Abstract
Coenzyme Q10 (CoQ10) deficiency appears to have a particularly heterogeneous clinical presentation. However, there appear to be 5 recognisable clinical phenotypes: encephalomyopathy, severe infantile multisystemic disease, nephropathy, cerebellar ataxia, and isolated myopathy. However, although useful, clinical symptoms alone are insufficient for the definitive diagnosis of CoQ10 deficiency which relies upon biochemical assessment of tissue CoQ10 status. In this article, we review the biochemical methods used in the diagnosis of human CoQ10 deficiency and indicate the most appropriate tissues for this evaluation.
Key Words: Antioxidant, Cholesterol, Coenzyme Q10, Disease, Mitochondria, Muscle, Plasma, Tissue
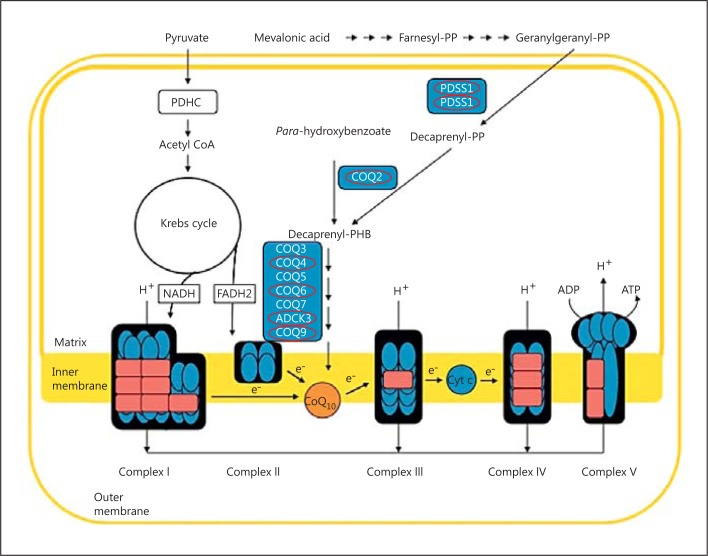
Coenzyme Q10 (CoQ10) plays an important role in oxidative phosphorylation where it acts as an electron carrier in the mitochondrial electron transport chain (ETC; fig. 1), accepting electrons derived from complex I (NADH ubiquinone reductase; EC 1.6.5.3) and complex II (succinate ubiquinone reductase; EC 1.3.5.1) and transferring them to complex III (ubiquinol cytochrome c reductase; EC 1.10.2.2) [Ernster and Dallner, 1995]. The reduced form of CoQ10, ubiquinol, has an important cellular antioxidant function; it protects membranes and plasma lipoproteins against free radical-induced oxidation [Ernster and Forsmark-Andrée, 1993]. In addition, CoQ10 is also involved in DNA replication and repair through its role in pyrimidine synthesis [Lopez-Martin et al., 2007], modulation of apoptosis via its regulation of the mitochondrial permeability transition pore [Cotan et al., 2011], and body temperature regulation via its obligatory cofactor role for the uncoupling proteins [Echtay et al., 2001].
Fig. 1.
Structure of mitochondrial electron transport chain, showing the electron carrier function of coenzyme Q10 (Q). Red circles indicate proteins with mutations causing CoQ10 deficiency; see table 1.
In view of its role as an electron carrier in the ETC and its antioxidant function, a deficit in CoQ10 status could conceivably contribute to disease pathophysiology by causing a failure in energy metabolism and compromising cellular antioxidant status.
The first cases of CoQ10 deficiency were reported in 1989 by Ogasahara et al. The patients were 2 sisters born to unrelated parents who presented with recurrent rhabdomyolysis associated with seizures and mental retardation. Since this time, a number of patients have been described, and CoQ10 deficiency appears to have a particularly heterogeneous clinical presentation. However, there appear to be 5 distinct clinical phenotypes: encephalomyopathy, severe infantile multisystemic disease, nephropathy, cerebellar ataxia, and isolated myopathy [Emmanuele et al., 2012]. In most cases, the family history suggests an autosomal recessive mode of inheritance. Since 2006, mutations in 7 genes, encoding components closely related with the CoQ10 biosynthetic pathway, have been associated with human CoQ10 deficiency (table 1). However, in the preponderance of patients with CoQ10 deficiency, it has not been possible to identify the underlying genetic cause [Rahman et al., 2012]. The genetic diagnosis of CoQ10 deficiency is complicated by the fact that the CoQ10 biosynthetic pathway has yet to be fully elucidated in humans, and the possibility arises that the cause of the deficit may result from pathogenic mutations in genes not directly related to CoQ10 synthesis [Emmanuele et al., 2012].
Table 1.
Gene mutations and associated clinical phenotypes of patients with CoQ10 deficiency
| Gene | Molecular genetics | Description |
|---|---|---|
| PDSS1 | 10p12.1 | Multisystem disease with early-onset deafness, optic atrophy, mild mental retardation, peripheral neuropathy, obesity, livedo reticularis, and cardiac valvulopathy; Mollet et al. [2007] |
| ASP308GLU (D308E) | ||
| PDSS2 | 6q21 | Fatal encephalomyopathy and nephrotic syndrome; Lopez et al. [2006] |
| GLN322TER (Q322X); SER382LEU (S382L) | ||
| COQ2 | 4q21.23 | Early-onset infantile encephalomyopathy, nephropathy; Salviati et al. [2005], Diomedi-Camassei et al. [2007] |
| Fatal infantile multiorgan disease including anemia, pancytopenia, liver failure, and renal insufficiency; Mollet et al. [2007] | ||
| COQ4 | 9q34.11 heterozygous 3.9-Mb deletion | Encephalomyopathic disorder, including poor growth, hypotonia, and delayed psychomotor development with moderate mental retardation and an inability to walk at age; Salviati et al. [2012] |
| COQ6 | 14q24.3 | Early-onset nephrotic syndrome with sensorineural deafness; Heeringa et al. [2011] |
| GLY255ARG (G255R); ALA353ASP (A353D); TRP447TER (Q447X); 1-bp del, c.1383delG; ARG162TER (R162X); TRP188TER (W188X) | ||
| CABC1 (COQ8/ADCK3) | 1q42.13 | Autosomal recessive childhood-onset cerebellar ataxia with cerebellar atrophy, seizures, developmental delay, and hyperlactatemia; Mollet et al. [2008], Lagier-Tourenne et al. [2008] |
| GLU551LYS (E551K); ARG213TRP (R213W); GLY272VAL (G272V); GLY272ASP (G272D); 1-bp ins, c.1812insG; IVS11+2 T≥C; 22-bp del/3-bp ins; TYR514CYS (Y514C); 3-bp del, 1750ACC 3-bp del, c.1750delAAC; 993C-T c. 993C>T, Ex8 → (Lys314_Gln360del); GLY549SER (G549S) | ||
| COQ9 | 16q13 | Infant with severe fatal CoQ10 deficiency; Duncan et al. [2009] |
| ARG244TER (R244X) |
The purpose of this article is to critically review the current biochemical methods used in the diagnosis of human CoQ10 deficiency and to indicate the most appropriate tissues for this evaluation.
Methods Used to Determine Tissue CoQ10 Status
The most common laboratory procedures used for diagnosis of CoQ10 deficiency are based on high-pressure liquid chromatography with ultraviolet (HPLC-UV) or electrochemical (HPLC-ED) detection systems. Other procedures, such as tandem-mass spectrometry, have been employed for tissue CoQ10 assessment; however, this method will not be discussed further in this article and the reader is referred to the review by Barshop and Gangoiti [2007] which discusses this analytical procedure. Although it is possible to determine the reduced (ubiquinol) and oxidized forms of CoQ10 concomitantly by HPLC-ED analysis, diagnostically, the determination of total tissue CoQ10 status is sufficiently accurate to detect human CoQ10 deficiencies. Simultaneous measurement of both reduced and oxidized forms of CoQ10 usually requires a complex pre-analytical management of samples, and chromatographically, this is more complicated than the measurement of total CoQ10. Furthermore, the propensity of ubiquinol to oxidise to CoQ10 unless frozen immediately at −80°C may detract from the clinical utility of this determination [Molyneux et al., 2008]. Therefore, the simultaneous assessment of both reduced and oxidised forms of CoQ10 is probably more suitable for research purposes rather than for clinical diagnosis. However, in appropriately handled tissue samples the ratio of ubiquinol: CoQ10 has been used as a marker of oxidative stress [Niklowitz et al., 2004; Kaya et al., 2012].
In the following paragraphs, details of the HPLC methods employed to determine tissue CoQ10 status in the laboratories of the authors will be outlined.
HPLC-UV Conditions
Total tissue CoQ10 status is quantified by reverse-phase HPLC with UV detection at 275 nm according to the method of Duncan et al. [2005]. CoQ10 is separated on a HPLC column (Techsphere ODS 5 μm, 150 × 4.6 mm). The mobile phase consists of ethanol:methanol:60% perchloric acid; 700:300:1.2 to which 7 g of sodium perchlorate are added [Boitier et al., 1998]. The flow rate is maintained at 0.7 ml/min. Ubiquinone species are detected at 275 nm. This HPLC method has been used to determine the CoQ10 status of skeletal muscle and blood mononuclear cells which were prepared and extracted according to the method of Duncan et al. [2005].
HPLC-ED Conditions
The total CoQ10 concentration is quantified by reverse-phase HPLC with electrochemical detection (Coulochem II, ESA, Mass., USA) according to a previously reported procedure [Montero et al., 2008]. Briefly, CoQ9 and CoQ10 are separated in a nucleosil C-18 column (5 μm, 25 × 0.4 cm, Teknokroma, Barcelona, Spain). The mobile phase consists of 20 mM of lithium perchlorate in ethanol/methanol (40/60). Electrochemical detector cells were set to −600 mV (conditioning cell, Model 5021) and +600 mV (analytical cell, Model 5010).
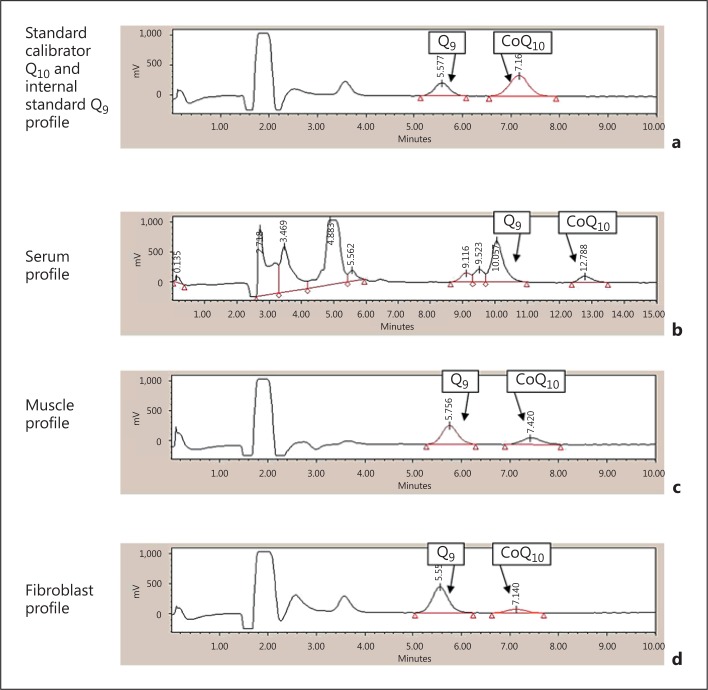
This HPLC method has been employed to assess the CoQ10 status of plasma, skeletal muscle, and fibroblasts (fig. 2) which were prepared and extracted according to the method of Montero et al. [2008].
Fig. 2.
Reverse-phase HPLC-ED chromatograms of CoQ9 and CoQ10 in a standard calibrator Q10 and internal standard Q9 profile, b serum profile, c muscle profile, and d fibroblast profile.
Internal Standards
A major difficulty encountered when assessing CoQ10 status in tissue is the lack of commercially available non-physiological internal standards (IS). In most cases coenzyme Q9 is the IS of choice [Okamoto et al., 1988]. Unfortunately, as a result of dietary contamination and synthesis by intestinal microorganisms, CoQ9 has been detected in human tissue and plasma samples contributing up to 2-7% of the total ubiquinone pool [Weber et al., 1997]. To avoid the possible influence of endogenous ubiquinones when evaluating tissue CoQ10 status, the non-physiological ubiquinones, di-ethoxy-CoQ10 [Edlund, 1988] and di-propoxy-CoQ10 [Duncan et al., 2005], have been employed as IS in these determinations.
Tissue Assessment
Plasma, Blood Mononuclear Cells, and Platelet Assessment
Clinical assessment of CoQ10 deficiency is generally based on plasma measurements, and the reference interval established for plasma CoQ10 status appears to range from 0.5 to 1.7 µM [Molyneux et al., 2008]. Plasma CoQ10 levels are also monitored following supplementation therapy to assess absorption and bioavailability of CoQ10 formulations. Plasma CoQ10 levels as high as 10.7 µM have been reached following supplementation with solubilised formulations of ubiquinol [Bhagavan and Chopra, 2007]. Higher than ‘normal’ levels of plasma CoQ10 appear requisite to facilitate tissue uptake and allow transfer across the blood brain barrier, although these levels may vary for different tissue [Bhagavan and Chopra, 2007]. In Parkinson's disease, plasma CoQ10 levels of 4.6 µM were reported by Shults et al. [2002] to be the most efficacious in slowing functional decline in patients. In contrast, a plasma level of 2.8 µM showed the highest therapeutic benefit in the treatment of congestive heart disease patients [Belardinelli et al., 2006]. Gender does not appear to influence plasma CoQ10 status [Molyneux et al., 2005]; however, the effect of age upon plasma CoQ10 levels is as yet uncertain, with studies reporting both a positive correlation with age and others finding no age effects [Miles et al., 2004; Duncan et al., 2005]. Plasma CoQ10 status is influenced by both dietary supply and hepatic biosynthesis [Hargreaves et al., 2005]. This is in contrast to other tissues which are dependent upon de novo biosynthesis [Kalen et al., 1987]. The effect of diet is of particular importance, since CoQ10 has a relatively long circulatory half-life (approx. 24 h), and dietary intake may contribute up to 25% of the total amount of plasma CoQ10 [Weber et al., 1997].
Plasma CoQ10 status is highly dependent upon the concentration of lipoproteins which are the major carriers of CoQ10 in the circulation, with approximately 58% of total plasma CoQ10 being associated with low-density lipoprotein or LDL fraction [Karlsson et al., 1992; McDonnell and Archbold, 1996]. Therefore, in view of its dependence upon both dietary intake and lipoprotein concentration, plasma CoQ10 status may not truly reflect tissue levels [Duncan et al., 2005]. It has been suggested that plasma CoQ10 levels should be expressed as a ratio to either total plasma cholesterol or LDL cholesterol in order to be of diagnostic value [Kontush et al., 1997; Tomasetti et al., 1999]. Furthermore, expressing plasma CoQ10 as a ratio to total cholesterol appears to exclude any influence of age on this parameter [Wolters and Hahn, 2003; Molyneux et al., 2005].
Assessment of blood mononuclear cells has been suggested as an alternative surrogate to evaluate endogenous CoQ10 status [Duncan et al., 2005]. Mononuclear cells are easily isolated from EDTA/Li-Heparin blood, and the CoQ10 status of these cells has been reported to correlate with that of skeletal muscle [Duncan et al., 2005]. Blood mononuclear cells are also reported to reflect changes in cellular status following supplementation [Turunen et al., 2004]. This is illustrated by the patient described in the study of Duncan et al. [2005] who was found to have a CoQ10 deficiency in blood mononuclear cells (20 pmol/mg of protein: reference interval 37-133 pmol/mg). However, following CoQ10 supplementation at 300 mg/day for 2 months, the mononuclear cell CoQ10 status of the patient increased to 42 pmol/mg, and this was accompanied by an improvement in mobility; the patient went from being a ‘bottom shuffler’ to being able to walk upright with the aid of assistance. Platelets have also been used as surrogates to evaluate endogenous CoQ10 levels in clinical studies [Shults et al., 1997; Mortensen et al., 1998]. Furthermore, the CoQ10 status of platelets was also found to increase following CoQ10 supplementation indicating these cell fragments may also be used to monitor the effect of CoQ10 supplementation on endogenous levels [Niklowitz et al., 2004].
Skeletal Muscle
Skeletal muscle is generally considered as the tissue of choice for CoQ10 assessment, and this tissue has been used in diagnosis of CoQ10 deficiency since the first cases of this deficiency were reported by Ogasahara et al. [1989]. However, in view of the importance of this tissue in the diagnosis of CoQ10 deficiency, there appears to be no universally accepted units in which to represent skeletal muscle CoQ10 status, and therefore it is difficult to compare reference ranges between laboratories (table 2). As is shown in table 2, skeletal muscle CoQ10 status can be represented in either units of µg/g fresh weight of tissue or as pmol/mg of protein (nmol/g of protein). Interestingly, although HPLC-UV and HPLC-ED detection methods were used to determine skeletal muscle CoQ10 status in the studies reported by Rahman et al. [2001] and Montero et al. [2008], respectively, the reference ranges reported in these studies are markedly similar. In the study by Montero et al. [2008], a patient was described in whom a decreased skeletal muscle CoQ10 status was suspected in view of a severe reduction in the activities of the ETC CoQ10-dependent enzymes, complexes II-III (succinate: cytochrome c reductase; EC. 1.3.5.1 + 1.10.2.2) and I-III (NADH: cytochrome c reductase; EC 1.6.5.3 + 1.10.2.2). However, the patient was found to have a normal level of skeletal muscle CoQ10 when related to protein (125 nmol/g; reference values: 110-480 nmol/g). When the muscle CoQ10 content was related to citrate synthase (CS) activity, the mitochondrial marker enzyme [Selak et al., 2000], evidence of a CoQ10 deficiency, was apparent (1.16 nmol/CS units; reference values 2.68-8.47 nmol/CS units). A possible explanation for this observation offered by the authors was the possibility that the high degree of muscle injury the patient was experiencing [rhabdomyolysis and elevated plasma creatine kinase levels (250,000 UI; reference values 50-250 UI)] may have resulted in a depletion of skeletal muscle protein. Therefore, when the CoQ10 status was related to total muscle protein content, this may result in a ‘false-negative’ result. Since approximately 50% of cellular CoQ10 is present in the mitochondria [Ernster and Dallner, 1995], expressing muscle CoQ10 status in relation to CS activity may have important diagnostic value (table 2). This is especially important in mitochondrial myopathies where excessive proliferation of mitochondria has been reported in muscle [DiMauro, 2004] and therefore expressing CoQ10 to CS activity which takes into account that the mitochondrial enrichment of the sample may highlight evidence of a deficiency which may not be identifiable if CoQ10 status is solely related to total protein.
Table 2.
Reference values for CoQ10 levels in muscle and fibroblasts expressed in the different units reported in the literature
| Muscle |
Fibroblast |
Authors | |||
|---|---|---|---|---|---|
| Method for analysis | CoQ10 (nmol/g protein) | CoQ10 (nmol/CS units)a | CoQ10/gram of tissue | CoQ10 | |
| HPLC-UV (275 nm) | 1,440–2,260 (1,811)b | 43–51 (48) ng/mg of protein | Ogasahara et al. [1989] | ||
| HPLC-UV | 140–580 (241) | 39–75 (62) nmol/g protein | Duncan et al. [2009] | ||
| HPLC-ED | 117–312 (214) | Miles et al. [2004] | |||
| HPLC-ED | 110–480 (231) | 2.7–8.5 (5.4) | 48–112 (67) nmol/g protein | Montero et al. [2008] | |
| HPLC-UV (275 nm) | 2.7–7.0 (4.7) | Horvath et al. [2006] | |||
| HPLC-UV (275 nm) | 140–580 (213) | Rahman et al. [2001] | |||
| HPLC-UV (275 nm) | 24.0-39.5 (31.5) nmol/g of wet tissue | Sacconi et al. [2010] | |||
| HPLC-ED | 20-79 (37.4) μmol/g tissue | Terracciano et al. [2012] | |||
| HPLC-ED | 21.7-88.7 (33.0) μmol/g | Pastore et al. [2012] | |||
| HPLC-ED | 18.5–45.7 (32.1) μg/g | 34–70.4 (52.2) ng/mg | Lopez et al. [2006] | ||
| HPLC-ED | 50.3–66.7 (58.5) ng/mg protein | Lagier-Tourenne et al. [2008] | |||
| HPLC-tandem mass spectrometry | 12.6–51.8 (32.2) pmol/mg | 56–184 (120) nmol/g protein | Mollet et al. [2008] | ||
| HPLC-tandem mass spectrometry | 2.0–2.8 (2.4) nmol/CS units | Buján et al. [2013] | |||
Data is presented as range (mean) values. Regarding the reference values in muscle and fibroblasts, most authors report consistent reference intervals, although noticeable differences are present in others. In view of this, the use of validated protocols together with an external quality control program seems necessary to minimize such differences.
CS = Citrate synthase.
These data are reported as ng/mg of muscle mitochondrial protein.
Decreased activity of either ETC complex I-III and/or complex II-III is also indicative of a CoQ10 deficiency as the activity of these linked enzymes is dependent upon endogenous CoQ10 [Rahman et al., 2001]. Furthermore, the study by Montero et al. [2008] has suggested that complex II-III activity may be a more sensitive marker of a diminution in CoQ10 status than that of complex I-III. However, normal levels of complex I-III or II-III activity do not exclude a decrease in muscle CoQ10 status as has previously been observed in patients with the ataxic phenotype of CoQ10 deficiency [Lamperti et al., 2003]. In view of the essential role ubiquinol plays in pyrimidine synthesis [Lopez et al., 2006], mitochondrial DNA depletion syndrome may also be associated with a decrease in CoQ10 status as has been reported by Montero et al. [2009]. Therefore, the assessment of muscle CoQ10 status in patients who present with multiple ETC deficiencies should not be discouraged. Furthermore, in view of the association between mitochondrial DNA mutations and muscle coenzyme Q10 deficiency, assessment of muscle CoQ10 status should be considered in addition to the determination of ETC enzyme activities in patients with suspected mitochondrial disease [Sacconi et al., 2010]. Decreased glycerol 3-phosphate dehydrogenase and/or dihydroorotate cytochrome c reductase activity may also indicate evidence of decreased muscle CoQ10 levels as these enzymes have been reported to be especially sensitive to perturbations in CoQ10 status [Rotig et al., 2000].
Fibroblasts
Assessment of fibroblast CoQ10 status should also be considered in the diagnosis of CoQ10 deficiency. Published reference ranges for fibroblast CoQ10 status are shown in table 2. In view of the suggested tissue specificity of CoQ10 deficiency, a normal level of CoQ10 in fibroblasts does not exclude a deficit in CoQ10 status in other tissues [Ogasahara et al., 1989]. Indeed, normal levels of fibroblast CoQ10 have been reported in patients with genetically confirmed defects in CoQ10 biosynthesis [Lagier-Tourenne et al., 2008]. In contrast, however, fibroblast assessment has been used to reveal evidence of a CoQ10 deficiency in a patient with a normal muscle CoQ10 status [Montero et al., 2008]. Fibroblasts also provide a means of assaying CoQ10 biosynthesis by studying the incorporation of 14C-p-hydroxybenzoate, 3H-mevalonate, and/or 3H-decaprenyl-pyrophosphate into CoQ10 [Lopez et al., 2006; Quinzii et al., 2006]. These radiolabelled incorporation studies can be used to confirm a deficiency in CoQ10 biosynthesis, identify the position of the defect in the biosynthetic pathway in some cases, as well as to discriminate between primary and secondary CoQ10 deficiencies.
Cerebral Spinal Fluid
In view of the preponderance of neurological dysfunction associated with CoQ10 deficiency [Mancuso et al., 2010], the ability to assess cerebral CoQ10 status would be of considerable diagnostic value. Cerebral spinal fluid (CSF) is considered the appropriate surrogate to assess cerebral CoQ10 status. However, in view of the low levels of CoQ10 detected in CSF with HPLC-UV, detection would be insufficiently sensitive for this analysis [Duncan et al., 2005]. Tentative reference ranges for CSF CoQ10 levels of 1.18-4.91 nM established from a patient cohort aged 9-18 years, n = 15 [Artuch et al., 2004] and 5.7-9 nM established from a patient cohort aged 0.1-22 years, n = 17 [Duberley et al., 2012], respectively, have been reported. The discrepancies in these ranges may in part result from the different analytical techniques and sample preparations employed for this determination. In the study by Artuch et al. [2004], CSF samples were filtered by passing through a 10,000-NMWL column prior to analysis by HPLC-EC detection. In contrast, tandem spectrometry was employed to determine the CoQ10 status in unfiltered CSF in the study by Duberley et al. [2012]. A further factor, which may also have contributed to this disparity, is the different ages of the ‘disease control’ patients used to establish these reference ranges. Although Isobe et al. [2010] reported no correlation between age and CSF CoQ10 status, this study was undertaken solely in adults aged 65.8 ± 12.4 years (mean ± SD), and CSF was not investigated from children. Therefore, in order to establish a more reliable and robust reference interval for CSF CoQ10 status, further studies are required that evaluate the effects of age, gender, as well as the rostral-caudal gradient upon CSF levels of this ubiquinone.
Discussion/Conclusion
The actual prevalence of human CoQ10 deficiency is at present unknown, but it is suspected that this condition is under-diagnosed [Rahman et al., 2012]. This is compounded by the lack of specialist centres which are able to determine tissue CoQ10 status together with the extreme clinical heterogeneity of this condition [Rahman et al., 2012]. It is recommended that the CoQ10 status is determined in the muscle biopsies of all patients with suspected mitochondrial disease. Once evidence of a CoQ10 deficiency is detected, further studies will be required to elucidate the underlying cause of this defect. Genetic investigations and radiolabelled biosynthetic studies in fibroblasts may help to distinguish between primary or secondary causes of the deficiency. However, in a number of patients with CoQ10 deficiency it has not been possible to elucidate the underlying cause of the defect [Rahman et al., 2012].
Since muscle biopsies may not always be available, there is a need for a less invasive means to assess tissue CoQ10 status. Although there are some concerns over the diagnostic value of plasma CoQ10 levels, platelet and blood mononuclear cell determinations may offer an alternative means for this assessment.
It has been suggested that there may be tissue specific isoenzymes in the CoQ10 biosynthetic pathway; therefore the CoQ10 status of one tissue may not reflect that of another [Ogasahara et al., 1989]. Since neurological dysfunction is a constant clinical feature in CoQ10 deficiency syndromes, although some defects may be expressed in muscle or peripheral tissue, other defects (such as those in cerebral CoQ10 biosynthesis) may not be expressed and may remain undiagnosed. The ability to accurately assess CSF CoQ10 status may therefore enhance the diagnosis yield of patients with neurological dysfunction and previously undiagnosed cerebral CoQ10 deficiency. In view of the differences in the tissue of choice for CoQ10 assessment, units in which CoQ10 is expressed and the reference intervals used for this diagnosis between laboratories a more unified approach is required for monitoring patients and their treatment. The establishment of an external quality control (Ex-QC) scheme for the measurement of tissue CoQ10 status is suggested for laboratories offering this clinical diagnostic service. At present, a trial Ex-QC scheme is running between laboratories in the UK and Spain and, if successful, will be offered on a more global scale.
Acknowledgements
This work was supported by the Center for Biomedical Network Research on Rare Diseases (CIBERER-ISCIII) and by the project FIS: PI11/02350. Part of this work was undertaken at the University College of London Hospitals who received a proportion of their funding from the Department of Health's NIHR Biomedical Research Centres funding scheme.
References
- Artuch R, Aracil A, Mas A, Monros E, Vilaseca MA, et al. Cerebrospinal fluid concentrations of idebenone in Friedreich ataxia patients. Neuropediatrics. 2004;35:95–98. doi: 10.1055/s-2004-815830. [DOI] [PubMed] [Google Scholar]
- Barshop BA, Gangoiti JA. Analysis of coenzyme Q in human blood and tissues. Mitochondrion. 2007;7S:s89–s93. doi: 10.1016/j.mito.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Belardinelli R, Mucaj A, Lacalaprice F, Solenghi M, Seddaiu G, et al. Coenzyme Q10 and exercise training in chronic heart failure. Eur Heart J. 2006;27:2675–2681. doi: 10.1093/eurheartj/ehl158. [DOI] [PubMed] [Google Scholar]
- Bhagavan HN, Chopra RK. Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion. 2007;7S:s78–s88. doi: 10.1016/j.mito.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Boitier E, Degoul F, Desguerre I, Charpantier C, Francois D, et al. A case of mitochondrial encephalomyopathy associated with a muscle coenzyme Q10 deficiency. J Neurol Sci. 1998;156:41–46. doi: 10.1016/s0022-510x(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Buján N, Arias A, Montero R, García-Villoria J, Lissens W, et al. High performance liquid chromatography-tandem mass spectrometry assay for the analysis of CoQ10 biosynthesis in cultured fibroblasts. J Inherit Metab Dis. 2013. in press.
- Cotan D, Cordero MD, Garrido-Maraver J, Oropesa-Avila M, Rodriguez-Hernandez A, et al. Secondary coenzyme Q10 deficiency triggers mitochondria degradation by mitophagy in MELAS fibroblasts. FASEB J. 2011;25:2669–2687. doi: 10.1096/fj.10-165340. [DOI] [PubMed] [Google Scholar]
- DiMauro S. Mitochondrial diseases. Biochimica et Biophysica Acta. 2004;1654:80–88. doi: 10.1016/j.bbabio.2004.03.014. [DOI] [PubMed] [Google Scholar]
- Diomedi-Camassei F, Di Giandomenico S, Santorelli FM, Cardi G, Piemonte F, et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- Duberley KE, Hargreaves IP, Chaiwatanasirikul K, Heales SJ, Rahman S, et al. Development of a mass spectrometry method for quantification of coenzyme Q10 in CSF. J Inherit Metab Dis. 2012;35(Suppl 1):S124. [Google Scholar]
- Duncan AJ, Heales SJ, Mills K, Eaton S, Land JM, et al. Determination of Coenzyme Q10 in blood mononuclear cells, skeletal muscle and plasma by HPLC using di-propoxy-Coenzyme Q10 as an internal standard. Clin Chem. 2005;51:2380–2382. doi: 10.1373/clinchem.2005.054643. [DOI] [PubMed] [Google Scholar]
- Duncan AJ, Bitner-Glindzicz M, Meunier B, Costello H, Hargreaves IP, et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echtay KS, Winkler E, Frischmuth K, Klingenberg M. Uncoupling proteins 2 and 3 are highly active H+ transporters and highly nucleotide sensitive when activated by coenzyme Q (ubiquinone) Proc Natl Acad Sci USA. 2001;98:1416–1421. doi: 10.1073/pnas.98.4.1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund PO. Determination of coenzyme Q10 a-tocopherol and cholesterol in biological samples by coupled-column liquid chromatography with coulometric and ultraviolet detection. J Chromatogr. 1988;425:87–97. doi: 10.1016/0378-4347(88)80009-4. [DOI] [PubMed] [Google Scholar]
- Emmanuele V, Lopez LC, Berardo A, Naini A, Tadesse S, et al. Heterogeneity of coenzyme Q10 deficiency: patient study and literature review. Arch Neurol. 2012;69:978–83. doi: 10.1001/archneurol.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernster L, Dallner G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim Biophys Acta. 1995;1271:195–204. doi: 10.1016/0925-4439(95)00028-3. [DOI] [PubMed] [Google Scholar]
- Ernster L, Forsmark-Andrée P. Ubiquinol: an endogenous antioxidant in aerobic organisms. Clin Investig. 71(8 Suppl):S60–S65. doi: 10.1007/BF00226842. [DOI] [PubMed] [Google Scholar]
- Hargreaves IP, Duncan AJ, Heales SJR, Land JM. The effect of HMG-CoA reductase inhibitors on Coenzyme Q10 availability: biochemical/clinical implications. Drug Saf. 2005;28:659–676. doi: 10.2165/00002018-200528080-00002. [DOI] [PubMed] [Google Scholar]
- Heeringa SF, Chernin G, Chaki M, Zhou W, Sloan AJ, et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121:2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath R, Schneiderat P, Schoser BG, Gempel K, Neuen-Jacob E, et al. Coenzyme Q10 deficiency and isolated myopathy. Neurology. 2006;66:253–255. doi: 10.1212/01.wnl.0000194241.35115.7c. [DOI] [PubMed] [Google Scholar]
- Isobe C, Abe T, Terayama Y. Levels of reduced and oxidized coenzyme Q10 and 8-hydroxy-2-deoxyguanosine in the cerebrospinal fluid of patients with living Parkinson's disease demonstrate that mitochondrial oxidative stress damage and/or oxidative DNA damage contributes to the neurodegenerative process. Neuroscience Letts. 2010;469:159–163. doi: 10.1016/j.neulet.2009.11.065. [DOI] [PubMed] [Google Scholar]
- Kalen A, Norling B, Appelkvist EL, Dallner G. Ubiquinone biosynthesis by the microsomal fraction of rat liver. Biochim Biophys Acta. 1987;926:70–78. doi: 10.1016/0304-4165(87)90183-8. [DOI] [PubMed] [Google Scholar]
- Karlsson J, Diament B, Edlund PO, Lund B, Folkers K, et al. Plasma ubiquinone, α-tocopherol and cholesterol in man. Int J Vitam Nutr Res. 1992;62:160–164. [PubMed] [Google Scholar]
- Kaya Y, Cebi A, Soylemez N, Demir H, Alp HH, et al. Correlations between oxidative DNA damage, oxidative stress and coenzyme Q10 in patients with coronary artery disease. Int J Med Sci. 2012;9:621–626. doi: 10.7150/ijms.4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontush A, Reich A, Baum K, Spranger T, Finckh B, et al. Plasma ubiquinol-10 is decreased in patients with hyperlipidaemia. Atherosclerosis. 1997;129:119–126. doi: 10.1016/s0021-9150(96)06021-2. [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C, Tazir M, Lopez LC, Quinzii CM, Assoum M, et al. ADCK3 an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamperti C, Naini A, Hirano M, De Vivo DC, Bertini E, et al. Cerebellar ataxia and coenzyme Q10 deficiency. Neurology. 2003;60:1206–1208. doi: 10.1212/01.wnl.0000055089.39373.fc. [DOI] [PubMed] [Google Scholar]
- Lopez LC, Schuelle M, Quinzii CM, Kanki T, Rodenburg RJ, et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2)mutations. Am J Hum Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Martin JM, Salviati L, Trevisson E, Montini G, Dimauro S, et al. Missense mutation of COQ2 gene causes defects of bioenergetics and de novo pyrimidine synthesis. Hum Mol Genet. 2007;16:1091–1097. doi: 10.1093/hmg/ddm058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M, Orsucci D, Volpi L, Calsolaro V, Siciliano G. Coenzyme Q10 in neuromuscular and neurodegenerative disorders. Curr Drug Targets. 2010;11:111–121. doi: 10.2174/138945010790031018. [DOI] [PubMed] [Google Scholar]
- McDonnell MG, Archbold GP. Plasma ubiquinol/cholesterol ratios in patients with hyperlipidemia, those with diabetes mellitus and in patients requiring dialysis. Clin Chim Acta. 1996;253:117–126. doi: 10.1016/0009-8981(96)06357-7. [DOI] [PubMed] [Google Scholar]
- Miles MV, Horn PS, Morrison JA, Tang PH, DeGrauw T, et al. Plasma coenzyme Q10 reference intervals but not redox status, are affected by gender and race in self-reported healthy adults. Clin Chim Acta. 2004;347:139–144. doi: 10.1016/s0009-8981(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Mollet J, Giurgeal I, Schlemmer D, Dallner G, Chretien D, et al. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest. 2007;17:765–772. doi: 10.1172/JCI29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollet J, Delahodde A, Serre V, Chretien D, Schlemmer D, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molyneux SL, Florkowski CM, Lever M, George PM. Biological variation of coenzyme Q10. Clin Chem. 2005;51:455–457. doi: 10.1373/clinchem.2004.043653. [DOI] [PubMed] [Google Scholar]
- Molyneux SL, Young JM, Florkowski CM, Lever M, George PM. Coenzyme Q10: is there a clinical role and a case for measurement? Clin Biochem Rev. 2008;29:71–81. [PMC free article] [PubMed] [Google Scholar]
- Montero R, Sánchez-Alcázar JA, Briones P, Hernández AR, Cordero MD, et al. Analysis of coenzyme Q10 in muscle and fibroblasts for the diagnosis of CoQ10 deficiency syndromes. Clin Biochem. 2008;41:697–700. doi: 10.1016/j.clinbiochem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Montero R, Sanchez-Alcazar JA, Briones P, Navarro-Sastre A, Gallard E. Coenzyme Q10 deficiency associated with a mitochondrial DNA depletion syndrome: a case report. Clin Biochem. 2009;42:742–745. doi: 10.1016/j.clinbiochem.2008.10.027. [DOI] [PubMed] [Google Scholar]
- Mortensen SA, Heidt P, Sehested J. Clinical perspectives in treatment of cardiovascular diseases with coenzyme Q10 in Lenza G, Barnabei, Rabbi A, Battion M (eds): Highlights in Ubiquinone Research, pp 226-227 (Taylor and Francis, London 1998)
- Niklowitz P, Menke T, Andler W, Okun JG. Simultaneous analysis of coenzyme Q10 in plasma, erythrocytes and platelets: comparison of the antioxidant level in blood cells and their environment in healthy children and after oral supplementation in adults. Clin Chim Acta. 2004;342:219–226. doi: 10.1016/j.cccn.2003.12.020. [DOI] [PubMed] [Google Scholar]
- Ogasahara S, Engel A, Frens D, Mack D. Muscle coenzyme Q deficiency in familial mitochondrial encephalomyositis. Proc Natl Acad Sci USA. 1989;86:2379–2382. doi: 10.1073/pnas.86.7.2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Fukunaga Y, Ida Y, Kishi T. Determination of reduced and total ubiquinones in biological materials by liquid chromatography with electrochemical detection. J Chromatogr. 1988;430:11–19. doi: 10.1016/s0378-4347(00)83129-1. [DOI] [PubMed] [Google Scholar]
- Pastore A, Di Giovamberadino G, Petrillo S, Boenzi S, Bertini S, et al. Pediatric reference intervals for muscle coenzyme Q10. Biomarkers. 2012;17:764–766. doi: 10.3109/1354750X.2012.727029. [DOI] [PubMed] [Google Scholar]
- Qunzii C, Naini A, Salviati L, Trevisson E, Navas P, et al. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes a primary coenzyme Q10 deficiency. Am J Hum Genet. 2006;78:345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Hargreaves I, Clayton P, Heales S. Neonatal presentation of coenzyme Q10 deficiency. J Pediatr. 2001;139:456–458. doi: 10.1067/mpd.2001.117575. [DOI] [PubMed] [Google Scholar]
- Rahman S, Clarke CF, Hirano M. 176th ENMC International Workshop: diagnosis and treatment of coenzyme Q10 deficiency. Neuromuscular Disord. 2012;22:76–86. doi: 10.1016/j.nmd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotig A, Appelkvist EL, Geromel V, Chretien D, Kadhom N, et al. Quinone-responsive multiple respiratory chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356:391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- Sacconi S, Trevisson E, Salviat L, Ayme S, Rigal O, et al. Coenzyme Q10 is frequently reduced in muscle of patients with mitochondrial disease. Neuromuscular Disord. 2010;20:44–48. doi: 10.1016/j.nmd.2009.10.014. [DOI] [PubMed] [Google Scholar]
- Salviati L, Sacconi S, Murer L, Zacchello G, Franceschini L, et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: a CoQ10-responsive condition. Neurology. 2005;65:606–608. doi: 10.1212/01.wnl.0000172859.55579.a7. [DOI] [PubMed] [Google Scholar]
- Salviati L, Trevisson E, Rodriquez-Hernandez MA, Casarin A, Pertegata V, et al. Haploinsufficiency of COQ4 causes coenzyme Q10 deficiency. J Med Genet. 2012;49:187–196. doi: 10.1136/jmedgenet-2011-100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak MA, de Chadarevian JP, Melvin JJ. Mitochondrial activity in Pompe's disease. Pediatr Neurol. 2000;23:54–57. doi: 10.1016/s0887-8994(00)00145-4. [DOI] [PubMed] [Google Scholar]
- Shults CW, Haas RH, Passor D, Beal MF. Coenzyme Q10 is reduced in mitochondria from Parkinsonian patients. Ann Neurol. 1997;42:261–265. doi: 10.1002/ana.410420221. [DOI] [PubMed] [Google Scholar]
- Shults CW, Oakes D, Kieburtz K, Beal FL, Haas R, et al. The effects of coenzyme Q10 in early Parkinson Disease. Arch Neurol. 2002;59:1541–1550. doi: 10.1001/archneur.59.10.1541. [DOI] [PubMed] [Google Scholar]
- Terracciano A, Renaldo F, Zanni G, D' Amico A, Pastore A, et al. The use of muscle biopsy in the diagnosis of undefined ataxia with cerebellar atrophy in children. Eur J Paediatric Neurol. 2012;16:248–256. doi: 10.1016/j.ejpn.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasetti M, Littarru GP, Stocker R, Alleva R. Coenzyme Q10 enrichment decreases oxidative DNA damage in human lymphocytes. Free Radic Biol Med. 1999;27:1027–1032. doi: 10.1016/s0891-5849(99)00132-x. [DOI] [PubMed] [Google Scholar]
- Turunen M, Olsson J, Dallner G. Metabolism and function of coenzyme Q. Biochimi Biophys Acta. 2004;1660:171–199. doi: 10.1016/j.bbamem.2003.11.012. [DOI] [PubMed] [Google Scholar]
- Weber C, Bysted A, Holmer G. The coenzyme Q10 content of the average Danish diet. Internat J Vit Nutr Res. 1997;67:123–129. [PubMed] [Google Scholar]
- Wolters M, Hahn A. Plasma ubiquinone status and response to six month supplementation combined with multivitamins in healthy elderly women – results of a randomized, double blind, placebo controlled study. Int J Vitam Res. 2003;73:207–214. doi: 10.1024/0300-9831.73.3.207. [DOI] [PubMed] [Google Scholar]