differences between affective psychoses and schizophrenia and relationship to clinical outcome. Bipolar Disorders. 2011;13:545–555. doi: 10.1111/j.1399-5618.2011.00953.x. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophrenia Research. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- Garrett A, Kelly R, Gomez R, Keller J, Schatzberg AF, Reiss AL. Aberrant brain activation during a working memory task in psychotic major depression. American Journal of Psychiatry. 2011;168:173–182. doi: 10.1176/appi.ajp.2010.09121718. [DOI] [PubMed] [Google Scholar]
- Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. American Journal of Psychiatry. 1998;155:1711–1717. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- Haber SN, Calzavara R. The cortico-basal ganglia integrative network: the role of the thalamus. Brain Research Bulletin. 2009;78:69–74. doi: 10.1016/j.brainresbull.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J, Whalley HC, Marwick K, McKirdy J, Sussmann J, Romaniuk L, Johnstone EC, Wan HI, McIntosh AM, Lawrie SM. Hippocampal function in schizophrenia and bipolar disorder. Psychological Medicine. 2010;40:761–770. doi: 10.1017/S0033291709991000. [DOI] [PubMed] [Google Scholar]
- Hallahan BP, Craig MC, Toal F, Daly EM, Moore CJ, Ambikapathy A, Robertson D, Murphy KC, Murphy DGM. In vivo brain anatomy of adult males with Fragile X syndrome: an MRI study. Neuroimage. 2011;54:16–24. doi: 10.1016/j.neuroimage.2010.08.015. [DOI] [PubMed] [Google Scholar]
- Hamilton LS, Altshuler LL, Townsend J, Bookheimer SY, Phillips OR, Fischer J, Woods RP, Mazziotta JC, Toga AW, Nuechterlein KH, Narr KL. Alterations in functional activation in euthymic bipolar disorder and schizophrenia during a working memory task. Human Brain Mapping. 2009;30:3958–3969. doi: 10.1002/hbm.20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG. Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. Journal of Neuroscience. 2007;27:13835–13842. doi: 10.1523/JNEUROSCI.2571-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander E, Anagnostou E, Chaplin W, Esposito K, Haznedar MM, Licalzi E, Wasserman S, Soorya L, Buchsbaum M. Striatal volume on magnetic resonance imaging and repetitive behaviors in autism. Biological Psychiatry. 2005;58:226–232. doi: 10.1016/j.biopsych.2005.03.040. [DOI] [PubMed] [Google Scholar]
- Hong S, Hikosaka O. The globus pallidus sends reward-related signals to the lateral habenula. Neuron. 2008;60:720–729. doi: 10.1016/j.neuron.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III--the final common pathway. Schizophrenia Bulletin. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivleva EI, Bidesi AS, Thomas BP, Meda SA, Francis A, Moates AF, Witte B, Keshavan MS, Tamminga CA. Brain gray matter phenotypes across the psychosis dimension. Psychiatry Research. 2012;204:13–24. doi: 10.1016/j.pscychresns.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates WR, Burnette CP, Bessette BA, Folley BS, Strunge L, Jabs EW, Pearlson GD. Frontal and caudate alterations in velocardiofacial syndrome (deletion at chromosome 22q11.2) Journal of Child Neurology. 2004;19:337–342. doi: 10.1177/088307380401900506. [DOI] [PubMed] [Google Scholar]
- Khan AR, Wang L, Beg MF. FreeSurfer-initiated fully-automated subcortical brain segmentation in MRI using Large Deformation Diffeomorphic Metric Mapping. Neuroimage. 2008;41:735–746. doi: 10.1016/j.neuroimage.2008.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Hamilton JP, Gotlib IH. Reduced caudate gray matter volume in women with major depressive disorder. Psychiatry Research: Neuroimaging. 2008;164:114–122. doi: 10.1016/j.pscychresns.2007.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolschijn PCMP, van Haren NEM, Lensvelt-Mulders GJLM, Hulshoff Pol HE, Kahn RS. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Human Brain Mapping. 2009;30:3719–3735. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R, Dubois B. Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cerebral Cortex. 2006;16:916–928. doi: 10.1093/cercor/bhj043. [DOI] [PubMed] [Google Scholar]
- Liu IY, Howe M, Garrett A, Karchemskiy A, Kelley R, Alegria D, Reiss A, Chang K. Striatal volumes in pediatric bipolar patients with and without comorbid ADHD. Psychiatry Research: Neuroimaging. 2011;194:14–20. doi: 10.1016/j.pscychresns.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Harms MP, Wang L, Barch D, Thompson P, Kim J, Miller MI, Csernansky JG. Basal ganglia shape abnormalities in the unaffected siblings of schizophrenia patients. Biological Psychiatry. 2008;64:111–120. doi: 10.1016/j.biopsych.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamah D, Wang L, Barch D, de Erausquin GA, Gado M, Csernansky JG. Structural analysis of the basal ganglia in schizophrenia. Schizophrenia Research. 2007;89:59–71. doi: 10.1016/j.schres.2006.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand WR, Yurgelun-Todd D. Striatal structure and function in mood disorders: a comprehensive review. Bipolar Disorders. 2010;12:764–785. doi: 10.1111/j.1399-5618.2010.00874.x. [DOI] [PubMed] [Google Scholar]
- Matsuo K, Rosenberg DR, Easter PC, Mac Master FP, Chen HH, Nicoletti M, Caetano SC, Hatch JP, Soares JC. Striatal volume abnormalities in treatment-naïve patients diagnosed with pediatric major depressive disorder. Journal of Child and Adolescent Psychopharmacology. 2008;18:121–131. doi: 10.1089/cap.2007.0026. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Whalley HC, McKirdy J, Hall J, Sussmann JED, Shankar P, Johnstone EC, Lawrie SM. Prefrontal function and activation in bipolar disorder and schizophrenia. American Journal of Psychiatry. 2008a;165:378–384. doi: 10.1176/appi.ajp.2007.07020365. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Muñoz Maniega S, Lymer GKS, McKirdy J, Hall J, Sussmann JED, Bastin ME, Clayden JD, Johnstone EC, Lawrie SM. White matter tractography in bipolar disorder and schizophrenia. Biological Psychiatry. 2008b;64:1088–1092. doi: 10.1016/j.biopsych.2008.07.026. [DOI] [PubMed] [Google Scholar]
- Miller JM, Vorel SR, Tranguch AJ, Kenny ET, Mazzoni P, van Gorp WG, Kleber HD. Anhedonia after a selective bilateral lesion of the globus pallidus. American Journal of Psychiatry. 2006;163:786–788. doi: 10.1176/ajp.2006.163.5.786. [DOI] [PubMed] [Google Scholar]
- Nugent AC, Luckenbaugh DA, Wood SE, Bogers W, Zarate CA, Drevets WC. Automated subcortical segmentation using FIRST: Test-retest reliability, interscanner reliability, and comparison to manual segmentation. Human Brain Mapping. 2013;34:2313–2329. doi: 10.1002/hbm.22068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong D, Walterfang MA, Malhi G, Styner M, Velakoulis D, Pantelis C. Shape alteration in the caudate nucleus in individuals with bipolar affective disorder. Australian and New Zealand Journal of Psychiatry. 2012;46:340–351. doi: 10.1177/0004867412440191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ongür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF. Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Research: Neuroimaging. 2010;183:59–68. doi: 10.1016/j.pscychresns.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashos IA, Tupler LA, Blitchington T, Krishnan KR. Magnetic-resonance morphometry in patients with major depression. Psychiatry Research: Neuroimaging. 1998;84:7–15. doi: 10.1016/s0925-4927(98)00042-0. [DOI] [PubMed] [Google Scholar]
- Pearlson GD, Wong DF, Tune LE, Ross CA, Chase GA, Links JM, Dannals RF, Wilson AA, Ravert HT, Wagner HN. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Archives of General Psychiatry. 1995;52:471–477. doi: 10.1001/archpsyc.1995.03950180057008. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Holmes AJ, Dillon DG, Goetz EL, Birk JL, Bogdan R, Dougherty DD, Iosifescu DV, Rauch SL, Fava M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. American Journal of Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radenbach K, Flaig V, Schneider-Axmann T, Usher J, Reith W, Falkai P, Gruber O, Scherk H. Thalamic volumes in patients with bipolar disorder. European Archives of Psychiatry and Clinical Neurosciences. 2010;260:601–607. doi: 10.1007/s00406-010-0100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Kim H, Makris N, Cosgrove GR, Cassem EH, Savage CR, Price BH, Nierenberg AA, Shera D, Baer L, Buchbinder B, Caviness VS, Jenike MA, Kennedy DN. Volume reduction in the caudate nucleus following stereotactic placement of lesions in the anterior cingulate cortex in humans: a morphometric magnetic resonance imaging study. Journal of Neurosurgery. 2000;93:1019–1025. doi: 10.3171/jns.2000.93.6.1019. [DOI] [PubMed] [Google Scholar]
- Rieckmann A, Karlsson S, Fischer H, Bäckman L. Caudate dopamine D1 receptor density is associated with individual differences in frontoparietal connectivity during working memory. Journal of Neuroscience. 2011;31:14284–14290. doi: 10.1523/JNEUROSCI.3114-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimol LM, Hartberg CB, Nesvåg R, Fennema-Notestine C, Hagler DJ, Pung CJ, Jennings RG, Haukvik UK, Lange E, Nakstad PH, Melle I, Andreassen OA, Dale AM, Agartz I. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biological Psychiatry. 2010;68:41–50. doi: 10.1016/j.biopsych.2010.03.036. [DOI] [PubMed] [Google Scholar]
- Singh A, Mahgoub N, Klimstra S. Apathy associated with a unilateral globus pallidus lesion: a case report. International Journal of Geriatric Psychiatry. 2011;26:765–766. doi: 10.1002/gps.2563. [DOI] [PubMed] [Google Scholar]
- Smith MJ, Wang L, Cronenwett W, Mamah D, Barch DM, Csernansky JG. Thalamic morphology in schizophrenia and schizoaffective disorder. Journal of Psychiatric Research. 2011;45:378–85. doi: 10.1016/j.jpsychires.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinks R, Nopoulos P, Ward J, Fuller R, Magnotta VA, Andreasen NC. Globus pallidus volume is related to symptom severity in neuroleptic naive patients with schizophrenia. Schizophrenia Research. 2005;73:229–233. doi: 10.1016/j.schres.2004.05.020. [DOI] [PubMed] [Google Scholar]
- Strasser HC, Lilyestrom J, Ashby ER, Honeycutt NA, Schretlen DJ, Pulver AE, Hopkins RO, Depaulo JR, Potash JB, Schweizer B, Yates KO, Kurian E, Barta PE, Pearlson GD. Hippocampal and ventricular volumes in psychotic and nonpsychotic bipolar patients compared with schizophrenia patients and community control subjects: a pilot study. Biological Psychiatry. 2005;57:633–639. doi: 10.1016/j.biopsych.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Strub RL. Frontal lobe syndrome in a patient with bilateral globus pallidus lesions. Archives of Neurology. 1989;46:1024–1027. doi: 10.1001/archneur.1989.00520450096027. [DOI] [PubMed] [Google Scholar]
- Sui J, Pearlson G, Caprihan A, Adali T, Kiehl KA, Liu J, Yamamoto J, Calhoun VD. Discriminating schizophrenia and bipolar disorder by fusing fMRI and DTI in a multimodal CCA+ joint ICA model. Neuroimage. 2011;57:839–855. doi: 10.1016/j.neuroimage.2011.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauscher-Wisniewski S, Tauscher J, Logan J. Caudate volume changes in first episode psychosis parallel the effects of normal aging: a 5-year follow-up study. Schizophrenia Research. 2002;58:185–188. doi: 10.1016/s0920-9964(01)00406-6. [DOI] [PubMed] [Google Scholar]
- Toal F, Bloemen OJN, Deeley Q, Tunstall N, Daly EM, Page L, Brammer MJ, Murphy KC, Murphy DGM. Psychosis and autism: magnetic resonance imaging study of brain anatomy. British Journal of Psychiatry. 2009;194:418–425. doi: 10.1192/bjp.bp.107.049007. [DOI] [PubMed] [Google Scholar]
- Verstynen TD, Badre D, Jarbo K, Schneider W. Microstructural organizational patterns in the human corticostriatal system. Journal of Neurophysiology. 2012;107:2984–2995. doi: 10.1152/jn.00995.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelbel GT, Bates ME, Buckman JF, Pandina G, Hendren RL. Caudate nucleus volume and cognitive performance: are they related in childhood psychopathology? Biological Psychiatry. 2006;60:942–950. doi: 10.1016/j.biopsych.2006.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Mamah D, Harms MP, Karnik M, Price JL, Gado MH, Thompson PA, Barch DM, Miller MI, Csernansky JG. Progressive deformation of deep brain nuclei and hippocampal-amygdala formation in schizophrenia. Biological Psychiatry. 2008;64:1060–1068. doi: 10.1016/j.biopsych.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PW, Ketter TA. Biology and recent brain imaging studies in affective psychoses. Current Psychiatry Reports. 2000;2:298–304. doi: 10.1007/s11920-000-0071-x. [DOI] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Raouf S, D'Esposito M, Carter CS. Impaired prefrontal-basal ganglia functional connectivity and substantia nigra hyperactivity in schizophrenia. Biological Psychiatry. 2013;74:122–129. doi: 10.1016/j.biopsych.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]


