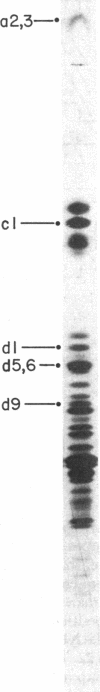
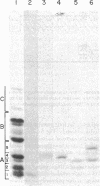
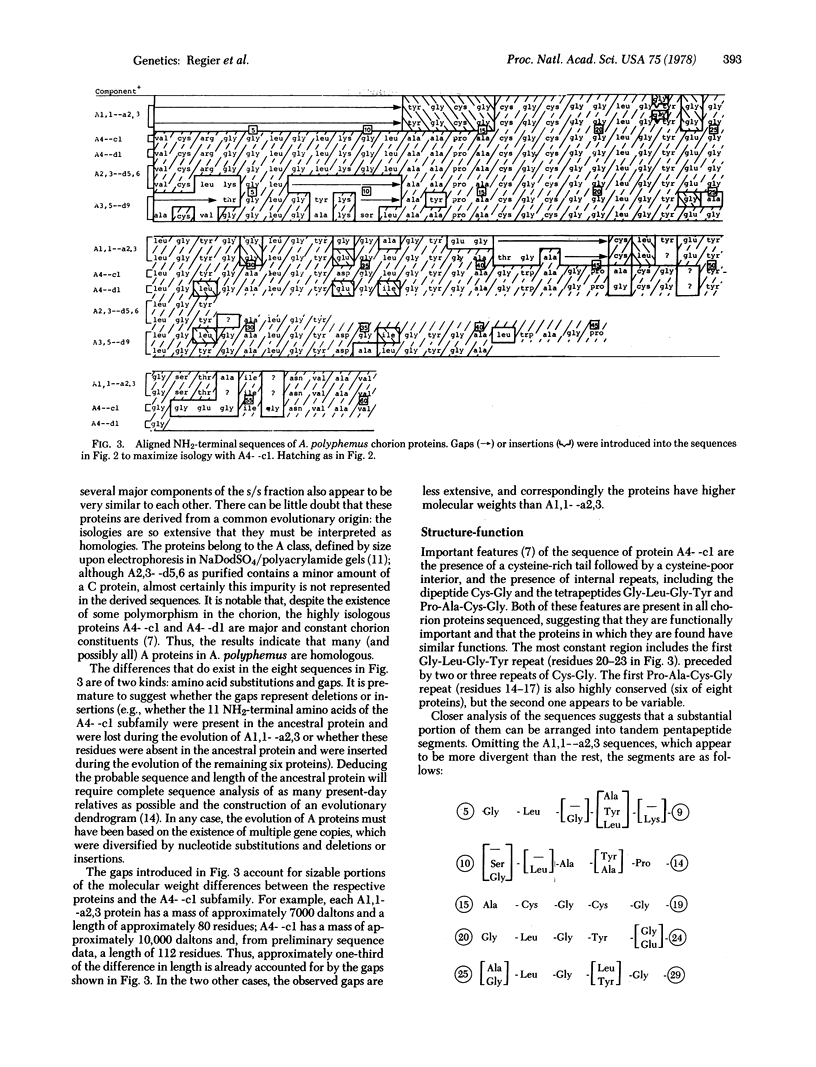
Abstract
Five polypeptide components have been isolated from the eggshell (chorions) of a silkmoth. Two are homogeneous on sodium dodecyl sulfate and isoelectric focusing gels, and three contain predominantly two proteins each. Amino acid analyses show that all five components are similar to each other. These proteins have been sequenced from the amino terminus. Homogeneous components yielded single sequences; heterogeneous components yielded two residues at some positions, consistent with their containing two major electrophoretic components. Striking similarities are apparent among all these sequences. These similarities can be increased dramatically by separating each of the three protein mixtures into two sequences and introducing a small number of gaps or insertions. This is due in part to bringing into register a portion that contains short repeating subunits found in all sequences. All proteins are also characterized by a region of high cysteine content near the amino terminus followed by a longer low-cysteine region. The data suggest that these proteins share a common evolutionary origin and are encoded by a multigene family.
Full text
PDF
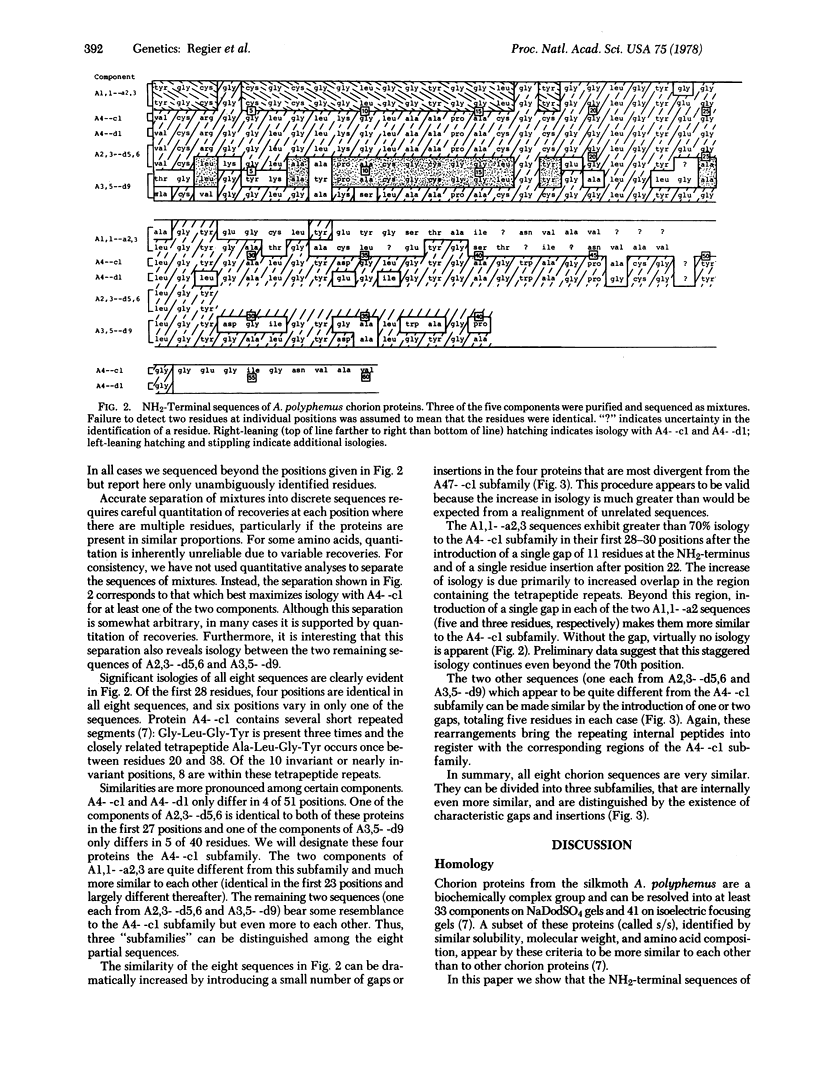

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Brown D. D., Sugimoto K. The structure and evolution of ribosomal and 5S DNAs in Xenopus laevis and Xenopus mulleri. Cold Spring Harb Symp Quant Biol. 1974;38:501–505. doi: 10.1101/sqb.1974.038.01.054. [DOI] [PubMed] [Google Scholar]
- Clarkson S. G., Birnstiel M. L. Clustered arrangement of tRNA genes of Xenopus laevis. Cold Spring Harb Symp Quant Biol. 1974;38:451–459. doi: 10.1101/sqb.1974.038.01.049. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A., Kafatos F. C., Maniatis T. The primary structure of rabbit beta-globin mRNA as determined from cloned DNA. Cell. 1977 Apr;10(4):571–585. doi: 10.1016/0092-8674(77)90090-3. [DOI] [PubMed] [Google Scholar]
- Eggert F. M., Allen G. A., Burgess R. C. Amelogenins. Purification and partial characterization of proteins from developing bovine dental enamel. Biochem J. 1973 Mar;131(3):471–484. doi: 10.1042/bj1310471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Titani K., Neurath H., Walsh K. A. Application of sequenator analyses to the study of proteins. Biochemistry. 1972 Nov 21;11(24):4493–4502. doi: 10.1021/bi00774a011. [DOI] [PubMed] [Google Scholar]
- Hood L., Campbell J. H., Elgin S. C. The organization, expression, and evolution of antibody genes and other multigene families. Annu Rev Genet. 1975;9:305–353. doi: 10.1146/annurev.ge.09.120175.001513. [DOI] [PubMed] [Google Scholar]
- Kabat D. Gene selection in hemoglobin and in antibody-synthesizing cells. Science. 1972 Jan 14;175(4018):134–140. doi: 10.1126/science.175.4018.134. [DOI] [PubMed] [Google Scholar]
- Paul M., Goldsmith M. R., Hunsley J. R., Kafatos F. C. Specific protein synthesis in cellular differentiation. Production of eggshell proteins by silkmoth follicular cells. J Cell Biol. 1972 Dec;55(3):653–680. doi: 10.1083/jcb.55.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul M., Kafaots F. C. Specific protein synthesis in cellular differentiation. II. The program of protein synthetic changes during chorion formation by slikmoth follicles, and its implementation in organ culture. Dev Biol. 1975 Jan;42(1):141–159. doi: 10.1016/0012-1606(75)90320-6. [DOI] [PubMed] [Google Scholar]
- Swart L. S. Homology in the amino-acid sequences of the high-sulphur proteins from wool. Nat New Biol. 1973 May 2;243(122):27–29. [PubMed] [Google Scholar]
- Terhorst C., Parham P., Mann D. L., Strominger J. L. Structure of HLA antigens: amino-acid and carbohydrate compositions and NH2-terminal sequences of four antigen preparations. Proc Natl Acad Sci U S A. 1976 Mar;73(3):910–914. doi: 10.1073/pnas.73.3.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg E. S., Birnstiel M. L., Purdom I. F., Williamson R. Genes coding for polysomal 9S RNA of sea urchins: conservation and divergence. Nature. 1972 Nov 24;240(5378):225–228. doi: 10.1038/240225a0. [DOI] [PubMed] [Google Scholar]