Abstract
Laing early onset distal myopathy and myosin storage myopathy are caused by mutations of slow skeletal/β-cardiac myosin heavy chain encoded by the gene MYH7, as is a common form of familial hypertrophic/dilated cardiomyopathy. The mechanisms by which different phenotypes are produced by mutations in MYH7, even in the same region of the gene, are not known. To explore the clinical spectrum and pathobiology we screened the MYH7 gene in 88 patients from 21 previously unpublished families presenting with distal or generalised skeletal muscle weakness, with or without cardiac involvement. Twelve novel mutations have been identified in thirteen families. In one of these families the grandfather of the proband was found to be a mosaic for the MYH7 mutation. In eight cases de novo mutation appeared to have occurred, which was proven in three. The presenting complaint was footdrop, sometimes leading to delayed walking or tripping, in members of 17 families (81%), with other presentations including cardiomyopathy in infancy, generalised floppiness and scoliosis. Cardiac involvement as well as skeletal muscle weakness was identified in 9 of 21 families. Spinal involvement such as scoliosis or rigidity was identified in 12 (57%). This report widens the clinical and pathological phenotypes, and the genetics of MYH7 mutations leading to skeletal muscle diseases.
Keywords: MYH7, Laing distal myopathy, MPD1
Introduction
Slow skeletal muscle/β-cardiac myosin heavy chain, encoded by the MYH7 gene is expressed in cardiac muscle and all slow skeletal muscle fibres (Jandreski et al, 1987). Mutations in MYH7 are common causes of hypertrophic cardiomyopathy (CMH1; MIM# 192600), with 207 distinct mutations found to cause hypertrophic cardiomyopathy alone (Walsh, 2010). As well as causing cardiomyopathy, mutations in MYH7 also cause skeletal muscle diseases with or without cardiac involvement. These skeletal muscle diseases are myosin storage myopathy (MSM; MIM# 608358) and Laing early onset distal myopathy (MPD1; MIM# 160500). Overall, 327 MYH7 mutations have been reported in myoMAPR database to cause human disease (HTTP://bmf2.colorado.edu/myomapr/).
Myosin Storage Myopathy (MSM) was the first skeletal muscle disease identified as being caused by mutations in MYH7 (Tajsharghi et al, 2003). It was clinically defined in 1971 in a brother and sister with a congenital myopathy in which fine granular material staining intensely with the myosin ATPase reaction had accumulated within the muscle fibres (Cancilla et al, 1971). It was subsequently shown that this family had a heterozygous mutation in MYH7 confirming this original family as having MSM (Dye et al, 2006).
MYH7 mutations were first found to be associated with MPD1 in 2004 when five different heterozygous mutations were identified in six families with a distinct distal myopathy phenotype (Meredith et al, 2004). The muscle pathology phenotype was varied, but excessive fibre size variability was the most common feature (Lamont et al, 2006). It was initially postulated that the position of the mutation within the gene dictated the phenotype, but the distinctions have been blurred, with overlap of mutation position and phenotype. The recent description of a large Spanish family has corroborated this fact, showing that the even within one family there can be variation in clinical and pathologic phenotype (Muelas et al, 2010).
Patients and methods
Patient cohort
The molecular testing of the MYH7 gene was performed either at the Neurogenetic testing laboratory, Royal Perth Hospital, Perth, Western Australia, or at the centre in the country where the patient was seen. Hence, six families were analysed in WA and the remaining 15 families were analysed in the country of origin. If a pathogenic mutation was found in MYH7, the referring clinician was asked to forward clinical details, including phenotypic details, family history and results of laboratory tests such as muscle biopsy.
Mutation screening
Genomic DNA was extracted as per standard protocols from blood or tissue samples from patients and where possible, parents or other family members. The relatives of index cases were examined by the physician caring for the index case, and informed consent obtained for molecular genetic testing. All overseas cases and the first four of the Perth cases were analysed amplification of exons 30 to 40 of MYH7 by polymerase chain reaction (PCR) with Hot Star Taq polymerase (Qiagen), followed by bi-directional sequencing using Big Dye Terminator (BDT) mix Version 3.1 (Applied Biosystems). The last two samples analysed in Perth were screened using next generation Ion Torrent sequencing technology (Life Technologies), as part the development of improved diagnostic screening methods. The entire MYH7 coding sequence was amplified in 6 overlapping amplicons. These amplicons were then prepared and sequenced as per the manufacturers instructions (Life Technologies). Any variations in MYH7 identified by Ion Torrent sequencing were later confirmed by Sanger sequencing. All variations were analysed with reference to the MYH7 GenBank cDNA sequence Accession number NM 000257.2. All sequence variation descriptions were checked using the Mutalyzer programme (Wildeman et al 2008). All sequence variants have been submitted to the ClinVar sequence variation database (http://www.clinvar.com/) (Landrum et al., 2014). ClinVar accession numbers are provided in Table 1.
TABLE 1.
MUTATION AND CLINICAL PHENOTYPE
| Family; country of origin |
Mutation (protein) |
Mutation (cDNA)* | Number of affected family members |
First symptom and age at presentation |
Ankle dorsiflexion weakness |
Long finger extensor weakness |
Site of other weakness | Other | ClinVar accession number |
|---|---|---|---|---|---|---|---|---|---|
| 1 UK |
p.Leu1481Pro^ | c.4442T>C | Six over 3 generations | “Toe walker” when commenced walking at 1 year | Y | Y, by 8 years | Cervical spine, trunk, scapular, ankle inversion by 15 years | TA contractures and hamstrings | SCV000120158 |
| 2 USA |
p.Glu1508del | c.4522_4524delGAG | One | Scoliosis aged 10 years | Y | Y, by 21 years | Neck flexors, hip extensors | TA contractures, cervical paraspinal, hips | SCV000120159 |
| 3 Belgium |
p.Glu1508del | c.4522_4524delGAG | Two over 2 generations | high stepping gait in both, at 4 yrs and 7 yrs respectively | Y | Y, by 15yrs | Weakness of deltoid, neck flexor, sternocleidomastoid, triceps, wrist extension, hip adductor, quads, glutei, posterior tibial, toe flexors | TA contractures | SCV000120159 |
| 4 Canada |
p.Gln1541Pro^ | c.4622A>C | Eight over 4 generations | Foot drop and awkward gait in early childhood | Y | In 30’s to 40’s | Proximal leg | 3 had calf hypertrophy | SCV000119900 |
| 5 Australia |
p.Thr1599Pro^ | c.4795A>C | Six over 3 generations | Foot drop when started walking at one year | Y | Y as adult | Nil | Nil | SCV000119901 |
| 6 UK |
p.Arg1608Pro^ | c.4823G>C | Two over 2 generations | Floppy baby during first months of life | Y | Y | Generalised. Loss of neck control, wheelchair dependant by 15 yrs | Widespread joint contractures | SCV000119902 |
| 7 UK |
p.Leu1612Pro^ | c.4835T>C | One | Footdrop aged 4 years | Y | N | Neck extensor contractures, mild hamstring contractures | SCV000119903 | |
| 8 USA |
p.Lys1617del | c.4849_4851delAAG | Nine over 5 generations | Proband presented in infancy with bilateral footdrop | Y | Y by 6th decade | Neck flexors/extensors, finger flexors, generalised | SCV000119904 | |
| 9 UK |
p.Lys1617del | c.4849_4851delAAG | Six over 3 generations | Footdrop when started walking at one year | Y | Y | Hip and knee flexion, shoulder and elbow, neck flexion | TA contractures | SCV000119904 |
| 10 UK |
p.Lys1617del | c.4849_4851delAAG | One | Ankle weakness at 5 years | Y | Y by 31 years | Shoulder girdle, wrist flexion/extension, proximal leg | TA contractures | SCV000119904 |
| 11 USA |
p.Lys1617del | c.4849_4851delAAG | One | Always ran on her toes, presented aged 2 years; tripping a lot by 7 years | Y | Not by 10 years | Neck flexion, mild diffuse weakness | - | SCV000119904 |
| 12 UK |
p.Lys1617del | c.4849_4851delAAG | One | Unusual gait, toe-walking at 2.5 years | Y | Not by 10 years | Hip girdle, finger extensor weakness | TA contractures | SCV000119904 |
| 13 Spain |
p.Ala1636Pro^ | c.4906G>C | 20 over 4 generations | Abnormal gait, tripping easily, onset from early childhood to 4th decade | Y | Y in some patients | Neck flexors. Limb girdle (predominantly affecting pelvic muscles) and proximal leg | TA contractures, calf hypertrophy in some, myalgia | SCV000120167 |
| 14 USA |
p.Leu1646Pro ^ | c.4937T>C | Six over 3 generations | Tripping easily, 5 years in one, 7 years in another | Y | Y by 33 years | Scapular winging, hip flexion, | TA contractures | SCV000119905 |
| 15 Italy |
p.Arg1662Pro ^ | c.4985G>C | Three | Footdrop at 3 years | Y | Not by 7 years | nil | nil | SCV000119906 |
| 16 UK |
p.Glu1669del^ | c.5005_5007delGAG | Seven over 3 generations | Footdrop at 3 years | Y | Y by 72 years | Extensor digitorum brevis | Neck extensor contractures | SCV000119907 |
| 17 Finland |
p.Lys1729dup ^ | c.5186_5188dupAGA | Three over 3 generations | Early childhood with delay in walking walker | Y | N | Neck flexor weakness | Mild calf hypertrophy | SCV000119908 |
| 18 UK |
p.Leu1793del ^ | c.5378_5380delTGC | One | Mildly floppy baby, walking delayed til 18 months Presented with dilated cardiomyopathy aged 18 months | Y | Y | Hip girdle, with Trendelenburg gait | - | SCV000119909 |
| 19 Israel |
p.Glu1801Lys | c.5401G>A | Two, single generation | Delayed walking (20 months) and foot drop | Y by 6 years | N by 23 years | All lower limb muscles 4/5, dorsiflexion 1/5 | High arched palate | SCV000119910 |
| 20 USA |
p.Glu1914Lys ^ | c.5740G>A | One | Born at 33 weeks with hydrops fetalis Dilated cardiomyopathy and pulmonary hypertension at birth | Y by 3.5 years | - | Proximal and distal weakness | Diffuse hypotonia, microcephaly | SCV000119911 |
| 21 USA |
p.Glu1914Lys ^ | c.5740G>A | One | 8 weeks of life with dilated cardiomyopathy | Toe walks | N by 5 years | Diffuse hypotonia, poor endurance, neck flexion | Borderline microcephaly | SCV000119911 |
Y = yes, N = no, TA = tendo Achilles,
= novel mutation, not previously described,
MYH7 cDNA Accession number used = NM_000257.2
Muscle immunohistochemistry
Tibialis anterior muscle biopsy of two patients from family 17 (III:1, IV:1). were analysed for myosin immunohistochemistry using a double staining method (Raheem et al, 2010), with an antibody against slow myosin (Novocastra Laboratories Ltd. Newcastle Upon Tyne NE12 8EW, UK) and an antibody against fast IIA myosin A4.74 (Developmental Studies Hybridoma Bank, University of Iowa, Iowa city, IA 52242, USA). Slow myosin was visualized with a peroxidase-based detection kit (UltraViewTM Universal DAB detection kit, Ventana Medical Systems Inc., Tucson, AZ 85755, USA) and myosin A4.74 with alkaline phosphatase red detection system (UltraViewTM Universal Alkaline Phosphatase Red detection kit, Ventana Medical Systems Inc., Tucson, AZ 85755, USA).
Results
This paper reports on 88 individuals from 21 previously unpublished families in whom a MYH7 mutation has been identified (Table 1). The families are from the UK, USA, Scandinavia, Spain, Belgium, Italy, Israel and Australia. Twelve previously unpublished mutations are described, from thirteen families. There are three previously published mutations in the remaining eight families. Although referred to as “families”, in eight of 21 there was only a single case. Testing of both parents in three of these cases proved the mutations to be de novo. In family 6 the father (II:2) of the proband was found to a somatic mosaic. The full numbered pedigrees for all families except family 8 are to be found in Supp. Figure S1. Insufficient pedigree information was available for family 8.
Clinical features
Table 1 outlines the age of onset, presenting complaint and areas of muscle weakness. In 81% of families the presenting complaint in all or most of the members was footdrop, variously described as a high-stepping gait or excessive tripping. This led to delayed walking in three, but in six others the child walked by 12 months of age despite the footdrop. In a further eight families the footdrop came on later, ranging from two years, to the 4th decade in family 4 (subjects I:2 and II:1). Subject II:1 in family 2 came to medical attention aged 10 years with scoliosis, but on history had since early childhood walked heavily and slapped her feet. Subject III:3 in family 6 was diagnosed as being a “floppy baby” with generalised weakness, and he required a wheelchair by 15 years, representing the more severe end of the spectrum. Two cases presented as infants with cardiomyopathy. Subject II:3 from family 20 was born at 33 weeks with hydrops fetalis and pulmonary hypertension, which has not been described before with a MYH7 mutation. The other, subject II:1 from family 21, presented in cardiac failure aged eight weeks. Both of these children had diffuse muscle hypotonia and weakness.
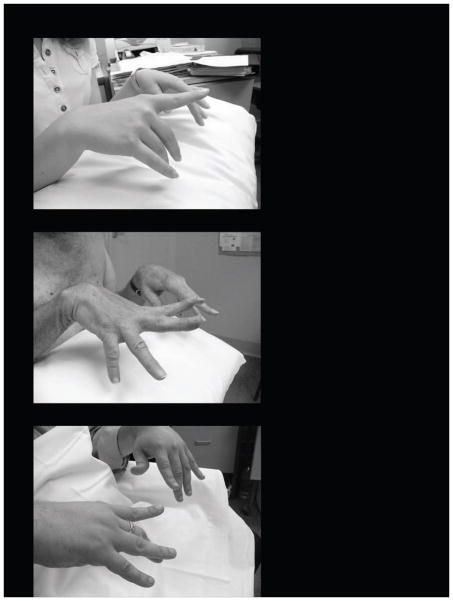
Variable weakness of the long finger extensors was reported in 14 of the 20 families (Figure 1). The age at which this happened was variable, appearing in infancy in two as part of diffuse weakness (de novo probands of families 20 and 21). However, in most it appeared later as a discrete event, between the ages of eight years to early 30’s. In the remaining seven families, the probands were less than 10 yrs old and may develop finger extensor weakness in the future. The site of other muscle weakness was also dependent on the age of the patient, and disease duration. Neck flexion weakness was common, seen in more than 50%. This was part of diffuse weakness in three. In five families the weakness remained mainly confined to the lower limb, plus or minus long finger extension and neck flexion weakness. However, in most cases there was more widespread weakness, including shoulder girdle and trunk. It is worth noting that although the weakness began distally, with the passage of time many proximal muscles became weak too. It is also notable that mild facial weakness was easily overlooked, but often patients were unable to fully bury their eyelashes. Tightness of the tendo Achilles was noted in nine families (43%).
Figure 1.
Weakness of the long finger extensors without involvement of the thenar eminence is typical of MPD1, but may not occur until years after the weakness of the ankle and toe dorsiflexors has occurred. Rarely finger extension weakness does not develop at all.
1a – 17 year old female
1b – 65 year old man
1c – 28 year old man
Table 2 outlines the cardiac, spinal and respiratory findings of this cohort. Five families did not have any of these three systems involved and do not appear in the table. Thirteen of 21 families, or 62%, had a “spinal phenotype” in some of their members. Spinal rigidity or kyphoscoliosis was seen in twelve families. Family 1 had spinal rigidity or kyphoscoliosis manifesting in different members of the family. Exaggerated lumbar lordosis was seen in members of families 3, 9, 14 and 19. Cardiac involvement due to the MYH7 mutation was seen in nine families, with the age of onset varying widely. The de novo probands from families 18, 20 and 21 all developed cardiomyopathy early on. The probands of family 18 and 20 required cardiac transplantation at 3 and 3.5 years respectively. The proband of family 21 had their cardiac status stabilised with medication when last followed up at 5 years of age. Cardiac problems developed later in one or more members of five families, ranging from mild to moderate in severity. The echocardiographic findings are described in Table 2. In the Finnish family, one of three members (II:3) had a “heart attack” aged 44 years and died, but no further details are available. Respiratory findings were less common, seen only in six members of six families. In two families (2 and 20) the abnormalities could have been secondary to cardiac disease, namely shortness of breath when climbing stairs, and pulmonary hypertension. However, in families 1, 6, 11 and 12, there was a decline in forced vital capacity, leading to the need for non-invasive ventilation in one teenager (III:3 in family 6).
TABLE 2.
SPINAL, CARDIAC AND RESPIRATORY FEATURES
| Family; country of origin | Mutation | Spinal phenotype | Cardiac involvement | Respiratory involvement |
|---|---|---|---|---|
| 1 UK |
p.Leu1481Pro^ | Cervical spine rigidity in proband; Scoliosis in sibling | No | Forced vital capacity fallen to 55% by age 34 years, and 22% by age 51 years |
| 2 USA |
p.Glu1508del | Presented with scoliosis, developed neck extensor contractures | Proband (II:1) DCM with MR, LV dysfunction, LVAD implanted aged 23 yrs most recent LV ejection fraction 0.21 | Shortness of breath with walking or climbing stairs |
| 3 Belgium |
p.Glu1508del | Kyphoscoliosis, exaggerated lumbar lordosis | No | No |
| 6 UK |
p.Arg1608Pro^ | Marked thoracic scoliosis requiring surgical fixation at 9 years | Proband (III:3) had mildly impaired LV function by 19 years; β-blocker commenced; 6 years later the LV systolic and diastolic dimensions are 2.5 and 3.6 cms respectively | Forced vital capacity reduced to 43% of predicted by 13 years. Required non-invasive ventilation soon after |
| 7 UK |
p.Leu1612Pro | Late in first decade of life developed spinal rigidity and scoliosis, cervical spine rigidity | No | No |
| 8 USA |
p.Lys1617del | Spinal fusion for scoliosis in proband | No | No |
| 9 UK |
p.Lys1617del | One person had scoliosis needing surgical fixation, one patient had marked lumbar lordosis | No | No |
| 11 USA |
p.Lys1617del | No | No | Forced vital capacity 72% and forced expiratory volume (1 min) 57% by 10 years |
| 12 UK |
p.Lys1617del | Spinal rigidity by 10 years | Proband (II:1) DCM detected aged 11.5 years, initial LV diameter 44cm, treated with ACE inhibitor and decreased to 41mm LV diameter by 15 years; FS = 29% | Forced vital capacity declined to 35% of predicted by 15 years |
| 13 Spain |
p.Ala1636Pro^ | Thoracic scoliosis in two | Single person (III:13) with aortic valvulopathy due to rheumatic fever | No |
| 14 USA |
p.Leu1646Pro^ | Thoracic scoliosis and lumbar lordosis in one | No | No |
| 16 UK |
p.Glu1669del^ | Neck extensor contractures | No | No |
| 17 Finland |
p.Lys1729dup^ | No | Single member (II:3) had “heart attack” aged 44 years | No |
| 18 UK |
p.Leu1793del^ | Marked kyphoscoliosis by 18 years | Proband (II:1) had DCM requiring orthoptic heart transplant aged 3 years | No |
| 19 Israel |
p.Glu1801Lys | Marked lumbar lordosis | Proband (III:1) had HCM by 23 yrs: diastolic IVS thickness 1.2cm (N<1.1); FS = 20% (N>28%) | No |
| 20 USA |
p.Glu1914Lys^ | No | Proband (II:3) presented at birth with HCM, required cardiac transplantation aged 3.5 yrs; explanted heart = NCCM | Pulmonary hypertension secondary to cardiac disease |
| 21 USA |
p.Glu1914Lys^ | No | Proband (II:1) had DCM by 8 weeks, stable on medication at 5 years with EF 65% | No |
= previously undescribed
DCM = Dilated cardiomyopathy
HCM = Hypertrophic cardiomyopathy
NCCM = Noncompaction cardiomyopathy
MR = Mitral regurgitation
LV = Left ventricular
LVAD = Left ventricular assist device
ACE = Angiotensin converting enzyme
FS = fractional shortening
IVS = interventricular septum
EF = ejection fraction
Muscle biopsy
Table 3 outlines the findings on muscle biopsy in twelve families. In five families the diagnosis was made clinically and confirmed on molecular testing, without resorting to biopsy. In three families, the biopsy was done, but only the conclusions were available, not detailed descriptions. Skeletal muscle biopsy had been performed on two brothers from family 14 (III:3 and III:4), and the findings were said to support the diagnosis of Central Core Disease (CCD). The single affected child in family 20 (II:3) had two biopsies, and the diagnosis was “Congenital Fibre Type Disproportion with myosin accumulation”. The single affected child in family 18 (II:1) had a pathological diagnosis of Congenital Fibre Type Disproportion (CFTD).
TABLE 3.
PATHOLOGICAL FEATURES ON MUSCLE BIOPSY
| Family/mutation | Abnormal fibre size | Fibre grouping |
Fibre size variability |
Fibre type predominance |
Internal nuclei |
Cores | Sarcoplasmic inclusion body, other |
Necrosis, phagocytosis, regeneration |
Connective tissue, fat |
|---|---|---|---|---|---|---|---|---|---|
| 1 p.Leu1481Pro ^ |
Type I fibres smaller than type II | - | - | Type I predominance | - | - | - | - | - |
| 2 p.Glu1508del |
Angulated atrophic fibres of both types, but more type 1 than 2 Some hypertrophic, more type 2 | Type 1 fibre grouping present | Marked excess | - | ↑ | - | - | nil | nil |
| 3 p.Glu1508del |
Atrophic type 1 fibres | Present | Increased | Type I fibre predominance | ↑↑↑ | Minicores | - | - | Slight endomysial fibrosis |
| 4 p.Gln1541Pro^ |
- | - | - | Type I predominance | ↑↑↑ | - | - | - | nil |
| 6 p.Arg1608Pro |
Atrophic type 1 fibres | Present | Increased | - | ↑ | Some cores; multiminicore myopathy suggested | - | - | - |
| 8 p.Lys1617del |
Many atrophic angular fibres | Present | Marked increase | Type 2 predominance | ↑↑↑ | Patchy areas of loss of oxidative activity | - | Several fibres showing these three features | Mild increase in connective tissue |
| 9 p.Lys1617del |
Rounded atrophic fibres, as well as hypertrophic fibres | - | Marked increase | Marked type 1 predominance | - | - | - | nil | Increase in both |
| 10 p.Lys1617del |
Both round and angulated atrophic fibres of both types, as well as hypertrophic fibres | - | Marked increase | Type 1 fibre predominance | - | “Moth eaten” changes and vacuolisation on oxidative stains | Desmin staining in regenerating fibres | All three | Endomysial fibrosis |
| 11 p.Lys1617del |
Atrophic fibres, more commonly type 1 than 2 | - | Increased | - | Few | - | Diffuse upregulation of desmin in some fibres | Occasional necrotic and regenerating fibres | - |
| 12 p.Lys1617del |
Occasional atrophic fibres | - | Mildly increased | - | - | Some irregularity of oxidative staining | - | - | - |
| 13 p.Ala1636Pro^ |
Both round and angulated atrophic fibres, less frequent hypertrophic fibres, type 1 fibres smaller than 2 in some | - | Mildly to markedly increased | Moderate to marked type 1 fibre predominance | ↑-↑↑ | Cores, minicores and “moth-eaten” changes | Present in 2 of 5 biopsies | Regeneration in some biopsies | Mild to moderate increase in connective tissue |
| 17 p.Lys1729dup^ |
Marked atrophy | Present | - | - | - | - | - | Fibrosis | - |
| 19 Glu1801Lys |
- | - | - | Type 1 predominance | - | - | - | - | - |
= novel mutation, not previously described
In the remaining thirteen families detailed findings were available. An increase in fibre size variability was seen in nine, namely 70%. In six of these, it was commented that either all or most of the atrophic fibres were type 1, but in others it was not specified. Type 1 fibre predominance was seen in seven of thirteen biopsies. Type 2 fibre predominance was seen in one family. Fibre type grouping was seen in five families, with increased central nuclei in seven. Abnormalities of oxidative enzyme staining were reported in three biopsies. Actual cores and minicores were seen in another three. A small number showed necrosis, phagocytosis or regeneration, and four reported a mild increase in connective tissue. It does not appear in the table, but there was nuclear clumping in two biopsies, and occasional fibre splitting in two other biopsies.
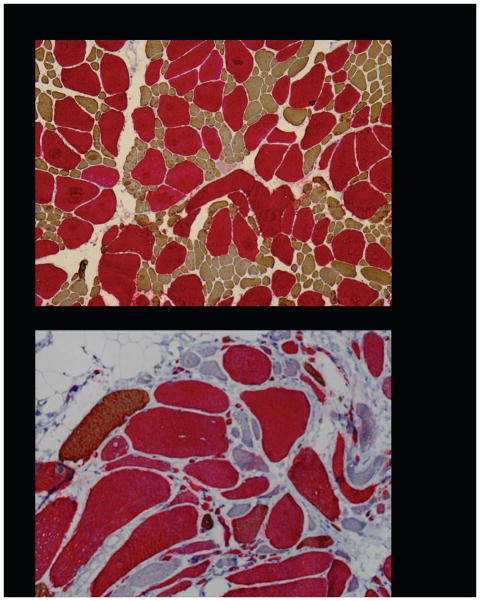
Figure 2 shows myosin immunohistochemistry with double staining method in two patients from family 17 (III:1, IV:1). In both samples there are rare normal-sized and many very atrophic slow type 1 fibres. At a milder stage of disease (Figure 2a) fast IIa fibres are of normal size and practically all slow type 1 fibres are small corresponding to congenital fibre type disproportion. At a more advanced disease stage (Figure 2b) atrophic slow type 1 fibres also express fast IIa myosin indicating they are hybrid fibres. Further, atrophic fast type IIa and IIx fibres are found as well.
Figure 2. Myosin immunohistochemistry with double staining method.
2a - Biopsy from a Finnish patient with the p.Glu1508del mutation shows the earlier stages of pathology: practically all type 1 fibres (brown) are small as is seen in Congenital Fibre Type Disproportion. Almost all fast IIa (red) fibres are normal-sized and there are no light blue IIx fast fibres in this tibialis anterior biopsy.
2b - Biopsy from a Finnish patient with the p.Lys1617del mutation illustrates more advanced pathology with extensive fibrosis and fat and almost SMA-like large group atrophies. All type1 fibres (brown) have variable degrees of reddish indicating hybrid fibres (arrows) expressing also fast IIa myosin (red). There are also atrophic fast type IIa and IIx (light blue) fibres which do not express slow myosin at all.
Genetic testing results
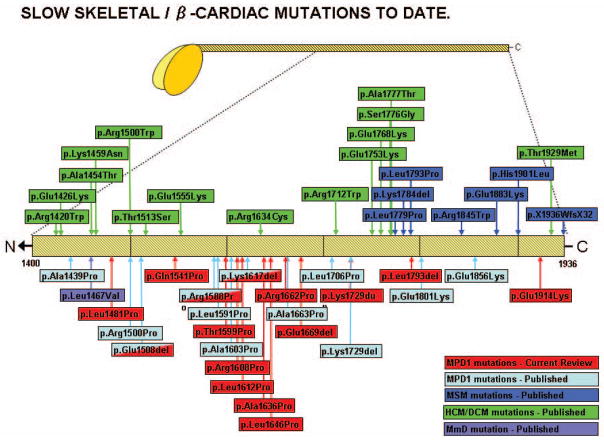
The causative mutation for each family is given in Table 1. There are twelve previously unpublished mutations and three recurrent. Of the recurrent mutations, the p.Lys1617del mutation was seen in five families, originating from different localities in the UK and the USA. Haplotyping was not available so it is not known if this was due to a founder mutation. The p.Glu1508del mutation was seen in two families, family 2 from the USA and family 3 from Belgium. The p.Glu1801Lys mutation was seen in family 19 from Israel. Of the previously unpublished mutations, each one was seen only in one family, with the exception of the p.Glu1914Lys mutation which was seen in the singleton cases from family 20 and 21 (II:3 and II;1 respectively). These two were unrelated and were shown to be de novo mutations, with both sets of parents being shown not to carry the mutation. The grandfather (II:2) of the index case in family 6 was found to be a somatic mosaic for the novel mutation found in that family. On review examination he was found to be mildly weak. This may be another source for presumed “de novo” cases. Figure 3 is a schematic of the MYH7 gene, indicating the position of gene mutations described in this review, as well as previously published mutations causing hypertrophic or dilated cardiomyopathy, myosin storage myopathy or previously published mutations causing Laing distal myopathy.
Figure 3.
A schematic of the MYH7 gene, indicating the position of gene mutations causing hypertrophic or dilated cardiomyopathy, myosin storage myopathy or Laing distal myopathy, as well as mutations described in this review.
Discussion
Clinical phenotype
The typical presentation of footdrop in MPD1 can lead to an initial misdiagnosis of Hereditary Motor and Sensory Neuropathy, as happened in the original Australian family (Laing et al, 1995). However, in MPD1, as well as in many other distal myopathies, the extensor digitorum brevis muscles are spared while anterior leg compartment muscles are atrophic, differentiating myopathies from neurogenic muscle atrophy (Udd 2011). Although footdrop is the usual presentation for MPD1, it is non-specific as it is seen in other distal myopathies such as both Tibial and Nonaka distal myopathies, and nemaline myopathy secondary to mutations in TPM3 (Udd 2011). The weakness of finger extension that usually follows is more specific. The thumb is usually spared, with variable involvement of the other digits (Figure 1). This “dropped fingers” pattern of weakness is not seen commonly in other distal myopathies, with the exception of distal nebulin myopathy (Wallgren-Pettersson et al. 2007), certain types of filaminopathy (Guergueltcheva et al, 2011) and as the presenting symptom in Welander distal myopathy with late onset (Borg et al, 1998). In the present series, some patients did not develop finger extensor weakness until their fourth decade of life, meaning this valuable clue may not be present early in the disease when a diagnosis is being sought. However, upper limb weakness can also precede lower limb weakness (Udd, 2009), and although there is a tendency for early onset, the possibility of much older onset has been described elsewhere, and in this present cohort (Muelas et al, 2010, Tasca et al, 2012).
It was initially thought that skeletal and cardiac muscle problems did not coexist in MPD1. Subsequently a boy was reported with early onset leg weakness and cardiomyopathy from the age of 7 years (Darin et al, 2007). Cardiac involvement may present early (Homayoun et al, 2011) or later such as the individual presenting with cardiomyopathy aged 53 yrs (Overeem et al, 2007). All of the mutations in these patients with MPD1 and cardiomyopathy are not in the MYH7 rod domain, but in the myosin head like “pure” cardiomyopathy mutations. Even within a single family such as the large published Spanish family, cardiac presentation was variable, with abnormalities in only 5 of 27 family members, including dilated cardiomyopathy, left ventricular impairment and ECG abnormalities in adulthood (Muelas et al, 2010).
There is an axial phenotype associated with MYH7 mutations, including thoracic or thoracolumbar scoliosis, spinal rigidity, fixed neck extension, and exaggerated lumbar lordosis. In 55% of families in this report there were either one or two family members with spinal problems. An English family with MPD1 had an initial diagnosis of Bethlem myopathy because scoliosis and spinal rigidity was present (Lamont et al 2006). A French MPD1 family manifested kyphoscoliosis in three of five affected family members (Dubourg et al, 2011).
Although not common, calf hypertrophy has been reported, as well as tibialis anterior hypertrophy or combined calf and tibialis anterior hypertrophy (Overeem et al, 2007, Tasca et al, 2012) In the large published Spanish family, 11 out of 27 patients had calf hypertrophy (Muelas et al, 2010).
Patients with Laing distal myopathy generally retain a high degree of functionality, despite early presentation, and sometimes significant focal weakness. One member of family 14 (III:4) worked as a roof tiler until his mid 30’s, despite footdrop from the age of 7 years, and another (III:3) was a competitive swimmer in her teens despite presenting as a five year old with ankle weakness. The proband from family 16 (III:9) presented with foot drop aged 3 years, and when seen again aged 72 years had developed complete paralysis of ankle dorsiflexion but no weakness in any other muscle group. This very slow deterioration despite early onset has been recognised in many reports (Dubourg et al, 2011; Hedera et al, 2003; Voit et al, 2001, Cullup et al, 2012). However, there is no doubt that the muscle weakness can be more rapidly progressive. The proband of family 6 (III:3) progressed from being a “floppy baby” but achieving motor milestones within normal parameters to requiring a wheelchair for mobility by age 15 years. This variability can even be within the same family (Muelas et al, 2010).
Muscle pathology
The commonest biopsy abnormality was excessive variation in fibre size but this is not very informative and has been repeatedly reported. More interestingly, the diagnosis of CFTD was made in several families. In the large Spanish family 10 of 14 muscle biopsies showed abnormally small type I fibres with type 1 predominance, fulfilling the criteria for CFTD (Muelas et al, 2010), and other families with MYH7 mutations have been similarly described (Ortolano et al, 2011; Sobrido et al, 2005, Clarke et al 2013). However, this is not specific as CFTD biopsy findings have also been described in mutations of ACTA1 (Laing et al, 2004), SEPN1 (Clarke et al, 2006), RYR1 and TPM3 (Clarke et al, 2008). As with excessive variation in fibre size, it is nonspecific.
In the more advanced stages the atrophic slow myosin type 1 fibre may appear as hybrid fibres, expressing fast IIa myosin as well. This is difficult to assess by ATPase staining of fibre types, as the hybrid fibres (type 2C by ATPase) cannot be well differentiated when atrophic. However, the finding of atrophic slow fibres being hybrid fibres seems to be pathognomonic for MPD1. Immunohistochemical staining for slow and fast myosins in previous published families have reported co-expression of slow and fast myosin in type 2C fibres in one (Muelas et al, 2010), in all type 2 fibres in another (Homayoun et al, 2011) and in type 1 fibres in a third family (Lamont et al, 2006). This has been attributed to a possible conversion of type 1 to type 2 fibres as a result of defective slow myosin protein generation. Besides the atrophic slow fibres, fast IIa (2A) and IIx (2B) fibres may undergo atrophic changes, which suggests that in the later stages of the disease all types of fibres are affected. This may be due to local cross-fibre molecular signalling events since the IIa and IIx fibres should not have any primary defect to cause catabolic atrophy.
The other pathological feature to emerge from this cohort was that of core pathology. In the large Spanish family cores or multicores were seen in 10 of 14 biopsies, frequently in seven and occasionally in three (Muelas et al, 2010). A Norwegian patient was reported as having both core and multiple mini-core changes, together with type I atrophy (Dubourg et al, 2011). Central cores in clinically unaffected skeletal muscles have been reported previously in hypertrophic cardiomyopathy patients with MYH7 mutations (Fananapazir et al, 1993). For many years the proband of family 12 (II:1) was described as a typical example of minicore myopathy, in Dubowitz Muscle Disorders in Childhood 2nd edition WB Saunders London 1995.
Overall, no typical abnormality in muscle pathology has emerged to help in a definitive diagnosis of MPD1, in contrast to the allelic MSM which is defined by the presence of the subsarcolemmal hyaline bodies which stain positively for slow heavy chain myosin (MyHC I). However, an MSM family has been described with the appearance of CFTD in two family members but hyaline bodies were seen in a third (Ortolano et al, 2011). Thus even in MSM histological findings are not completely reliable.
Genetic abnormalities
This mutation update presents twelve novel and three recurrent MYH7 mutations identified in 21 families with skeletal muscle disease caused by MYH7 mutations. This takes the total number of myopathy-related MYH7 mutations to 36 (Supp. Table S1). Of the recurrent mutations, two families had the previously described p.Glu1508del mutation (Dubourg et al, 2011). This mutation was originally described in French, Norwegian and Finnish families. It has now been identified in a Belgian family (family 3), and a family from the USA of Polish extraction (family 2). The p.Glu1801Lys mutation was first found in a Moldavian family, and in this paper was found in an Israeli Jewish family (family 19). The third recurrent mutation is the most common mutation, p.Lys1617del. This was identified in a further five families, from the UK and the USA.
In addition to the five families in this study, the p.Lys1617del mutation has been identified in eight published MPD1 families to date, making it the most frequent mutation causing MPD1 (Lamont et al, 2006; Meredith et al, 2004). As the residue is located in a 3-repeat microsatellite comprising three lysines (AAG), it is likely that this plays a role in the mutation’s recurrence through polymerase slippage (Viguera et al, 2001). This is despite microsatellites not being considered highly mutable until they are over the size of ten repeats (Weber et al, 1990). Other mutations, p.Glu1508del, p.Lys1729del, p.Lys1729ins, p.Leu1793Pro, p.Glu1801Lys have also been seen in more than one family, suggesting that particular areas of MYH7 may be more prone to mutations than others.
De Novo mutations
The current study has further shown that new sporadic MYH7 mutations occur frequently. In eight of the 21 families (38%) there was no family history of the disease. In four of these families (1, 12, 20 and 21) the mutation was proven to be de novo by showing both parents did not carry the mutation, and haplotyping confirmed they were the parents. In family 6 it was demonstrated that subject II:2 was a somatic mosaic, and on further examination he did have mild muscle weakness. This could be another cause of “apparent” de novo mutations. Three of eight MYH7 mutations were shown to be de novo in a previous publication (Lamont et al, 2006), and Homayoun et al demonstrated a de novo mutation in their case (Homayoun et al, 2011). Dubourg et al also showed that one of their three families with the p.Glu1508del mutation had a de novo mutation (Dubourg et al, 2011). Of the 21 previously published MPD1 families, 7 (33%) have been proven to represent de novo mutations, meaning a lack of family history should not argue against a diagnosis of MPD1. The high new mutation rate further suggests that there should be no particular ethnic predisposition to the disease and this prediction is borne out by the wide geographic and ethnic distribution of cases reported to date.
Missense mutations to proline
There are now 15 missense mutations to proline in MYH7 associated with MPD1, including eight families in this report. A further two have been associated with MSM (p.Leu1779Pro and p.Leu1793Pro) (Chai et al, 2007, Dye et al, 2006). The missense mutations to proline involve amino-acid residues at all positions of the heptad repeat, suggesting that insertion of proline at any of these positions may be equally deleterious, as previously proposed on the premise that any such change would introduce a kink in the protein structure (O’Neil & DeGrado, 1990; Piela et al, 1987). However, Smith et al suggest that proline substitutions in external positions of a coiled coil may in fact have only minor effects (Smith et al 2004).
Charge changing mutations
To date four mutations have been published which cause MPD1 or MSM involving glutamate to lysine amino acid changes (Glu>Lys). These mutations are all associated with a mixed phenotype of cardiomyopathy and skeletal myopathy (Tajsharghi et al, 2007; Udd, 2009). There were three Glu>Lys mutations in this study, and they all were associated with this mixed phenotype. A Glu>Lys mutation is accompanied by a large residue charge reversal (from a negative to a positively charged amino acid). Considering that correct formation of the thick filament relies on ordered charge attractions between regions of opposite charge on adjacent β-MyHC rods (McLachlan & Karn, 1982), it is likely that the charge reversal contributes to the molecular pathogenesis of the diseases. Furthermore all of these mutations are located such that they are positioned in the outer residues of the heptad repeat, and thus involved in the ordered arrangement of β-MyHC dimers during thick filament assembly domains (McLachlan & Karn, 1982). Though it is unknown how one set of Glu>Lys mutations can cause solely a cardiomyopathy and the other a mixed phenotype, the regions in which they occur do not overlap: Glu>Lys mutations causing pure HCM or DCM are located within residues 1356–1768, while those causing a mixed skeletal/cardiac phenotype are confined to residues 1793–1914. Thus, the location of a charge-reversing Glu>Lys mutation may play a role in the precise disease phenotype.
Pathobiology
What the specific molecular pathobiological processes are that cause a MYH7 mutation to result in myosin storage myopathy, distal myopathy or one of these mixed with cardiomyopathy remains largely unexplained.
Remarkably different phenotypes have been associated with different mutations of the same amino-acid residue of β-MyHC. Mutations in the head domain were previously only associated with cardiomyopathy, but now they have been found to cause a distal myopathy with cardiomyopathy (Darin et al, 2007) and cardiomyopathy associated with an extraordinary hypertrophic distal myopathy affecting the tibialis anterior (Overeem et al, 2007). In another example the p.Arg1500Pro missense mutation, found in the rod rather than the head domain of MHY7, can cause an MPD1 phenotype (Meredith et al, 2004), whereas the p.Arg1500Trp can cause DCM (Karkkainen et al. 2004). Biophysical experiments suggest that the two mutants may have different mechanisms of pathogenesis but exactly how they cause the two distinct disease phenotypes is still unclear (Armel and Leinwand, 2009). Similarly, the missense mutation p.Leu1793Pro causes MSM (Dye et al, 2006) while the heterozygous deletion of this residue (pLeu1793del) was identified in a boy in this series (Family 20, II:3) with a distal myopathy who underwent a cardiac transplant at age three years. Conversely, the same mutation at the residue can cause either MSM or MPD1 (Stalpers et al, 2011, Tasca et al, 2012).
Substantial variation in disease phenotype is also seen between family members carrying the same mutation (Muelas et al, 2010; Udd 2009). In the present series, Family 13 had a wide range of age at presentation, from 5 years in III:1 to 25 years in IV:7. However, disease phenotype was not as variable in the remainder of the families. A report from Uro-Coste described a mother presenting with proximal weakness at age 30 and her daughter presenting with cardiomyopathy at birth, (Uro-Coste et al, 2009). They both carried the previously described p.Leu1793Pro mutation. This phenotypic variation could be due to the effect of modifier genes. The possibility that Next Generation sequencing (NGS) techniques could help identify these modifiers in the future, is exciting as it may have therapeutic potential. Also, understanding of the epigenetic controls of the severity of MPD1 would present an opportunity for further diagnostic and therapeutic development, if they could be manipulated. NGS techniques will also allow for the entire MYH7 gene to be investigated, rather a limited screen of the head domain for cardiomyopathies and the rod domain for MPD1. This could lead to even more overlap between cardiac and skeletal muscle phenotypes, and a more complete spectrum of MYH7 disorders.
MYH7 is expressed in every slow muscle fibre in every muscle of the body. It is not known how the specific distribution patterns of affected muscles in MPD1 arise. Perhaps another protein may be involved in the disease pathogenesis. The mutations may interfere with the interaction between myosin and proteins that bind the light meromyosin (LMM) region of the myosin tail. Proteins that are known to interact with the LMM include myomesins 1–3 and M-protein (Obermann et al 1998; Schoenauer et al 2008) myomasp/LRRC39, myosin-binding protein C and titin (TTN) (Buvoli et al 2012). Buvoli et al suggest that the most likely bonding partner affected is myomasp/LRRC39 (Buvoli et al 2012).
It may be that animal models will not answer the questions of the pathobiology of Laing distal myopathy, because the pattern of muscles and muscle use in other animals is different to man. Nevertheless a pig model of MYH7 myopathy has recently been described (Murgiano et al, 2012). The mutation in the pig is c.4320_4321insCCCGCC (p.Ala1440_Ala1441insProAla), therefore involving the familiar insertion of a forbidden proline into the MYH7 rod domain and within the now known spread of rod domain MYH7 myopathy mutations following the publication of a p.Ala1439Pro MYH7 mutation in a Korean family who presented with a distal phenotype, which also became proximal after several decades (Park et al, 2013).
Incidence
A survey of published cases of “myosinopathies” giving skeletal muscle weakness suggests that MPD1 is more common than MSM. Taking this series into account, there have been 184 MPD1 patients from 42 families published, compared to 53 MSM patients from 12 families. We identified no MSM patients in this series, although that could have been because of referral bias. MPD1 is common because of the high rate of new mutations, as well as a phenotype which often allows survival into adult life and hence the chance of offspring.
Conclusion
The description of newly identified MPD1 cases has not only broadened the phenotypic spectrum of MYH7 muscle disease. The ongoing identification of both novel and known MYH7 mutations is important in increasing our knowledge of the spectrum of mutations and in the elucidation of disease mechanisms.
Supplementary Material
Acknowledgments
Funding: Alan Beggs is supported by the Muscular Dystrophy Association (USA) grant MDA201302 and by the National Institutes of Health grant R01 AR044345 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Nigel Laing is supported by Australian National Health and Medical Research Council Fellowship APP1002147.
References
- Armel TZ, Leinwand LA. Mutations at the same amino acid in myosin that cause either skeletal or cardiac myopathy have distinct molecular phenotypes. J Mol Cell Cardiol. 2009;48:1007–1013. doi: 10.1016/j.yjmcc.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg K, Ahlberg G, Anvet M, Edstrom L. Welander distal myopathy – an overview. Neuromusc Disord. 1998;8:115–118. doi: 10.1016/s0960-8966(98)00008-x. [DOI] [PubMed] [Google Scholar]
- Buvoli M, Buvoli A, Leinwand LA. Effects of pathogenic proline mutations on myosin assembly. J Mol Biol. 2012;415:807–818. doi: 10.1016/j.jmb.2011.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cancilla PA, Kalyanaraman K, Verity MA, Munsat T, Pearson CM. Familial myopathy with probable lysis of myofibrils in type I fibres. Neurol. 1971;21:579–585. doi: 10.1212/wnl.21.6.579. [DOI] [PubMed] [Google Scholar]
- Chai J, Liu C, Lai P, Yee W. Myosin storage myopathy with a novel slow-skeletal myosin (MYH7) mutation in a Chinese patient. Neuromusc Disord. 2007;17:838. [Google Scholar]
- Clarke NF, Kidson W, Quijano-Roy S, Estournet B, Ferreiro A, Guicheney P, et al. SEPN1associated with congenital fibre-type disproportion and insulin resistance. Ann Neurol. 2006;59:546–552. doi: 10.1002/ana.20761. [DOI] [PubMed] [Google Scholar]
- Clarke NF, Kolski H, Dye DE, Lim E, Smith RLL, Patel R, et al. Mutations in TPM3 are a common cause of congenital fibre type disproportion. Ann Neurol. 2008;63:329–337. doi: 10.1002/ana.21308. [DOI] [PubMed] [Google Scholar]
- Clarke NF, Amburgey K, North KN, Teener J, Waddell LB, et al. A novel MYH7 mutation is associated with highly variable clinical and histological congenital myopathy phenotypes. 2013;23:432–436. [Google Scholar]
- Cullup T, Lamont PJ, Cirak S, Damian MS, Wallefeld W, Gooding R, et al. Mutations in MYH7 cause Multi-minicore disease with variable cardiac involvement. Neuromusc Disord. 2012;22:1096–1104. doi: 10.1016/j.nmd.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Darin N, Tajsharghi H, Ostman-Smith I, Gilljam T, Oldfors A. New skeletal myopathy and cardiomyopathy associated with a missense mutation in MYH7. Neurol. 2007;68:2041–2042. doi: 10.1212/01.wnl.0000264430.55233.72. [DOI] [PubMed] [Google Scholar]
- Dubourg O, Maisonobe T, Behin A, Suominen T, Raheem O, Penttila S, et al. A novel MYH7 mutation occurring independently in French and Norwegian Laing distal myopathy families and de novo in one Finnish patient. J Neurol. 2011;258:1157–1163. doi: 10.1007/s00415-011-5900-9. [DOI] [PubMed] [Google Scholar]
- Dye DE, Azzarelli B, Goebel HH, Laing NG. Novel slow-skeletal myosin (MYH7) mutation in the original myosin storage myopathy kindred. Neuromusc Disord. 2006;16:357–360. doi: 10.1016/j.nmd.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Fananapazir L, Dalakos MC, Cyran F, Cohn G, Epstein ND. Missense mutations in the β-myosin heavy-chain gene cause central core disease in hypertrophic cardiomyopathy. Proc Natl Acad Sci. 1993;90:3993–3997. doi: 10.1073/pnas.90.9.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guergueltcheva V, Peeters K, Baets J, Ceuterick-de Groote C, Martin JJ, Suls A, et al. Distal myopathy with upper limb predominance caused by filamin C haploinsufficiency. Neurology. 2011;77:2105–2114. doi: 10.1212/WNL.0b013e31823dc51e. [DOI] [PubMed] [Google Scholar]
- Hedera P, Petty EM, Bui MR, Blaivis M, Fink JK. The second kindred with autosomal dominant distal myopathy linked to chromosome 14q. Arch Neurol. 2003;60:1321–1325. doi: 10.1001/archneur.60.9.1321. [DOI] [PubMed] [Google Scholar]
- Homayoun H, Khavandgar S, Hoover JM, Mohsen AW, Voxkley J, Lacomis D, et al. Novel mutation in MYH7 gene associated with distal myopathy and cardiomyopathy. Neuromusc Disord. 2011;21:219–222. doi: 10.1016/j.nmd.2010.12.005. [DOI] [PubMed] [Google Scholar]
- Jandreski MA, Sole MJ, Liew CC. Two different forms of beta myosin heavy chain are expressed in human striated muscle. Hum Genet. 1987;77:127–31. doi: 10.1007/BF00272378. [DOI] [PubMed] [Google Scholar]
- Karkkainen S, Helio T, Jaaskelainen P, Miettinen R, Tuomainen P, Ylitalo K, et al. Two novel mutations in the beta-myosin heavy chain gene associated with dilated cardiomyopathy. Eur J Heart Fail. 2004;6:861–868. doi: 10.1016/j.ejheart.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Laing NG, Laing BA, Meredith C, Wilton SD, Robbins P, Honeyman K, et al. Autosomal dominant distal myopathy: linkage to chromosome 14. Am J Hum Genet. 1995;56:422–27. [PMC free article] [PubMed] [Google Scholar]
- Laing NG, Clarke NF, Dye DE, Liyanage K, Walker KR, Kobayashi Y, et al. Actin mutations are one cause of congenital fibre type disproportion. Ann Neurol. 2004;56:689–694. doi: 10.1002/ana.20260. [DOI] [PubMed] [Google Scholar]
- Lamont PJ, Udd B, Mastaglia FL, de Visser M, Hedera P, Voit T, et al. Laing early onset distal myopathy: slow myosin defect with variable abnormalities on muscle biopsy. J Neurol Neurosurg Psychiatry. 2006;77:208–15. doi: 10.1136/jnnp.2005.073825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrum MJ, Lee JM, Riley GR, Jang W, Rubinstein WS, Church DM, Maglott DR. ClinVar: public archive of relationships among sequence variation and human phenotype. Nucleic Acids Res. 2014 Jan 1;42(1):D980–5. doi: 10.1093/nar/gkt1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan AD, Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982;299:226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- Meredith C, Herrman R, Parry C, Liyanage K, Dye DE, Durling HJ, et al. Mutations in the slow skeletal muscle fibre myosin heavy chain gene (MYH7) cause Laing distal myopathy (MPD1) Am J Hum Genet. 2004;75:703–708. doi: 10.1086/424760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muelas N, Hackman P, Luque H, Garces-Sanchez M, Azorin I, Suominen T, et al. MYH7 gene tail mutation causing myopathic profiles beyond Laing distal myopathy. Neurol. 2010;75:732–741. doi: 10.1212/WNL.0b013e3181eee4d5. [DOI] [PubMed] [Google Scholar]
- Murgiano L, Tammen I, Harlizius B, Drogemuller C. A de novo germline mutation in MYH7 causes a progressive dominant myopathy in pigs. BMC Genet. 2012;13:99. doi: 10.1186/1471-2156-13-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermann WM, van der Ven PF, Steiner F, Weber K, Furst DO. Mapping of a myosin-binding domain and a regulatory phosphorylation site in M-protein, a structural protein of the sarcomeric M band. Molecular Biology of the Cell. 1998;9:829–840. doi: 10.1091/mbc.9.4.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortolano S, Tarrio R, Blanco-Arias P, Teijeira S, Rodriguez-Trelles F, Garcia-Murias M, et al. A novel MYH7 mutation links congenital fibre type disproportion and myosin storage myopathy. Neuromusc Disord. 2011;21:254–262. doi: 10.1016/j.nmd.2010.12.011. [DOI] [PubMed] [Google Scholar]
- O’Neil KT, DeGrado WF. A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids. Science. 1990;250:646–651. doi: 10.1126/science.2237415. [DOI] [PubMed] [Google Scholar]
- Overeem S, Schelhaas HJ, Blijham PJ, Grootscholten MI, ter Laak HJ, Timmermans J, et al. Symptomatic distal myopathy with cardiomyopathy due to a MYH7 mutation. Neuromusc Disord. 2007;17:490–493. doi: 10.1016/j.nmd.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Park JM, Kim YJ, Yoo JH, Hong YB, Park JH, Koo H, et al. A novel MYH7 mutation with prominent paraspinal and proximal muscle involvement. Neuromusc Disord. 2013;23:580–586. doi: 10.1016/j.nmd.2013.04.003. [DOI] [PubMed] [Google Scholar]
- Piela L, Nemethy G, Scheraga HA. Proline-induced constraints in alpha-helices. Biopolymers. 1987;26:1587–1600. doi: 10.1002/bip.360260910. [DOI] [PubMed] [Google Scholar]
- Raheem O, Huovinen S, Suominen T, Haapasalo H, Udd B. Novel myosin heavy chain immunohistochemical double staining developed for the routine diagnostic separation of I, IIA and IIX fibres. Acta Neuropathol. 2010;119:495–500. doi: 10.1007/s00401-010-0643-8. [DOI] [PubMed] [Google Scholar]
- Schoenauer R, Lange S, Hirschy A, Ehler E, Perriard JC, Agarkova I. Myomesin 3, a novel structural component of the M-band in striated muscle. Journal of molecular biology. 2008;376:338–351. doi: 10.1016/j.jmb.2007.11.048. [DOI] [PubMed] [Google Scholar]
- Smith TA, Steinert PM, Parry DA. Modelling effects of mutations in coiled-coil structures: case study using epidermolysis bullosa simplex mutations in segment 1a of K5/K14 intermediate filaments. Proteins. 2004;55:1043–1052. doi: 10.1002/prot.20089. [DOI] [PubMed] [Google Scholar]
- Sobrido MJ, Fernandez JM, Fontoira E, Cabello A, Castro M, Teijeira S, et al. Autosomal dominant congenital fibre type disproportion: a clinicopathological and imaging study of a large family. Brain. 2005;128:1716–1727. doi: 10.1093/brain/awh511. [DOI] [PubMed] [Google Scholar]
- Stalpers X, Verrips A, Braakhekke J, Lammens M, van den Wijngaard A, Mostert A. Scoliosis surgery in a patient with “de novo” myosin storage myopathy. Neuromusc Disord. 2011;21:812–815. doi: 10.1016/j.nmd.2011.05.005. [DOI] [PubMed] [Google Scholar]
- Tajsharghi H, Thornell LE, Lindberg C, Lindvall B, Henriksson KG, Oldfors A. Myosin storage myopathy associated with a heterozygous missense mutation in MYH7. Ann Neurol. 2003;54:494–500. doi: 10.1002/ana.10693. [DOI] [PubMed] [Google Scholar]
- Tajsharghi H, Oldfors A, Swash M. Myosin storage myopathy with cardiomyopathy. Neuromusc Disord. 2007;17:725. doi: 10.1016/j.nmd.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Tasca G, Ricci E, Penttilas S, Monforte M, Giglio V, Ottaviani P, et al. New phenotype and pathology features in MYH7-related distal myopathy. Neuromusc Disord. 2012;22:640–647. doi: 10.1016/j.nmd.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Udd B. 165th ENMC International Workshop: distal myopathies 6–8th February 2009 Naarden, The Netherlands. Neuromusc Disord. 2009;19:429–438. doi: 10.1016/j.nmd.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Udd B. Distal myopathies. In: Tawil RN, Venance S, editors. Neuromuscular Disorders. New York: Wiley & Blackwell; 2011. pp. 91–95. [Google Scholar]
- Uro-Coste E, Arne-Bes MC, Pellissier JF, Richard P, Levade T, Heitz F, et al. Striking phenotypic variability in two familial cases of myosin storage myopathy with a MYH7 Leu1793pro mutation. Neuromusc Disord. 2009;19:163–166. doi: 10.1016/j.nmd.2008.11.012. [DOI] [PubMed] [Google Scholar]
- Viguera E, Canceill D, Ehrlich SD. Replication slippage involves DNA polymerase pausing and dissociation. Embo J. 2001;20:2587–2595. doi: 10.1093/emboj/20.10.2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voit T, Kulz P, Leube B, Neuen-Jacob E, Schroder JM, Cavallotti D, et al. Autosomal dominant distal myopathy: further evidence of a chromosome 14 locus. Neuromusc Disord. 2001;11:11–19. doi: 10.1016/s0960-8966(00)00158-9. [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C, Lehtokari VL, Kalimo H, Paetau A, Nuutinen E, Hackman P, et al. Distal myopathy caused by homozygous missense mutations in the nebilun gene. Brain. 2007;130:1465–1476. doi: 10.1093/brain/awm094. [DOI] [PubMed] [Google Scholar]
- Walsh R, Rutland C, Thomas R, Loughna S. Cardiomyopathy: A systematic review of disease-causing mutations in myosin heavy chain 7 and their phenotypic manifestations. Cardiology. 2010;115(1):49–60. doi: 10.1159/000252808. [DOI] [PubMed] [Google Scholar]
- Weber JL. Informativeness of human (dC-dA)n. (dG-dT)n polymorphisms. Genomics. 1990;7:524–530. doi: 10.1016/0888-7543(90)90195-z. [DOI] [PubMed] [Google Scholar]
- Wildeman M, van Ophuizen E, den Dunnen JT, Taschner PE. Improving sequence variant descriptions in mutation data bases and literature using the Mutalizer sequence variation nomenclature checker. Hum Mutat. 2008;29:6–13. doi: 10.1002/humu.20654. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.