Abstract
Wild populations of Drosophila mojavensis exhibit considerable conformational variation in the NAD+-free form of alcohol dehydrogenase (alcohol:NAD+ oxidoreductase; EC 1.1.1.1). The variation appears genetic, as it does not occur within an inbred strain. The NAD+-bound form of alcohol dehydrogenase, present in the same individuals, does not exhibit the variation, suggesting that the binding of NAD+ acts to stabilize conformation. Such cofactor binding to enzymes may thus conceal considerable variation. A similar effect is suggested for binding of esterase subunits.
Full text
PDF
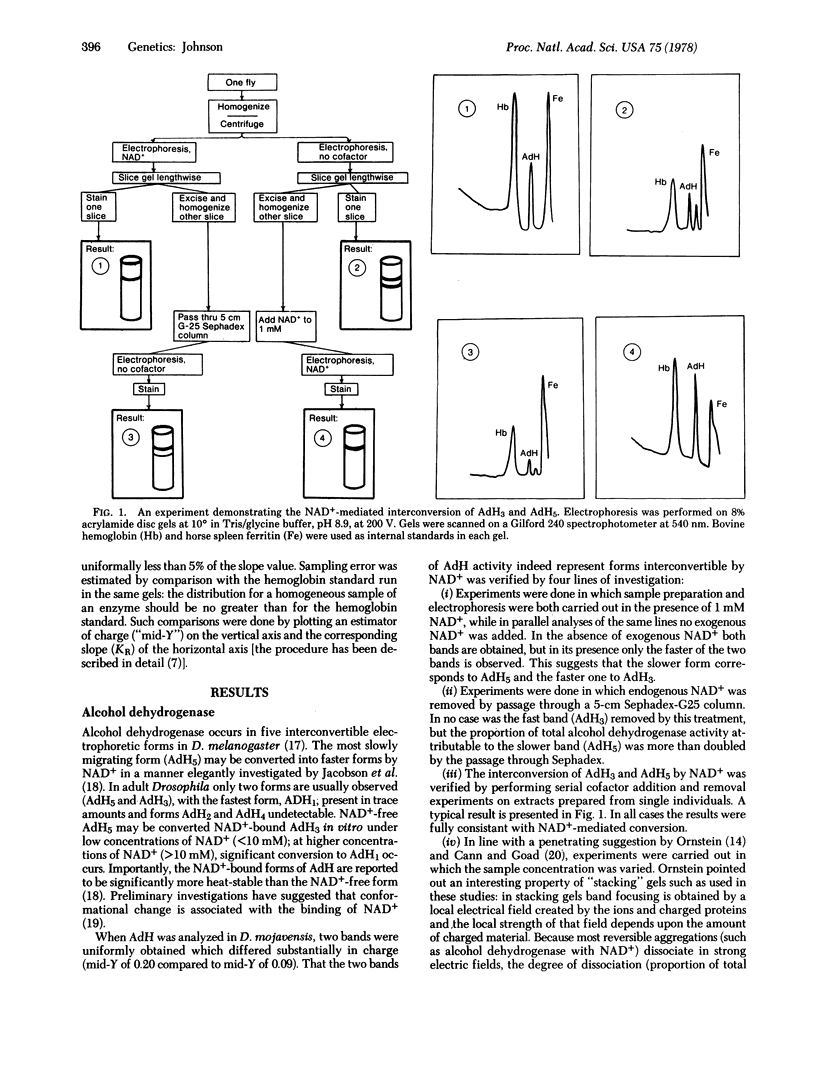
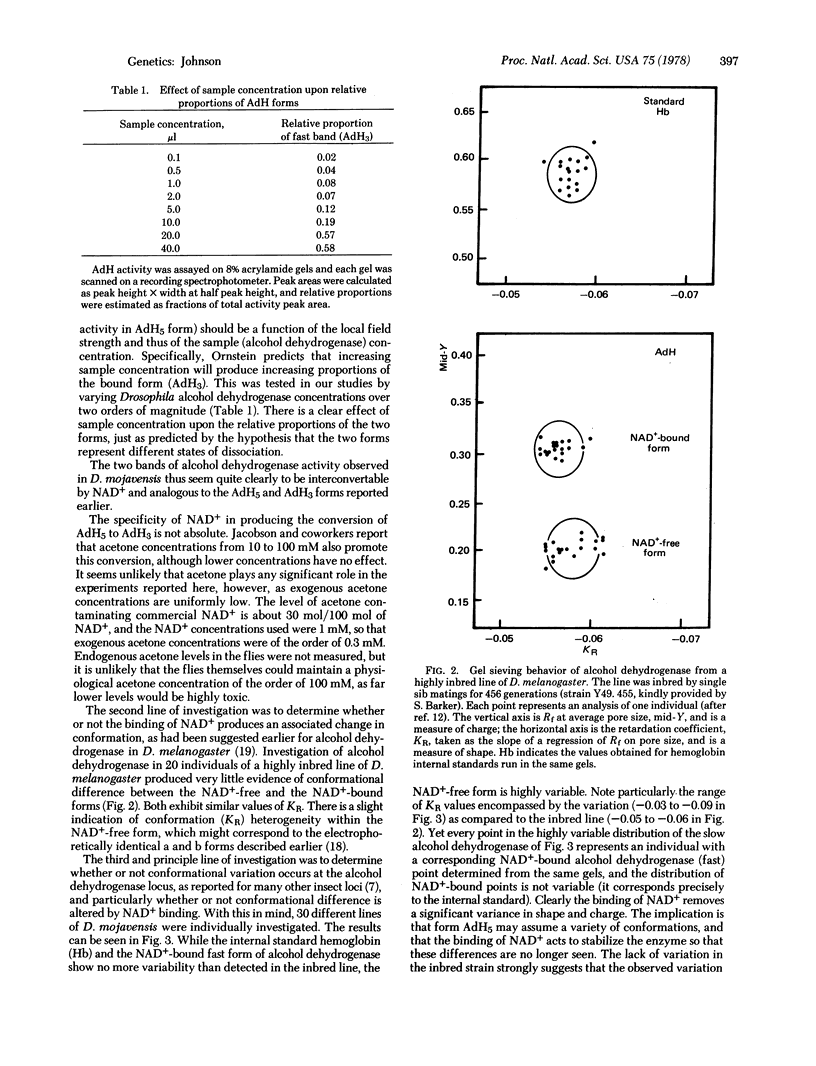
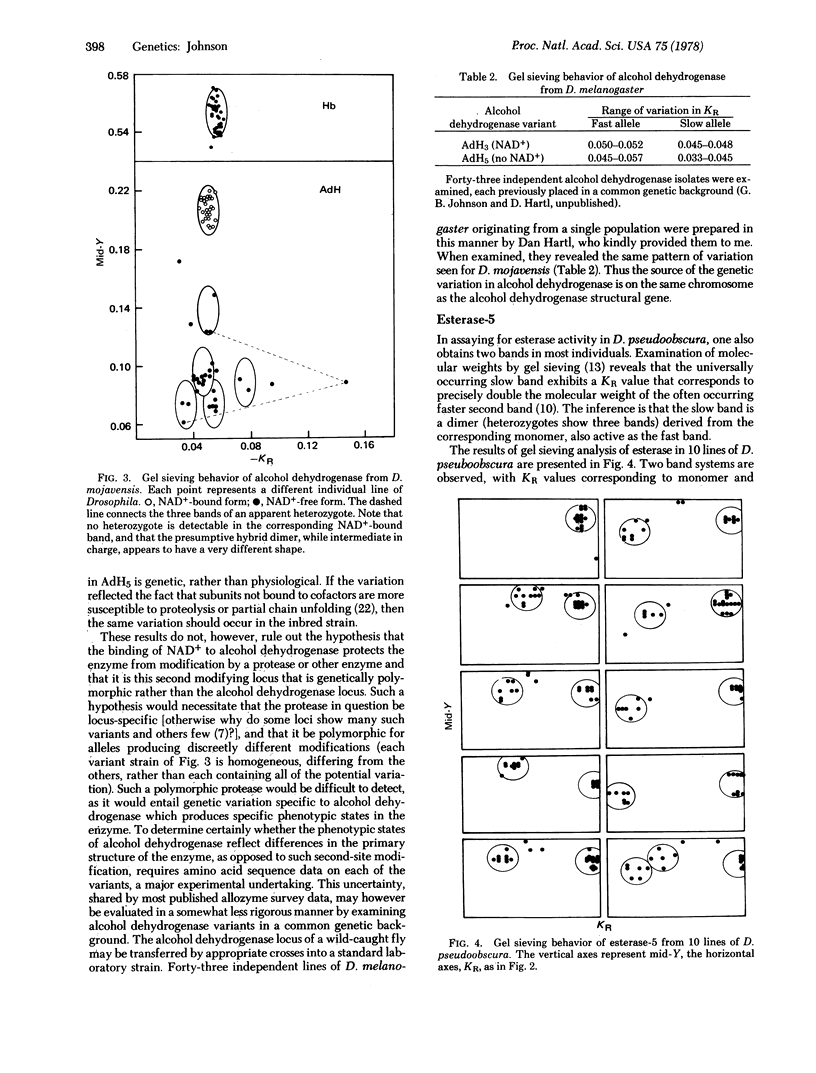

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein S. C., Throckmorton L. H., Hubby J. L. Still more genetic variability in natural populations. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3928–3931. doi: 10.1073/pnas.70.12.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cann J. R., Goad W. B. Two or more electrophoretic zones from a single macromolecule. Ann N Y Acad Sci. 1968 Jun 14;151(1):638–649. doi: 10.1111/j.1749-6632.1968.tb11924.x. [DOI] [PubMed] [Google Scholar]
- Harris H., Hopkinson D. A. Average heterozygosity per locus in man: an estimate based on the incidence of enzyme polymorphisms. Ann Hum Genet. 1972 Jul;36(1):9–20. doi: 10.1111/j.1469-1809.1972.tb00578.x. [DOI] [PubMed] [Google Scholar]
- Hubby J. L., Lewontin R. C. A molecular approach to the study of genic heterozygosity in natural populations. I. The number of alleles at different loci in Drosophila pseudoobscura. Genetics. 1966 Aug;54(2):577–594. doi: 10.1093/genetics/54.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson K. B. Alcohol dehydrogenase of Drosophila: interconversion of isoenzymes. Science. 1968 Jan 19;159(3812):324–325. doi: 10.1126/science.159.3812.324. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Murphy J. B., Knopp J. A., Ortiz J. R. Multiple forms of drosophila alcohol dehydrogenase. 3. Conversion of one form to another by nicotinamide adenine dinucleotide or acetone. Arch Biochem Biophys. 1972 Mar;149(1):22–35. doi: 10.1016/0003-9861(72)90295-0. [DOI] [PubMed] [Google Scholar]
- Johnson G. B. Characterization of electrophoretically cryptic variation in the alpine butterfly Colias meadii. Biochem Genet. 1977 Aug;15(7-8):665–693. doi: 10.1007/BF00484097. [DOI] [PubMed] [Google Scholar]
- Johnson G. B. Evaluation of the Stepwise Mutation Model of Electrophoretic Mobility: Comparison of the Gel Sieving Behavior of Alleles at the Esterase-5 Locus of DROSOPHILA PSEUDOOBSCURA. Genetics. 1977 Sep;87(1):139–157. doi: 10.1093/genetics/87.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. B. Hidden alleles at the alpha-glycerophosphate dehydrogenase locus in Colias butterflies. Genetics. 1976 May;83(1):149–167. doi: 10.1093/genetics/83.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson G. B. Use of internal standards in electrophoretic surveys of enzyme polymorphism. Biochem Genet. 1975 Dec;13(11-12):833–847. doi: 10.1007/BF00484414. [DOI] [PubMed] [Google Scholar]
- Knopp J. A., Jacobson K. B. Multiple forms of drosophila alcohol dehydrogenase. IV. Protein fluorescence studies. Arch Biochem Biophys. 1972 Mar;149(1):36–41. doi: 10.1016/0003-9861(72)90296-2. [DOI] [PubMed] [Google Scholar]
- Lewontin R. C., Hubby J. L. A molecular approach to the study of genic heterozygosity in natural populations. II. Amount of variation and degree of heterozygosity in natural populations of Drosophila pseudoobscura. Genetics. 1966 Aug;54(2):595–609. doi: 10.1093/genetics/54.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Singh R. S., Lewontin R. C., Felton A. A. Genetic heterogeneity within electrophoretic "alleles" of xanthine dehydrogenase in Drosophila pseudoobscura. Genetics. 1976 Nov;84(3):609–629. doi: 10.1093/genetics/84.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher D. R. Enzyme instability and proteolysis during the purification of an alcohol dehydrogenase from Drosophila melanogaster. Biochem J. 1977 May 1;163(2):317–323. doi: 10.1042/bj1630317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt W. B. Adaptation at specific loci. I. Natural selection on phosphoglucose isomerase of Colias butterflies: Biochemical and population aspects. Genetics. 1977 Sep;87(1):177–194. doi: 10.1093/genetics/87.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]



