Abstract
Despite our understanding of hormonal influences on central nervous system (CNS) function, there is still much to learn about the pathogenesis of menstrual cycle-linked disorders. A growing literature suggests that the influence of sex steroids on neurological and psychiatric disorders is in part mediated by an aberrant CNS response to neuroactive steroids. Although sex steroids such as estradiol, progesterone, and the progesterone derivative allopregnanolone (ALLO) influence numerous neurotransmitter systems, it is their potent effect on the brain's primary inhibitory and excitatory neurotransmitters γ aminobutyric acid (GABA) and glutamate that links the study of premenstrual dysphoric disorder (PMDD) and catamenial epilepsy (CE). After providing an overview of these menstrual cycle-linked disorders, this article focuses on the preclinical and clinical research investigating the role of estradiol and progesterone (via ALLO) in the etiology of PMDD and CE. Through exploration of the phenomenological and neurobiological overlap between CE and PMDD, we aim to highlight areas for future research and development of treatments for menstrual cycle-linked neuropsychiatric disorders.
Keywords: Estradiol, Progesterone, Allopregnanolone, Catemenial epilepsy, Premenstrual dysphoric disorder, Neuropsychiatric disorder, Menstrual cycle-linked disorders, γ-Aminobutyric acid, Glutamate, Seizure disorder
1. Introduction
Among patients with epilepsy, lifetime unipolar major depression is considerably more common than in the general population, 32% versus 16%, respectively [1,2], and in patients with chronic medical illnesses such as asthma and chronic obstructive pulmonary disease [3]. Although, in general, women are at greater risk for unipolar major depressive disorder (MDD) [4], it is not yet known whether this sex bias for MDD holds true in epileptic populations. Aberrant interaction between ovarian hormones and the central nervous system (CNS) has been proffered as a potential contributor to the increased prevalence of depression in women and may be a common mechanism linking menstrual cycle-related disorders such as catemenial epilepsy (CE) and premenstrual dysphoric disorder (PMDD) [5–7]. Supporting the relationship between ovarian hormone fluctuations, seizure disorders, and depression is a recent study demonstrating that parturient women with epilepsy are more likely to suffer from postpartum depression than their healthy postpartum counterparts [8]. There are no studies to date investigating the relationship between MDD and CE in perimenopausal or menopausal women, although these disorders have been studied independently in this population. Harden [9] showed that perimenopausal hormone therapy and postmenopausal hormone therapy are both associated with seizure exacerbation, and it is well known that women are at greater risk for MDD during the menopausal transition [10–12].
Despite their common temporal features, the relationships between epilepsy, ovarian hormone fluctuations, and mood disorders are still not well understood. Perhaps the most promising line of investigation over recent years has been the focus on sex steroid modulation of neuronal excitability as it relates to normal brain function as well as seizure susceptibility and risk for behavioral disturbances [13,14]. Neuroactive steroids such as progesterone and estradiol influence numerous neurotransmitter systems including serotonin, norepinephrine, acetylcholine, and dopamine. However, it is their potent effects on the brain's primary inhibitory and excitatory neurotransmitters γ-aminobutyric acid (GABA) and glutamate, respectively, that link the study of CE with menstrual cycle disorders such as premenstrual syndrome (PMS) and PMDD and are the focus of this present article. Through exploration of the potential phenomenological and neurobiological overlap between CE and PMS/PMDD, we aim to highlight areas for future research and development of new treatments for menstrual cycle-related neuropsychiatric disorders.
2. Catamenial epilepsy
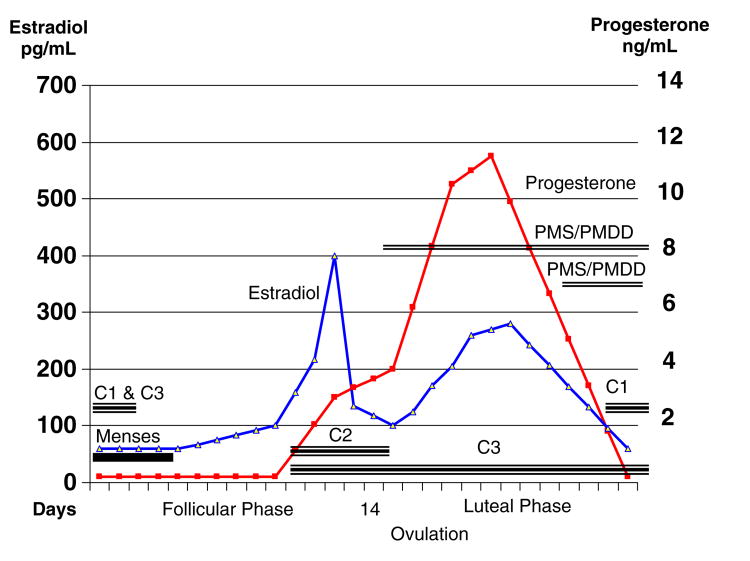
Catamenial epilepsy, which has been reported to affect 10–70% of women with focal and generalized epilepsy, refers to a menstrual cycle-related modification of a seizure disorder. The large variation in incidence is attributed to the lack of a uniform definition of CE prior to 1997 [5,6], as well as methodological differences in how seizure frequency is determined (patient self-report and retrospective reviews vs prospective assessments) [15]. In a well-designed study by Herzog et al. [7], seizure frequency was prospectively charted and plasma hormone levels were obtained in 184 women with intractable complex partial seizures. From these data, the most commonly accepted definition of CE was developed. Based on a twofold or greater increase in seizure frequency during a particular phase of the menstrual cycle, three patterns of CE were described [7]. In women with normal ovulatory cycles, there was a greater average daily seizure frequency in the perimenstrual phase (C1 pattern) and periovulatory phase (C2 pattern) compared with the midfollicular and midluteal phases. In those with anovulatory cycles, there was a greater average daily seizure frequency in the ovulatory, luteal, and menstrual phases compared with the midfollicular phase (C3 pattern) (see Fig. 1). According to their data, 30–40% of women with epilepsy experience catamenial exacerbation [7,16]. These data, however, are based on studies of women with refractory localized epilepsy and those who have been recruited into investigational or therapeutic trials of CE. Although these data were gathered prospectively and are not subject to biases inherent to retrospective study design, the women included in these studies represent a select population and may not reflect the prevalence of catamenial seizures in the general epileptic population. Although the etiology of CE is likely multifactorial and may involve fluctuations in blood levels of antiepileptic medications, changes in pH, water, and electrolyte imbalance [17], oscillations in ovarian hormones are thought to be a critical component in the pathogenesis of CE [15,18,19].
Fig. 1.
Premenstrual dysphoric disorder and catamenial epilepsy: symptom and seizure patterns. PMDD, Premenstrual Dysphoric Disorder; PMS, Premenstrual Syndrome; C1, Permenstrual seizure pattern; C2, Periovulatory seizure pattern; C3, Anovulatory seizure pattern.
3. Premenstrual dysphoric disorder
Premenstrual dysphoric disorder is a menstrual cycle-related mood disorder with an estimated prevalence of 1–7% [20]. This estimate is based on studies using prospective daily ratings, employing DSM-IV research criteria, and is much lower than reported in retrospective questionnaires. To fulfill the DSM-IV research criteria for PMDD, patients need to present with at least 5 of the following 11 symptoms, with one of the symptoms being in the first four: depression, irritability, anxiety/tension, affect lability, decreased interest, difficulty in concentrating, fatigue, feeling out of control, insomnia, change in appetite, breast tenderness and breast swelling. These symptoms must be severe enough to interfere with usual activities and must regularly occur during the last week of the luteal phase and remit within a few days of menses. Importantly, patients must be devoid of symptoms during the follicular phase to ensure that the premenstrual complaint is not an exacerbation of an underlying mood disorder. The diagnosis of PMDD must be confirmed by prospective daily diary ratings for at least 2 months to establish the temporal relationship between the onset of symptoms and the premenstrual period. Although the etiology of PMDD is not completely clear, Schmidt and colleagues [21] established the importance of sex steroids in symptom exacerbation in women with PMDD. In a double-blind cross-over design study, healthy women and those with PMDD underwent administration of leuprolide, a gonadotropin-releasing hormone agonist, to suppress ovulation for several months. This period of hypogonadism, which led to a significant reduction in PMDD symptoms, was followed by “add-back” estradiol or progesterone. Although hormone “add-back” had no effect on the healthy women, it led to a recurrence of sadness, anxiety, irritability, and impaired functioning in women with PMDD despite similar and physiological levels of plasma hormones in each group. These data suggest that women with PMDD have an aberrant CNS response to sex steroids, and this is now the prevailing theory for the etiology of PMDD symptoms [21].
4. Neuroactive steroids in CE
In general, estrogens and progesterone are thought to have opposing effects on seizure susceptibility, with estrogen being proconvulsant and progesterone being anticonvulsant [13]. The primary source of estradiol is the ovary, whereas progesterone is synthesized by the corpus luteum of the postovulatory ovary, the adrenals when under stress, and the placenta during pregnancy [22,23]. Estradiol, progesterone, and allopregnanolone (ALLO) synthesized in the periphery can cross the blood–brain barrier and modulate neuronal function, thus demonstrating “neuroactive” properties. However, the progesterone derivative ALLO is also considered a “neurosteroid,” as it is synthesized de novo in the CNS from cholesterol, primarily by glial mitochondria but also by some neurons. Impairment in mitochondrial function has been implicated in the pathophysiology of epilepsy. It is not yet known whether alteration in glial neurosteroidogenesis is a contributing factor. Although plasma steroids can pass into the brain, some investigators [24–27], but not all [28], have found discordant levels of neurosteroids in the CNS as measured in cerebrospinal fluid compared with the periphery, with CNS neurosteroid levels being orders of magnitude higher than plasma levels.
Neuroactive hormones are involved in a variety of biological functions throughout life, including gene expression (direct genomic effects), regulation of neurotransmitter release, and direct interactions with neurotransmitter receptors (nongenomic effects) [19]. The nongenomic effects of estrogens, which have a rapid onset and short duration of action, are likely implicated in the cyclic pattern of seizures associated with menstrual cycles [14]. Similar to estrogens, progesterone has genomic effects mediated by the parent steroid progesterone and nongenomic effects mediated by its neurosteroid derivatives ALLO and pregnanolone (PREG). Studies in animals have demonstrated that by disabling the genomic progesterone receptors via pharmacological agents or knockout mice, progesterone continues to have anticonvulsant effects in animal brains in the same way it does in animals without chemical or genetic manipulations [15,29]. Thus, the nongenomic effects of progesterone are likely to have greater implications in seizure frequency and severity in relation to the menstrual cycle than are the genomic effects of progesterone.
There are three biologically active estrogens: 17β-estradiol, estriol, and estrone. Each estrogen is considered the principal estrogen at different periods throughout life; that is, 17β-estradiol is the most potent estrogen in menstruating women, estriol is most abundant during pregnancy, and estrone is the major estrogen during menopause. Given that estradiol is the most potent and abundant form of estrogen in menstruating women, this form of estrogen is the most widely studied and thought to play a major role in the exacerbation of seizures in women with epilepsy [30].
The proconvulsant effects of estradiol have been demonstrated in both clinical and experimental observations in humans as well as animal models of epilepsy. A positive correlation between increased seizure frequency and estradiol-to-progesterone ratio during the perimenstrual and periovulatory phases compared with the midluteal phase has been observed in women with epilepsy [6]. Logothethis and colleagues [5] recorded increased EEG epileptiform activity, including increases in both spike frequency and seizures, when intravenous conjugated equine estrogens were infused during the premenstrual period in women with epilepsy. This observation was replicated by Jacono and colleagues [31] and led to the hypothesis that the increase in seizure activity observed in the periovulatory period is likely due to the estradiol surge that is relatively unopposed by progesterone during this portion of the menstrual cycle. This correlates with Herzog's clinical observations of the C2 and C3 patterns of CE [7] (Fig. 1). Increased seizure frequency in the C2 pattern in ovulating women and C3 pattern of anovulatory women is thought to be mediated by the proconvulsant effects of estradiol and lack of opposing progesterone in both of these contexts.
The epileptogenic properties of estradiol have been extensively studied in experimental animal models of epilepsy. Estradiol has been shown to have proconvulsant effects in ovariectomized rodents and to increase kindling audiogenic seizures and potentiate seizures induced by pentylenetetrazole, a GABAA receptor antagonist, and kainic acid, a non-NMDA ionotropic receptor agonist (for animal studies, see review by Reddy [13]). The mechanism by which estradiol increases neuronal excitability is not completely understood, but animal models have highlighted mechanisms that could be relevant to its proconvulsant effects. For example, there is evidence that estradiol increases NMDA receptor sensitivity to glutamate [32–35] and that increased sensitivity positively correlates with increased dendritic spine density in hippocampal CA1 pyramidal cells [35]. Another possible mechanism is that estradiol causes a downregulation of glutamic acid decarboxylase (GAD), the enzyme that mediates glutamate conversion to GABA, in ovariectomized rats [36]. Thus, exposure to estradiol in animal models is possibly facilitating excitatory neurotransmission in regions important in seizure vulnerability.
Progesterone is believed to have an important role in the pathogenesis of CE. As mentioned, progesterone is an endogenous precursor to lipophilic neuroactive steroids, ALLO and PREG, that can easily cross the blood–brain barrier in humans and modulate neuronal excitability via membrane-bound ion channels. The anticonvulsant effects of progesterone are likely mediated via its metabolite, ALLO. Progesterone is converted by 5α-reductase to 5α-dihydroprogesterone, which is then metabolized by 3α-hydroxysteroid dehydrogenase to form ALLO. ALLO acts on GABAA receptors and has anticonvulsant, anxiolytic, and anesthetic effects similar to the effects of benzodiazepines (BZDs), barbiturates, and alcohol [20,37].
In early studies in animals, Selye [38] first reported the anticonvulsant properties of progesterone in pentylenetetrazole-induced seizures. Over the past 60 years, this finding has been replicated in other animal models including amygdala kindling and maximal electroshock test as well as pentylenetetrazole-induced seizures [15]. The antiseizure effect of progesterone's metabolite, ALLO, was later confirmed by the pretreatment of animals with finasteride, a 5α-reductase inhibitor, prior to amygdala kindling and pentylenetetrazole administration, which resulted in the reduction of the antiseizure effect of progesterone (or ALLO) compared with non-pretreated animals [13]. This effect is also seen in genetically manipulated mice deficient in 5α-reductase, an enzyme important in the conversion of progesterone to ALLO [39].
Interestingly, women with epilepsy have a greater incidence of reproductive disorders that result in anovulatory cycles with low luteal phase levels of progesterone and ALLO. Polycystic ovarian syndrome and hypogonadotropic hypogonadism occur in 10–25 and 12% of women with temporal lobe epilepsy compared with 4–6 and 1.5% of the general population, respectively [40]. Although these reproductive disorders may partly be associated with the adverse effects of antiepileptic medications, they may also be a result of seizure-induced derangement of the hypothalamic circuitry that affects the release of gonadotropin-releasing hormone (for a review of these studies, see Fawley et al. [41]) and ultimately results in anovulation, low progesterone, and increased seizure frequency in the luteal phase. Restoration of the ovulatory cycle with clomiphene treatment is associated with a decrease in seizure frequency [42].
Although there are no studies to date showing a direct correlation between plasma progesterone levels and seizure exacerbation, given the anticonvulsant effects of progesterone and ALLO, it can be theorized that seizure frequency may be altered by progesterone levels during a normal menstrual cycle as suggested by the C1 catemenial seizure pattern described by Herzog et al. [7]. In patients with C1 pattern epilepsy, increased seizure frequency is thought to be mediated by the withdrawal of progesterone at the end of the luteal phase and subsequent changes in the GABAA receptor. Women with CE and ovulatory cycles have fewer seizures during the midluteal phase, when progesterone is highest, and have increased seizure frequency in the premenstrual phase, when progesterone levels abruptly decrease. The observed increase in seizure frequency as a result of declining progesterone levels resulted in further investigation of the possible proconvulsant effects of progesterone withdrawal. This effect has been confirmed in studies in both animals [37,43] and humans [13] and clinically correlates with the increased seizure susceptibility observed in alcohol and BZD withdrawal.
The mechanism underlying the effect of ALLO withdrawal is not completely understood but it is hypothesized that the reduction in ALLO increases seizure susceptibility via alteration of hippocampal GABAA receptor structure and function. Studies in animals have demonstrated that both chronic exposure to (i.e., 48 hours) and rapid removal of (i.e., “withdrawal”) ALLO increases the expression of a4 subunits of the GABAA receptor in several areas of the CNS, including CA1 pyramidal cells in the hippocampus [32]. Receptors containing the a4 subunit are relatively insensitive to BDZ modulation [43] and have faster deactivation kinetics [37], resulting in decreased neuronal inhibition and increased neuronal excitability, which correlates with increased anxiety-like behaviors [44] and seizure susceptibility [13]. Suggesting a common mechanism, animal models demonstrate similar increases in a4 subunit expression as a result of withdrawal from other potent allosteric GABAA modulators, such as ethanol and BDZ.
In addition to changes in 4α subunit expression of the GABAA receptor following hormone withdrawal, GABAA δ-subunit expression is also altered by changing levels of progesterone [45]. δ subunits of the GABAA receptors are expressed in extrasynaptic membranes and facilitate constant background inhibitory tone [46]. Maguire and colleagues [45] demonstrated a decrease in GABAA δ-subunit expression in the dentate gyrus during the estrous cycle in mice, when serum progesterone levels are relatively low. Altered membrane expression reduced tonic inhibition by 50%, which correlated with increased seizure susceptibility and anxiety-like behaviors in mice. During late diestrus, when progesterone levels are relatively high, the authors demonstrated increased expression of the δ-containing GABAA receptors, which correlated with increased tonic inhibition and decreased seizure susceptibility. Hormonal fluctuations influence δ-subunit GABAA receptor expression, which alters subtle, steady-state dampening of neuronal excitability and likely facilitates seizure susceptibility.
One would expect to find similar changes in the GABAA receptors during pregnancy or after delivery when major shifts in progesterone levels occur. In a series of animal experiments, Bitran and Smith [47] found that termination of pseudopregnancy by ovariectomy and subsequent withdrawal from ovarian steroids were associated with an increase in the number of BZD receptors and decreased GABA-stimulated chloride influx in the hippocampus of female rats. The behavioral correlate, measured with an elevated plus-maze, suggested that termination of pseudopregnancy by ovariectomy also precipitated anxiogenic-like effects in these female rats. Other studies in animals have also demonstrated pregnancy-related changes in other aspects of the GABAergic system. Smolen and colleagues [48] showed a significant reduction in GABA neurotransmitter turnover during parturition in mice, which may suggest that the GABAergic system is altered to serve as a compensatory anticonvulsant mechanism to offset the withdrawal of progesterone and ALLO after delivery [48].
Recently, Zhou and Smith [49] suggested that neurosteroid shifts, rather than absolute levels, play a critical role in triggering expression of the GABAA α4 subunit. Zhou and Smith [49] investigated the optimal conditions for steroid-induced upregulation of α4 expression in an in vitro model and found that ALLO and 17β-estradiol increase expression of the α4 subunit of the GABAA receptor, but that this effect is dependent on the background steroid milieu as well as the stage of neuronal development. This again highlights the potential importance of the extrasynaptic receptors in maintaining adequate CNS inhibitory tone [45].
5. Neuroactive steroids in PMS and PMDD
Examination of the relationship between hormonal levels and symptom development of PMS or PMDD across the menstrual cycle reveals a close link between hormonal changes occurring after ovulation and an increase in symptom severity during the luteal phase of the menstrual cycle [50]. However, some women experience peak symptoms of PMS or PMDD just prior to the onset of menses, when hormone levels are declining [51] (see Fig. 1). Furthermore, in a study of women with PMS, the late luteal phase was shortened by the administration of an anti-progesterone, mifepristone, and PMS symptoms still persisted [52]. These studies suggest that the temporal relationship between symptom onset and hormone fluctuation during the menstrual cycle cannot alone explain the pathogenesis of PMS and PMDD. However, the formation of the corpus luteum has been consistently reported to be required for symptom development. As expected, pharmacologically induced anovulation with a gonadotropin-releasing hormone agonist has been shown to improve mood in those with PMDD [53,54]. However, symptoms return in those with PMDD, but not in controls, once estrogen–progesterone therapy is added back [21]. This was interpreted as an abnormal hormonal response to normal hormonal fluctuations in women with PMDD.
Studies using hormone therapy in postmenopausal women have found estradiol and progesterone to result in different symptoms when administered as sequential hormone therapy compared with estrogen monotherapy [20]. Progesterone together with estrogen produces symptoms consistent with PMS, and higher doses of estrogen with progesterone have been shown to increase symptom severity. Estrogen alone, however, is an effective antidepressant in perimenopausal women with MDD [10–12] and may enhance overall well-being in menopausal women in general [50]. These data in humans are interesting and parallel nicely with the results of Murphy and Segal [36]. In their study, estradiol on its own resulted in increased dendritic spine density in ovariectomized rat. However, progesterone co-administration blocked the estradiol-induced increase in spine density.
The exact mechanism underlying the antidepressant effect of estradiol is unknown, but clinical and preclinical studies have shown estrogen to have multiple serotonin-enhancing effects [23,55–58]. Sequential estrogen and progesterone therapy resembles ovulatory cycles, and estrogen-only treatment is similar to anovulatory cycles [20]. Women taking sequential hormone therapy had a significant deterioration in mood and physical symptoms when progesterone was given at the end of the treatment cycle compared with women treated with estrogen only, who did not have any mood deterioration at the end of the treatment cycle, suggesting that estrogen alone has a CNS effect different from that of estrogen combined with progesterone or ALLO.
Studies examining the relationship between peripheral concentrations of neuroactive steroids and PMDD have yielded contradictory results. Most studies [59–61], but not all [62–64], have failed to show differences in plasma progesterone and estradiol (and ALLO) levels in patients with PMDD compared with healthy controls. Currently, there is no clear evidence to suggest a deficiency or excess of peripheral concentrations of neuroactive steroids in patients with PMDD. It is more likely that women with PMS and PMDD have an abnormal CNS response to neurosteroids and that this aberrant response occurs with normal cycle-to-cycle fluctuations in ALLO.
Although peripheral ALLO concentrations may not distinguish women with PMDD from their healthy counterparts, it has been suggested that peripheral concentrations of ALLO are associated with the severity of symptoms in PMDD patients. This theory is substantiated by data indicating that within the cycles of patients with PMDD, greater luteal phase concentrations of ALLO are associated with improved symptom ratings [61] and lower concentrations of ALLO during the luteal phase are associated with higher anxiety and irritability scores [65]. In contrast, Freeman et al. [66] found that improvement in symptoms in patients with PMDD treated with a selective serotonin reuptake inhibitor was associated with lower rather than higher levels of ALLO posttreatment. It is possible that these contradictory results can be explained by other factors, such as when and how neurosteroid levels were obtained/assayed and whether abnormal neurosteroid responses to stress and/or past psychiatric history were taken into consideration [65,67]. Studies have found that patients with PMS and PMDD, compared with healthy controls, have different psychological and neurosteroid responses to stress. Women with PMS/PMDD exhibit more distress in response to the same event during the premenstrual phase than in the postmenstrual phase, but they do not experience an increased frequency of stressful life events compared with healthy controls [68]. Those with PMDD have lower than expected stress-induced increased ALLO levels compared with healthy control subjects, 42% versus 83%, respectively [65], and a recent study of response to mental stress found that women with a history of depression (a group of women with PMDD and healthy controls) had blunted ALLO response to stress, whereas those in both groups without a history of depression did not [67]. Furthermore, this study found that in women with a history of depression there was no correlation between ALLO and progesterone levels, and ALLO response predicted severity of PMDD symptoms only in women with past depression. Thus, past depression may be a better predictor of dysregulated ALLO response to stress than PMDD, and further research is necessary regarding this relationship.
The most consistent overall finding in women with PMS and PMDD is an aberrant CNS response to neuroactive hormones and neurosteroids. The administration of oral contraceptives [69,70], estradiol–progesterone add-back therapy in women treated with gonadotropin-releasing hormone [21] and hormone therapy in postmenopausal women [71], has been shown to worsen mood symptoms in women with a history of PMS compared with healthy, appropriately matched controls. Perhaps the most informative finding is an association between mood deterioration during the luteal phase and reduced functional GABAA receptor sensitivity to positive allosteric GABAA modulators such as BZDs, alcohol, and neurosteroids in women with PMDD compared with healthy controls [72–75]. In addition, symptom severity correlated with a lesser sensitivity to neuroactive steroids and other GABAergic substances [72,76]. Interestingly, decreased sensitivity of the GABAA receptor during the luteal phase was not observed after women with PMDD were adequately treated with a selective serotonin reuptake inhibitor (SSRI). Following treatment with an SSRI, women with PMDD had improved symptoms and had a response to neurosteroids similar to that of women without PMDD [76]. Although blockade of serotonin reuptake into presynaptic nerve terminals may contribute to the efficacy of SSRIs in the treatment of PMDD [77], the primary therapeutic action of these agents in PMDD is hypothesized to be mediated through SSRI enhancement of 3α-hydroxysteroid dehydrogenase, the enzyme that facilitates the conversion of 5α-dihydroprogesterone to ALLO.
Animal models also demonstrate a decreased sensitivity to neurosteroids and BDZs and increased anxiety following ALLO withdrawal that coincide with increased expression of the GABAA α4 subunit in mouse CA1 hippocampus [43,78,79]. As previously discussed, it is likely that the upregulation of the hippocampal α4 subunit in the GABAA receptors results in decreased neuronal inhibition and promotes neuronal excitability, resulting in negative mood symptoms and seizures in those with PMDD and CE, respectively. Perhaps SSRI treatment leads to enhanced neurosteroidogenesis and, therefore, overall inhibitory tone, which “protects” the vulnerable individual from aberrant GABAergic responses to steroid shifts.
Alterations in inhibitory and excitatory tone over the menstrual cycle have been demonstrated in women with PMS/PMDD and healthy controls using transcranial magnetic stimulation (TMS) and an acoustic startle response. During the follicular phase both women with PMS and healthy controls showed similar evoked potentials measured by TMS, but during the luteal phase, healthy women showed more inhibition and subjects with PMS showed fasciculations [80]. These findings suggest that GABA inhibitory tone is enhanced during the luteal phase in healthy women and decreased in women with PMDD. Another possibility is that women with PMDD have enhanced excitatory tone during the luteal phase. By employing an acoustic startle paradigm (a measure of physiological arousal to a mildly adverse stimulus), Epperson et al. [81] demonstrated a heightened magnitude of acoustic startle response, but not increased response to affective stimuli, in women with PMDD compared with healthy controls, during the luteal phase of the menstrual cycle compared with the follicular phase. These data suggest that the neurosteroid milieu has a powerful modulatory effect on physiological reactivity and arousal in women with PMDD, but not in healthy controls. Alternatively, these findings could represent a failure in women with PMDD to appropriately respond to the normal balancing effects of hormones on physiological arousal. These data are further supported by studies in which, after induction of panic attacks via lactate infusion and carbon dioxide, the incidence of panic attacks was much higher in those with PMDD than in controls [82,83]. The normal physiological response to affective stimuli in all women, described in Epperson and colleagues' 2007 study [81], is interesting and may suggest that the abnormal neurosteroid milieu is mediating the negative mood state and affecting the physiological response in women with PMDD as opposed to the negative mood state altering physiology. These data, however, are preliminary; replication and further study of acoustic startle response in women with PMDD are needed to draw definitive conclusions.
Proton magnetic resonance spectroscopy (1H MRS) is another method recently used to examine the relationship between GABA and neurosteroids in women with PMDD. Epperson et al. [59] found that healthy women demonstrate a cyclic decline in cortical GABA from a peak in the follicular phase to a trough in the late luteal phase as estradiol, progesterone, and ALLO increase. Women with PMDD, however, do not show this decline in GABA over the menstrual cycle or a relationship between GABA and plasma neuroactive steroids, which suggests an aberrant relationship between this neurotransmitter system and neuroactive steroids in women with PMDD.
6. Treatment for CE and PMDD: Is there a connection?
Foremost, sound diagnostic procedures are mandatory prior to the administration of treatment. The DSM-IV provides research diagnostic criteria for PMDD, and Herzog [7] has established an accepted definition of CE. Prospective daily mood and seizure charting relative to the menstrual cycle is compulsory in the diagnosis of PMDD and CE, respectively (see Table 1).
Table 1. Daily record of severity of problems.
| Date (month/day/year): |
|---|
| Note if spotting or menses with S or M: |
| 1. Felt depressed, sad, “down,” or “blue”; or felt hopeless; or felt worthless or guilty |
| 2. Felt anxious, tense, “keyed up,” or on edge |
| 3. Had mood swings (i.e., suddenly feeling sad or tearful) or was sensitive to rejection or feelings were easily hurt |
| 4. Felt angry or irritable |
| 5. Had less interest in usual activities (work, school, friends, hobbies) |
| 6. Had difficulty concentrating |
| 7. Felt lethargic, tired, or fatigued; or had a lack of energy |
| 8. Had increased appetite or overate; or had cravings for specific foods |
| 9. Slept more, took naps, found it hard to get up when intended; or had trouble getting to sleep or staying asleep |
| 10. Felt overwhelmed or unable to cope or felt out of control |
| 11. Had breast tenderness, breast swelling, bloated sensation, weight gain, headache, joint or muscle pain, or other physical symptoms |
| 12. At work, school, home, or in daily routine, at least one of the problems noted above caused reduction of productivity or inefficiency |
| 13. At least one of the problems noted above caused avoidance of or less participation in hobbies or social activities |
| 14. At least one of the problems noted above interfered with relationships with others |
Note the degree to which you experience each of the problems listed below. Complete this assessment in the evening only prior to bedtime. Enter the number that corresponds to the severity as noted here: 1 = not at all, 4 = moderate, 2 = minimal, 5 = severe, 3 = mild, 6 = extreme.
The C1 and C2 patterns of CE and PMDD occur in those with ovulatory cycles, but ovulation is not necessary for symptom development in the C3 pattern of CE. Thus, the C1 and C2 patterns of CE may be more relevant to PMDD than the C3 pattern of CE. Although it is likely that ovulation is not the only factor mediating symptoms of PMDD and CE, therapeutic studies have focused on the manipulation of ovulation in the treatment of PMDD and CE.
Induction of anovulation via high-dose synthetic gonadotropin-releasing hormone (GnRH) has been shown to decrease premenstrual symptoms [20] and seizure frequency [84]. Treatment is, however, temporary, as GnRH agonists cannot be taken for more than 6–9 months due to the adverse effect of hypoestrogenism and its consequences for bone mineral density, menopausal and postmenopausal symptoms, and possibly cognitive function. Adding back estradiol and progesterone to GnRH therapy will alleviate the adverse effects of hypoestrogenism, but Schmidt and colleagues [21] demonstrated, in a double-blind cross-over study design, that although adding back estradiol and progesterone had no effect in healthy controls, it led to the recurrence of negative mood symptoms in women with PMDD.
Combined oral contraception pills (COCPs) also induce anovulation; thus, theoretically, they are a reasonable choice for the treatment of PMDD and CE. However, the effectiveness of COCPs for the treatment of these disorders has been controversial and currently there is no consensus on the use of COCPs in the treatment of PMDD and CE.
Multiple studies have investigated the relationship between COCPs and mood with both positive and negative results (see review article by Oinonen and Mazmanian [85]). This variability may be a result of not distinguishing between healthy subjects and subjects with PMS and not controlling for different types of COCPs (monophasic, biphasic, or triphasic) or the type of progestin derivative contained in the COCPs (estranes or gonanes).
Triphasic and monophasic COCPs were compared in women with and without PMS, and more negative mood changes were reported in women with PMS taking triphasic compared with monophasic preparations [86–88]. This finding is consistent with the literature, which suggests that women with PMDD have an abnormal CNS response to fluctuations in neurosteroids; therefore, we would expect women with PMDD to experience more negative mood symptoms in response to triphasic preparations in which the level of progestin is fluctuating.
COCPs containing ethinyl estradiol and drospirenone (EE-DRSP; Yasmin and Yaz 24) have recently been shown to be beneficial in the treatment of PMDD. In a double-blind placebo-controlled study by Freeman et al. [89], 82 women who were diagnosed with PMDD according to DSM-IV criteria were randomly assigned to receive either EE-DRSP or placebo. Following three menstrual cycles, there was a 10% greater reduction in PMS symptoms in the EE-DRSP group than in the placebo group. Despite an adequate study design to assess the efficacy of EE-DRSP for the treatment of PMDD, there was a large placebo response rate (43%). Subsequently, two randomized placebo-controlled multicenter trials, using a placebo run in cycle to exclude placebo responders, showed a 50% reduction in Daily Record of Severity of Problems scores from baseline in women meeting DSM-IV criteria for PMDD [90].
The mechanism by which EE-DRSP is therapeutic in the treatment of PMDD is unknown, but there is evidence to suggest that EE-DRSP decreases the neurosteroid dihydroepiandrosterone sulfate (DHEAS) [91], a GABAA receptor antagonist [92], and NMDA receptor agonist [93,94]. Although COCPs including EE-DRSP also lower progesterone and potent GABAA receptor agonists such as ALLO [91,95], EE-DRSP concomitantly decreases levels of DHEAS, which may lead to a more effective balance between neuronal excitation and inhibition. It has also been hypothesized that DRSP differs from synthetic progestins contained in most COCPs, and its pharmacological profile in preclinical studies has shown it to be closer to natural progesterone. Thus, it is possible that DRSP has a more anxiolytic effect similar to ALLO compared with synthetic progestin and, thus, is helpful in alleviating PMDD symptoms. In the same vein, ethynodiol diacetate is a progestin that is converted to ALLO [96] and, therefore, would be a reasonable progestin preparation to include in future studies of COCPs in the treatment of PMDD.
There are far fewer studies evaluating the benefit of COCPs in women with CE compared with women with PMS/PMDD. In one small study, an oral contraceptive medication containing norethisterone was found to be ineffective in women with CE [97]. Given the numerous interactions between COCPs and hepatic microsomal-inducing antiepileptic drugs (phenytoin, barbiturates, carbamazepine, topiramate (doses >200 mg/day), oxcarbazepine, and lamotrigine) [98], as well as the seizure-provoking potential of estrogen, COCPs are unlikely to be a beneficial treatment for CE.
Synthetic progestogens without estrogen have been used for the treatment of PMS/PMDD and CE. Medroxyprogesterone acetate (MPA) is a contraceptive agent that has been used for the treatment of uncontrolled catamenial seizures in small open-label studies. Mattson et al. [99] added MPA to the antiepileptic drug regimen of 14 women who had uncontrolled seizures. All women initially took a pill containing 10 mg MPA two to four times each day. Six women who experienced amenorrhea later received 120-to 150-mg intramuscular MPA injections every 6–12 weeks instead of oral MPA. Overall reductions in seizure frequency averaged 30%. Of the 11 women who developed amenorrhea, 7 reported fewer seizures during MPA therapy. In a subsequent small investigation, Mattson and colleagues administered MPA to women in addition to their usual antiepileptic drug regimen and followed seizure frequency prospectively for a year. Seizure frequency fell significantly from a mean of 8.3 seizures/month before MPA administration to 5.1 seizures/month after MPA administration, equaling 39% fewer seizures. Seven of the 11 women who experienced obvious improvement had 52% fewer seizures, and MPA was most helpful in women when menses was eliminated. Although these results show some modest effects on seizure reduction, the study sample is small and includes biases inherent to open, nonrandomized studies. These data also emphasize that progestogen-induced ablation of ovulation (likely in those without menses) was associated with the greatest reductions in seizure frequency. This observation, together with the inability of MPA to be converted to ALLO, indicates that the potential effectiveness of MPA resides in its ability to eliminate ovulation without the exposure to estrogens that is unavoidable when using COCPs to eliminate ovulation. Unlike GnRH agonists, which also eliminate ovulation, the use of MPA is not limited by hypoestrogenism [100], suggesting that such progestogens, or preferably natural progesterone (Prometruim), may prove useful in the treatment of CE.
The rationale for the use of progestogens as well as progesterone in women with PMDD is based on the unsubstantiated premise that progesterone deficiency is the cause of PMS/PMDD [101]. Wyatt et al. [101] conducted a systematic review of published randomized, placebo-controlled trials evaluating the efficacy of progestogens and progesterone in the management of PMS. In the four trials of progestogen therapy (378 women) reported, a statistically, but not clinically, significant improvement was noted in women taking progestogens compared with those taking placebo, with an odds ratio of 1.07 (1.03 to 1.11). The modest reduction in seizure frequency with progestogens in CE and clinically insignificant response to progestogens in PMDD could possibly be explained by the fact that synthetic progestogens are not converted to ALLO. Without ALLO's modulation of GABAA receptors, it is unlikely that progestogens could decrease seizure frequency or have anxiolytic effects in those with CE and PMDD, respectively.
Given that progesterone is converted to ALLO, it would seem likely that natural progesterone could be a promising treatment for CE and PMDD. Progesterone has been shown to be beneficial in the treatment of women with catamenial seizures in mostly open trials with very few subjects. Herzog et al. [102] administered progesterone lozenges, 200 mg three times daily, on Days 23 to 25 of each menstrual cycle to women with perimenstrual seizure exacerbation, and on Days 15 to 25 of each menstrual cycle to women with luteal phase seizure exacerbation. Progesterone was subsequently tapered and discontinued by Days 25 to 28. Although the design was open-label, one of the study strengths is that women prospectively documented seizure frequency and menstrual cycles. Eighteen women (72%) experienced a decline in seizure frequency during a 3-month treatment period compared with their seizure frequency 3 months prior to therapy. On average, daily seizure frequency declined by at least 50% [102]. In a 3-year follow-up to this study, 15 women who remained on progesterone therapy and their original antiepileptic drugs continued to show improved seizure control in comparison to their own baseline [103]. The interpretation of these data is limited by the open design and the likelihood that the 15 women who remained on the original treatment regimen probably represent those who had the most favorable response. Despite study limitations, these findings represented preliminary evidence that natural progesterone may be helpful in the treatment of CE. According to the National Institutes of Health website (http://crisp.cit.nih.gov), a prospective, randomized, placebo-controlled, double-blind investigation is underway to evaluate the efficacy of natural progesterone for the treatment of CE.
Controlled studies evaluated the efficacy of ganaxolone, a synthetic neurosteroid analog of ALLO with positive allosteric effects on GABAA receptors, for the treatment of seizures. It has been shown to be an effective anticonvulsant in animal models of epilepsy and to have therapeutic activity in adult patients with partial-onset seizures and in epileptic children with a history of infantile spasms. It is currently undergoing further development in infants with newly diagnosed infantile spasms, in adults with refractory partial onset seizures, and in women with CE [104].
Although it would appear that progesterone is a potential treatment for CE in open-label studies, early findings from open-label studies suggesting that progesterone was an effective treatment for PMS/PMDD were not substantiated in controlled clinical trials. Administration of oral micronized progesterone results in variable plasma levels of progesterone, ALLO, and PREG [105] and had inconsistent results in the treatment of PMS/PMDD in placebo-controlled trials [106]. A systematic review of 10 published randomized, placebo-controlled trials evaluating the efficacy of progesterone therapy (531 women) found little support for the use of progesterone in the management of premenstrual syndrome [101].
Given the anxiolytic effects of ALLO, it is surprising that progesterone therapy is not an effective treatment for PMDD. One possible explanation for these negative findings is that peripheral levels of ALLO may not be an accurate gauge of CNS levels. Another possibility is that women with PMDD have aberrant GABAA receptors or less than ideal subunit configurations and, thus, are more tolerant of ALLO and its anxiolytic effect during the luteal phase. In support of this is Sundstrom and colleagues' [74] finding that women with PMS/PMDD have a greater tolerance of the effects of ALLO during the luteal phase compared with women without PMS/PMDD. Perhaps the most interesting finding by Sundstrom et al. is that women with PMDD treated with SSRIs show a similar response to ALLO as women without PMDD [74]. Studies in animals have demonstrated an increase in brain content of ALLO, but not other neurosteroids, in response to fluoxetine and paroxetine [107]. Subsequently, in a study of unipolar depressed patients, cerebrospinal fluid ALLO content was found to be 60% lower than in appropriately matched controls, but following 8–10 weeks of treatment with either fluoxetine or fluvoxamine, cerebrospinal fluid ALLO content normalized and was similar to that of controls [108]. SSRIs, that is, fluoxetine, sertraline, and paroxetine, are Food and Drug Administration-approved medications for the treatment of PMDD. Although SSRIs are still thought to exert their therapeutic effect via the serotonergic neurotransmitter system, studies in animals and clinical evidence suggest that SSRIs may also be beneficial in the treatment of anxiety and mood disorders, in part, by increasing the availability or potency of neuroactive GABA-ergic steroids [109]. The mechanism by which this occurs is unknown; however, one hypothesis is that the therapeutic action of SSRIs is mediated through their effect on 3a-hydroxysteroid dehydrogenase enzyme, which facilitates the conversion of 5α-dihydroprogesterone to ALLO. It is possible that women with PMDD are deficient in this enzyme and/or ALLO and SSRIs are helping restore an adequate level of ALLO. Alternatively, ALLO-induced changes in GABAA receptor subunit configuration may lead to paradoxical anxiogenic effects of certain levels of ALLO [44].
BZDs are also potent allosteric modulators of GABAA receptors that increase inhibitory neurotransmission and thus have antiseizure and anxiolytic effects that may play an important role in the treatment of CE and PMDD. Although data to support the efficacy of BZDs in the treatment of CE and PMDD are limited, clobazam and alprazolam have been found to be superior to placebo in a published double-blind placebo-controlled study on the treatment of CE and PMDD, respectively [106,110]. In addition, BZDs are frequently used in clinical practice. Clinical practice and data from controlled studies seem to support the hypothesis that modulation of the GABAA receptor plays a significant role in the etiology of CE and PMDD. Their clinical utility in treating seizures has been limited by tolerance to their antiseizure effect with chronic exposure and, possibly, cross-tolerance to neuroactive steroids [111]. Clobazam, a 1, 5-BZD, is efficacious as an add-on therapy in those with monotherapy-resistant seizures, catamenial seizures, and cluster seizures [110]. Data to support the use of clobazam for the treatment of catamenial seizures are from a small, double-blind, placebo-controlled, cross-over design study that found clobazam to be superior to placebo. However, the criteria used to define those with CE were unclear [112]. Despite the relatively positive findings of this study, clobazam is not frequently used in clinical practice possibly because of the potential for abuse or dependence associated with this class of medications. Larger, well-defined study samples are needed to determine the potential risks and confirm the potential benefits of this medication.
When anxiety symptoms are an outstanding feature of PMDD, anti-anxiety drugs such as alprazolam, clonazepam, and lorazepam are prescribed. Alprazolam, a high-potency BZD with mood-enhancing and anxiolytic effects, has been shown to be somewhat effective in patients with PMS. In a double-blind placebo-controlled trial, 185 women with PMS were randomized to oral micronized progesterone, alprazolam, or placebo. Alprazolam was significantly better than placebo or progesterone for total premenstrual symptoms, daily symptom report (DSR), factors of mental function, pain, and mood. Thirty-seven percent of the alprazolam group experienced a 50% reduction in total DSR scores [106]. The advantages of BZDs are that they tend to work quickly and may be used either as needed or regularly. Dependence and tolerance are occasional problems with these drugs, but Freeman [106] found no clinically significant withdrawal symptoms when alprazolam administration was restricted to the luteal phase.
7. Future investigations for the treatment of PMDD and CE
Although there is a relative consensus on how to define and diagnose PMDD and CE, there are few effective treatment strategies. GnRH agonists are helpful but their clinical utility is limited by adverse effects, particularly with prolonged use. Effective treatment strategies thus far for both PMDD and CE involve modulation of the GABAA receptor. BZDs, ganaxolone, and oral micronized progesterone are all positive allosteric modulators of the GABAA receptor and, depending on how the medication/hormone is dosed, appear to alleviate some PMDD and CE symptoms in some subjects. Future research is needed to investigate the efficacy of progestogens and certain COCP preparations in the treatment of CE and PMDD as the pharmacological profile of these agents with respect to CNS effects is highly variable.
SSRIs are thought to act by increasing ALLO levels, in addition to blocking the serotonin transporter, and are clearly beneficial for the treatment of PMDD in continuous or intermittent (luteal phase) dosing [113]. Whether SSRIs could play a role in the treatment of CE as well is unknown. However, some SSRIs, that is, fluoxetine, have anticonvulsant effects in animal models [114], and noradrenergic and serotonergic deficiencies have been found to contribute to seizure predisposition [115], suggesting that antidepressants may have the potential to decrease seizure susceptibility in patients with epilepsy.
Recently, a different class of antidepressants, serotonin–norepinephrine reuptake inhibitors (SNRIs), have been studied in the treatment of PMDD. In a double-blind placebo-controlled trial, venlafaxine was significantly more efficacious than placebo for treatment of PMDD [89]. Further studies are needed to evaluate the potential of intermittent venlafaxine dosing for the treatment of PMDD. Another prospect for investigation is the use of YAZ 24 in the treatment of CE. In addition to inducing anovulation, which has been shown to be effective in the treatment of CE, YAZ 24 also decreases DHEAS. DHEAS has been demonstrated to have excitatory and proconvulsant effects in studies in animals, which is likely due to its antagonistic actions at the GABAA receptor [13,116]. In addition to its GABAA-antagonistic properties, DHEAS also positively modulates NMDA receptors and potentiates an excitatory CNS response [94,117]. By decreasing the excitatory CNS effect of DHEAS, YAZ 24 could potentially be an effective treatment for CE and possibly a productive area for future investigations.
8. Summary
In conclusion, studies in both humans and animals provide evidence that the effects of ovarian steroids on inhibition via the GABA system and excitation via the glutamate system influence anxiety state and seizure susceptibility. The effect of neurosteroids on the GABAA receptor and glutamate system results in behavioral outcomes relevant for PMDD and CE. Despite our understanding of hormonal influences on CNS function, there is still much to learn about the pathogenesis and treatment of menstrual cycle-linked disorders.
GnRH therapy has been shown to have antiseizure effects and to reduce symptoms of PMDD, but long-term use is limited by adverse effects. COCPs have mostly been studied in PMDD. COCP preparations containing EEDRSP are FDA-approved for the treatment of PMDD, but their therapeutic effect has yet to be studied in those with CE. SSRIs and a SNRI (venlafaxine) are also FDA-approved for the treatment of PMDD and might be an interesting area for research for the treatment of CE. Synthetic progestin and natural progesterone treatment have been studied in small open trials and have yielded optimistic results for the treatment of CE. Whether these findings will be confirmed in an ongoing randomized clinical trial is not yet known.
Noninvasive techniques such as MRS, TMS, and acoustic startle may assist us in understanding the neuroendocrine-induced changes in the balance between neuronal excitation and inhibition in women with PMDD and CE. These and other investigative tools can contribute to the examination of potential disruptions in the GABAergic system thought to be critical to the pathogenesis of menstrual cycle-related fluctuations in mood and seizure frequency.
References
- 1.Hermann BP, Seidenberg M, Bell B. Psychiatric comorbidity in chronic epilepsy: identification, consequences, and treatment of major depression. Epilepsia. 2000;41(Suppl. 2):S31–41. doi: 10.1111/j.1528-1157.2000.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12 month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:617–27. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipcić I, Popović-Grle S, Marcinko D, et al. Screening for depression disorders in patients with chronic somatic illness. Coll Antropol. 2007;31:139–43. [PubMed] [Google Scholar]
- 4.Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey: I Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29:85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- 5.Logothethis J, Harner R, Morrell R, Torres F. The role of estrogens in catamenial exacerbation of epilepsy. Neurology. 1959;9:352–60. doi: 10.1212/wnl.9.5.352. [DOI] [PubMed] [Google Scholar]
- 6.Backstrom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–47. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- 7.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–8. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 8.Turner KP, Franza A, Fumarola C, et al. Postpartum depression in women with epilepsy versus women without epilepsy. Epilepsy Behav. 2006;9:293–7. doi: 10.1016/j.yebeh.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 9.Harden C. The effect of menopause and perimenopause on the course of epilepsy. Epilepsia. 1999;40:1402. doi: 10.1111/j.1528-1157.1999.tb02012.x. [DOI] [PubMed] [Google Scholar]
- 10.Freeman EW, Sammel MD, Liu L, Garcia CR, Nelson D, Hollander L. Hormones and menopausal status as predictors of depression in women in transition to menopause. Arch Gen Psychiatry. 2004;61:62–70. doi: 10.1001/archpsyc.61.1.62. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt PJ. Mood, depression, and reproductive hormones in the menopausal transition. Am J Med. 2005;118(12B):54S–8S. doi: 10.1016/j.amjmed.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 12.Soares CN, Prauty, Born L, Steiner M. Treatment of menopauserelated mood disturbances. CNS Spectr. 2005;10:489–97. doi: 10.1017/s109285290002318x. [DOI] [PubMed] [Google Scholar]
- 13.Reddy DS. Pharmacology of endogenous neuroactive steroids. Crit Rev Neurobiol. 2003;15:179–234. doi: 10.1615/critrevneurobiol.v15.i34.20. [DOI] [PubMed] [Google Scholar]
- 14.Backstrom T, Andersson A, Andree L, et al. Pathogenesis of menstrual cycle-linked CNS disorders. Ann NY Acad Sci. 2003;1007:42–53. doi: 10.1196/annals.1286.005. [DOI] [PubMed] [Google Scholar]
- 15.Reddy DS. Role of neurosteroids in catamenial epilepsy. Epilepsy Res. 2004;62:99–118. doi: 10.1016/j.eplepsyres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 16.Herzog AG, Harden CL, Liporace J, et al. Frequency of catamenial seizure exacerbation in women with localization–related epilepsy. Ann Neurol. 2004;56:431–4. doi: 10.1002/ana.20214. [DOI] [PubMed] [Google Scholar]
- 17.Foldvary-Schaefer N, Falcone T. Catamenial epilepsy: pathophysiology, diagnosis, and management. Neurology. 2003;61:S2–S15. doi: 10.1212/wnl.61.6_suppl_2.s2. [DOI] [PubMed] [Google Scholar]
- 18.Scharfman HE, MacLusky NJ. The influence of gonadal hormones on neuronal excitability, seizures, and epilepsy in the female. Epilepsia. 2006;47:1423–40. doi: 10.1111/j.1528-1167.2006.00672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veliskova J. Estrogens and epilepsy: why are we so excited? Neuroscientist. 2007;13:77–88. doi: 10.1177/1073858406295827. [DOI] [PubMed] [Google Scholar]
- 20.Backstrom T, Andree L, Birzniece V, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs. 2003;17:325–42. doi: 10.2165/00023210-200317050-00003. [DOI] [PubMed] [Google Scholar]
- 21.Schmidt PJ, Nieman LK, Danaceau MA, Adams LF, Rubinow DR. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med. 1998;338:209–16. doi: 10.1056/NEJM199801223380401. [DOI] [PubMed] [Google Scholar]
- 22.Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- 23.Amin Z, Mason GF, Cavus I, Krystal JH, Rothman DL, Epperson CN. Interaction of neuroactive steroids and GABA in the development of neuropsychiatric disorders in women. Pharmacol Biochem Behav. 2006;84:635–43. doi: 10.1016/j.pbb.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 24.Backstrom T, Gee KW, Lan N, Sorensen M, Wahlstrom G. Steroids in relation to epilepsy and anaesthesia. Ciba Found Symp. 1990;153:225–30. doi: 10.1002/9780470513989.ch13. [DOI] [PubMed] [Google Scholar]
- 25.Cheney DL, Uzunov D, Costa E, Guidotti A. Gas chromatographic–mass fragmentographic quantitation of 3 alpha-hydroxy-5 alpha-pregnan-20-one (allopregnanolone) and its precursors in blood and brain of adrenalectomized and castrated rats. J Neurosci. 1995;15:4641–50. doi: 10.1523/JNEUROSCI.15-06-04641.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schumacher M, Weill-Engerer S, Liere P, et al. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Rasmusson AM, Pinna G, Paliwal P, et al. Decreased cerebrospinal fluid allogregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–13. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 28.Kim YS, Zhang H, Kim HY. Profiling neurosteroids in cerebrospinal fluids and plasma by gas chromatography/electron capture negative chemical ionization mass spectrometry. Anal Biochem. 2000;277:187–95. doi: 10.1006/abio.1999.4384. [DOI] [PubMed] [Google Scholar]
- 29.Mohammed S, Abolhassan A, Pourgholami MH. Evaluation of the anticonvulsant profile of progesterone in male amygdala-kindled rats. Epilepsy Res. 1998;30:195–202. doi: 10.1016/s0920-1211(98)00004-7. [DOI] [PubMed] [Google Scholar]
- 30.Morrell MJ. Epilepsy in women: the science of why it is special. Neurology. 1999;53(4 Suppl 1):S42–8. [PubMed] [Google Scholar]
- 31.Jacono JJ, Robertson JM. The effects of estrogen, progesterone, and ionized calcium on seizures during the menstrual cycle of epileptic women. Epilepsia. 1987;28:571–7. doi: 10.1111/j.1528-1157.1987.tb03690.x. [DOI] [PubMed] [Google Scholar]
- 32.Smith BD, Waterhouse DJ, Woodward SS. Locally applied estrogens potentiate glutamate-evoked excitation of cerebellar Purkinje cells. Brain Res. 1988;475:272–82. doi: 10.1016/0006-8993(88)90615-4. [DOI] [PubMed] [Google Scholar]
- 33.Wong M, Moss RL. Patch-clamp analysis of direct steroidal modulation of glutamate receptor-channels. J Neuroendocrinol. 1994;6:347–55. doi: 10.1111/j.1365-2826.1994.tb00592.x. [DOI] [PubMed] [Google Scholar]
- 34.Woolley CS, Weiland NG, McEwen BS, Schwartzkroin PA. Estradiol increases the sensitivity of hippocampal CA1 pyramidal cells to NMDA receptor-mediated synaptic input: correlation with dendritic spine density. J Neurosci. 1997;17:1848–59. doi: 10.1523/JNEUROSCI.17-05-01848.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woolley CS, McEwen BS. Estradiol mediates fluctuation in hippocampal synapse density during the estrous cycle in the adult rat. J Neurosci. 1992;12:2549–54. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Murphy DD, Segal M. Progesterone prevents estradiol-induced dendritic spine formation in cultured hippocampal neurons. Neuroendocrinology. 2000;72:133–43. doi: 10.1159/000054580. [DOI] [PubMed] [Google Scholar]
- 37.Smith SS. Withdrawal properties of a neuroactive steroid: implications for GABA(A) receptor gene regulation in the brain and anxiety behavior. Steroids. 2002;67:519–28. doi: 10.1016/s0039-128x(01)00170-2. [DOI] [PubMed] [Google Scholar]
- 38.Selye H. The antagonism between anesthetic steroid hormones and pentamethylenetetrazol (metrazol) J Lab Clin Med. 1942;27:1051–3. [Google Scholar]
- 39.Frye CA, Rhodes ME, Walf A, Harney J. Progesterone reduces phentylenetetrazol-induced ictal activity of wild type mice but not those deficient in type I 5α-reductase. Epilepsia. 2002;43(Suppl. 5):14–7. doi: 10.1046/j.1528-1157.43.s.5.19.x. [DOI] [PubMed] [Google Scholar]
- 40.Herzog AG, Seibel MM, Schomer DL, Vaitukaitis JL, Geschwind N. Reproductive endocrine disorders in women with partial seizures of temporal lobe origin. Arch Neurol. 1986;43:341–6. doi: 10.1001/archneur.1986.00520040029014. [DOI] [PubMed] [Google Scholar]
- 41.Fawley JA, Pouliotet WA, Dudek FE. Epilepsy and reproductive disorders: the role of the gonadotropin-releasing hormone network. Epilepsy Behav. 2006;8:477–82. doi: 10.1016/j.yebeh.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 42.Herzog AG. Clomiphene therapy in epileptic women with menstrual disorders. Neurology. 1988;38:432–4. doi: 10.1212/wnl.38.3.432. [DOI] [PubMed] [Google Scholar]
- 43.Gulinello M, Gong QH, Smith SS. Progesterone withdrawal increases the alpha4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology: a comparison with female rats. Neuropharmacology. 2002;43:701–14. doi: 10.1016/s0028-3908(02)00171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SS, Ruderman Y, Frye C, Homanics G, Yuan M. Steroid withdrawal in the mouse results in anxiogenic effects of 3alpha,5-beta-THP: a possible model of premenstrual dysphoric disorder. Psychopharmacology. 2006;186:323–33. doi: 10.1007/s00213-005-0168-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 46.Belelli D, Lambery JJ. Neurosteroids: endogenous regulators of the GABAA receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 47.Bitran D, Smith SS. Termination of pseudopregnancy in the rat produces an anxiogenic-like response that is associated with an increase in benzodiazepine receptor binding density and a decrease in GABA-stimulated chloride influx in the hippocampus. Brain Res Bull. 2005;64:511–8. doi: 10.1016/j.brainresbull.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 48.Smolen A, Smolen TN, Han PC. Alterations in regional brain GABA concentration and turnover during pregnancy. Pharmacol Biochem Behav. 1993;44:63–9. doi: 10.1016/0091-3057(93)90281-w. [DOI] [PubMed] [Google Scholar]
- 49.Zhou X, Smith SS. Steroid requirements for regulation of the alpha4 subunit of the GABA(A) receptor in an in vitro model. Neurosci Lett. 2007;411:61–6. doi: 10.1016/j.neulet.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Backstrom T, Snaders D, Leask RM. Mood, sexuality, hormones and the menstrual cycle: II. Hormone levels and their relation to premenstrual syndrome Psychsom Med. 1983;45:503–7. doi: 10.1097/00006842-198312000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Pearlstein TB, Bachmann GA, Zacur HA, Yonkers KA. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception. 2005;72:414–21. doi: 10.1016/j.contraception.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt PJ, Neiman LK, Grover GN, et al. Lack of effect of induced menses on symptoms in women with premenstrual syndrome. N Engl J Med. 1991;324:1174–9. doi: 10.1056/NEJM199104253241705. [DOI] [PubMed] [Google Scholar]
- 53.Hammarbäck S, Bäckström T. Induced anovulation as treatment of premenstrual tension syndrome: a double-blind cross-over study with GnRH-agonist versus placebo. Acta Obstet Gynecol Scand. 1988;67:159–66. doi: 10.3109/00016348809004191. [DOI] [PubMed] [Google Scholar]
- 54.Hammarbäck S, Ekholm UB, Bäckström T. Spontaneous anovulation causing disappearance of cyclical symptoms in women with the premenstrual syndrome. Acta Endocrinol (Copenh) 1991;125:132–7. doi: 10.1530/acta.0.1250132. [DOI] [PubMed] [Google Scholar]
- 55.Pecins-Thompson M, Brown NA, Kohama SG, Bethea CL. Ovarian steroid regulation of tryptophan hydroxylase mRNA expression in rhesus macaques. J Neurosci. 1996;16:7021–9. doi: 10.1523/JNEUROSCI.16-21-07021.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sumner BEH, Fink G. Effects of acute estradiol on 5-hydroxytryp-tamine and dopamine receptor subtype mRNA expression in female rat brain. Mol Cell Neurosci. 1993;4:83–92. doi: 10.1006/mcne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 57.Sumner BEH, Fink G. Estrogen increases the density of 5-hydroxytryptamine 2A receptors in cerebral cortex and nucleus accumbens in the female rat. J Steroid Biochem Mol Biol. 1995;54:15–20. doi: 10.1016/0960-0760(95)00075-b. [DOI] [PubMed] [Google Scholar]
- 58.Kugaya A, Epperson CA, Zoghbi S, et al. Increase in prefrontal cortex serotonin 2A receptors following estrogen treatment in postmenopausal women. Am J Psychiatry. 2003;160:1522–4. doi: 10.1176/appi.ajp.160.8.1522. [DOI] [PubMed] [Google Scholar]
- 59.Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry. 2002;59:851–8. doi: 10.1001/archpsyc.59.9.851. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt PJ, Purdy RH, Moore PH, Jr, Paul SM, Ribinow DR. Circulating levels of anxiolytic steroids in the luteal phase in women with premenstrual syndrome and in control subjects. J Clin Endocrinol Metab. 1994;79:1256–60. doi: 10.1210/jcem.79.5.7962316. [DOI] [PubMed] [Google Scholar]
- 61.Wang M, Seippel L, Purdy RH, Bäckström T. Relationship between symptom severity and steroid variation in women with premenstrual syndrome: study on serum pregnenolone, pregnenolone sulfate, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnan-20-one. J Clin Endocrinol Metab. 1996;81:1076–82. doi: 10.1210/jcem.81.3.8772579. [DOI] [PubMed] [Google Scholar]
- 62.Lombardi I, Luisi S, Quirici B, et al. Adrenal response to adrenocorticotropic hormone stimulation in patients with premenstrual syndrome. Gyn Endocrinol. 2004;18:79–87. doi: 10.1080/09513590310001652955. [DOI] [PubMed] [Google Scholar]
- 63.Monteleone P, Luisi S, Tonetti A, et al. Allopregnanolone concentrations and premenstrual syndrome. Eur J Endocrinol. 2000;142:269–73. doi: 10.1530/eje.0.1420269. [DOI] [PubMed] [Google Scholar]
- 64.Rapkin AJ, Morgan M, Goldman L, Brann DW, Simone D, Maheash VB. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–14. doi: 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- 65.Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–97. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- 66.Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–20. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 67.Klatzkin RR, Morrow AL, Light KC, Pedersen CA, Girdler SS. Histories of depression, allopregnanolone responses to stress, and premenstrual symptoms in women. Biol Psychol. 2006;71:2–11. doi: 10.1016/j.biopsycho.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 68.Schmidt PJ, Grover GN, Hoban MC, Rubinow DR. State-dependent alterations in the perception of life events in menstrual-related mood disorders. Am J Psychiatry. 1990;147:230–4. doi: 10.1176/ajp.147.2.230. [DOI] [PubMed] [Google Scholar]
- 69.Cullberg J. Mood changes and menstrual symptoms with different estrogen/combinations: a double blind comparison with placebo. Acta Psychiatry Scand. 1972;236:1–84. [PubMed] [Google Scholar]
- 70.Graham CA, Sherwin BB. The relationship between retrospective premenstrual symptom reporting and present oral contraceptive use. J Psychosom Res. 1987;31:45–53. doi: 10.1016/0022-3999(87)90097-3. [DOI] [PubMed] [Google Scholar]
- 71.Björn I, Sundström-Poromaa I, Bixo M, Nyberg S, Bäckström G, Bäckström T. Increase in estrogen dose deteriorates mood during progestin phase in sequential hormonal therapy. Clin Endocrinol Metab. 2003;88:2026–30. doi: 10.1210/jc.2002-020755. [DOI] [PubMed] [Google Scholar]
- 72.Sundström I, Ashbrook D, Bäckström T. Reduced benzodiazepine sensitivity in patients with premenstrual syndrome: a pilot study. Psychoneuroendocrinology. 1997;22:25–38. doi: 10.1016/s0306-4530(96)00035-2. [DOI] [PubMed] [Google Scholar]
- 73.Sundström I, Nyberg S, Bäckström T. Patients with premenstrual syndrome have reduced sensitivity to midazolam compared to control subjects. Neuropsychopharmacology. 1997;17:370–81. doi: 10.1016/S0893-133X(97)00086-9. [DOI] [PubMed] [Google Scholar]
- 74.Sundström I, Andersson A, Nyberg S, Ashbrook D, Purdy RH, Bäckström T. Patients with premenstrual syndrome have a different sensitivity to a neuroactive-steroid during the menstrual cycle compared to control subjects. Neuroendocrinology. 1998;67:126–38. doi: 10.1159/000054307. [DOI] [PubMed] [Google Scholar]
- 75.Nyberg S, Wahlström G, Bäckström T, Sundström Poromaa I. Altered sensitivity to alcohol in the late luteal phase among patients with premenstrual dysphoric disorder. Psychoneuroendocrinology. 2004;29:767–77. doi: 10.1016/S0306-4530(03)00121-5. [DOI] [PubMed] [Google Scholar]
- 76.Sundstrom I, Backstrom T. Citalopram increases pregnanolone sensitivity in patients with premenstrual syndrome: an open trial. Psychoneuroendocrinology. 1998;23:73–88. doi: 10.1016/s0306-4530(97)00064-4. [DOI] [PubMed] [Google Scholar]
- 77.Wyatt KM, Dimmock PW, O'Brien PM. Selective serotonin reuptake inhibitors for premenstrual syndrome. Cochrane Database Syst Rev. 2002;4:CD001396. doi: 10.1002/14651858.CD001396. [DOI] [PubMed] [Google Scholar]
- 78.Shen H, Gong QH, Yuan M, Smith SS. Short term steroid treatment increases delta GABA(A) receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–86. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith SS, Gong QH. Ethanol effects on GABA-gated current in a model of increased alpha4betadelta GABAA receptor expression depend on time course and preexposure to low concentrations of the drug. Alcohol. 2007;41:223–31. doi: 10.1016/j.alcohol.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry. 2003;54:757–62. doi: 10.1016/s0006-3223(02)01924-8. [DOI] [PubMed] [Google Scholar]
- 81.Epperson CN, Pittman B, Czarkowski C, Stiklus S, Krystal JH, Grillon C. Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32:2190–8. doi: 10.1038/sj.npp.1301351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Facchinetti F, Romano G, Fava M, Genazzani AR. Lactate infusion induces panic attacks in patients with premenstrual syndrome. Psychosom Med. 1992;54:288–96. doi: 10.1097/00006842-199205000-00005. [DOI] [PubMed] [Google Scholar]
- 83.Harrison WM, Sandberg D, Gorman JM, et al. Provocation of panic with carbon dioxide inhalation in patients with premenstrual dysphoria. Psychiatry Res. 1998;27:183–92. doi: 10.1016/0165-1781(89)90133-9. [DOI] [PubMed] [Google Scholar]
- 84.Bauer J, Wildt L, Rugel D, Stefan H. The effect of a synthetic GnRH analogue on catamenial epilepsy: a study in ten patients. J Neurol. 1992;239:284–6. doi: 10.1007/BF00810354. [DOI] [PubMed] [Google Scholar]
- 85.Oinonen KA, Mazmanian D. To what extent do oral contraceptives influence mood and affect? J Affect Disord. 2002;70:229–40. doi: 10.1016/s0165-0327(01)00356-1. [DOI] [PubMed] [Google Scholar]
- 86.Bancroft J, Boyle H, Warner P, Fraser HM. The use of an LHRH agonist, buserelin, in the long-term management of premenstrual syndromes. Clin Endocrinol. 1987;27:171–82. doi: 10.1111/j.1365-2265.1987.tb01142.x. [DOI] [PubMed] [Google Scholar]
- 87.Backstrom T, Hansson-Malmstrom Y, Lindhe BA, Cavalli-Bjorkman B, Nordenstrom S. Oral contraceptives in premenstrual syndrome: a randomized comparison of triphasic and monophasic preparations. Contraception. 1992;46:253–68. doi: 10.1016/0010-7824(92)90006-f. [DOI] [PubMed] [Google Scholar]
- 88.Kurshan N, Epperson CN. Oral contraceptives and mood in women with and without premenstrual dysphoria: a theoretical model. Arch Women's Ment Health. 2006;9:1–14. doi: 10.1007/s00737-005-0102-z. [DOI] [PubMed] [Google Scholar]
- 89.Freeman EW, Kroll R, Rapkin A, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med. 2001;10:561–9. doi: 10.1089/15246090152543148. [DOI] [PubMed] [Google Scholar]
- 90.Fenton C, Wellington K, Moen MD, Robinson DM. Drospirenone/ethinylestradiol 3mg/20microg (24/4 day regimen): a review of its use in contraception, premenstrual dysphoric disorder and moderate acne vulgaris. Drugs. 2007;67:1749–65. doi: 10.2165/00003495-200767120-00007. [DOI] [PubMed] [Google Scholar]
- 91.Paoletti AM, Lello S, Fratta S, et al. Psychological effects of oral contraceptive formulation containing 3 mg of drospirenone plus 30 micrograms of ethinyl estradiol. Fertil Steril. 2004;81:645–51. doi: 10.1016/j.fertnstert.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 92.Majewska MD, Demirgoren S, Spivak CE, London ED. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990;526:143–6. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- 93.Bergeron RJ, Weimar WR, Wu Q, Feng Y, McManis JS. Polyamine analogue regulation of NMDA MK-801 binding: a structure–activity study. J Med Chem. 1996;39:5257–66. doi: 10.1021/jm960545x. [DOI] [PubMed] [Google Scholar]
- 94.Monnet FP, Mahe V, Robel P, Baulieu EE. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci USA. 1995;92:3774–8. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Follesa P, Porcu P, Sogliano C, et al. Changes in GABAA receptor gamma 2 subunit gene expression induced by long-term administration of oral contraceptives in rats. Neuropharmacology. 2002;42:325–36. doi: 10.1016/s0028-3908(01)00187-3. [DOI] [PubMed] [Google Scholar]
- 96.Simic B, Kniewald J, Kniewald Z. Influence of ethynodiol diacetate on the formation of A-homo-3-oxa-5alpha-pregnane-4,20-dione in female rats. Endocrine Regul. 1998;32:125–31. [PubMed] [Google Scholar]
- 97.Dana-Haeri J, Richens A. Effect of norethisterone on seizures associated with menstruation. Epilepsia. 1983;24:377–81. doi: 10.1111/j.1528-1157.1983.tb04901.x. [DOI] [PubMed] [Google Scholar]
- 98.Crawford P. Best practice guidelines for the management of women with epilepsy. Epilepsia. 2005;46:117–24. doi: 10.1111/j.1528-1167.2005.00323.x. [DOI] [PubMed] [Google Scholar]
- 99.Mattson RH, Cramer JA, Caldwell BV, Siconolfi BC. Treatment of seizures with medroxyprogesterone acetate: preliminary report. Neurology. 1984;34:1255–8. doi: 10.1212/wnl.34.9.1255. [DOI] [PubMed] [Google Scholar]
- 100.Speroff L, Glass RH, Kase NG. Clinical gynecology, endocrinology and infertility. Baltimore, MD: Williams & Wilkins; 1994. [Google Scholar]
- 101.Wyatt K, Dimmock P, Jones P, Obhrai M, O'Brien S. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. Br Med J. 2001;323:776–80. doi: 10.1136/bmj.323.7316.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Herzog AG. Progesterone therapy in women with complex partial and secondary generalized seizures. Neurology. 1995;45:1660–2. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- 103.Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology. 1999;52:1917–8. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- 104.Nohria G. Ganaxolone. Neurotheraputics. 2007;4:102–5. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Freeman EW, Weinstock L, Rickels K, Sondheimer SJ, Coutifaris C. A placebo-controlled study of effects of oral progesterone on performance and mood. Brit J Clin Pharmacol. 1992;33:293–8. doi: 10.1111/j.1365-2125.1992.tb04038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Freeman EW, Rickels K, Sondheimer SJ, Polansky M. A double-blind trial of oral progesterone, alprazolam, and placebo in treatment of severe premenstrual syndrome. JAMA. 1995;274:51–7. [PubMed] [Google Scholar]
- 107.Uzunov DP, Cooper TB, Costa E, Guidotti A. Fluoxetine-elicited changes in brain neurosteroid content measured by negative ion mass fragmentography. Proc Natl Acad Sci. 1996;93:12599–604. doi: 10.1073/pnas.93.22.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Uzunova V, Sheline Y, Davis JM, Rasmusson, Uznov P, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology. 2006;186:362–72. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- 110.Trimble MR. On the use of tranquillisers in epilepsy. Epilepsia. 2002;43(Suppl. 2):25–7. doi: 10.1046/j.1528-1157.2002.043s2025.x. [DOI] [PubMed] [Google Scholar]
- 111.Reddy SR, Rogawski MA. Stress induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J Neurosci. 2002;42:3795–805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Feely M, Calvert R, Gibson J. Clobazam in catamenial epilepsy: a model for evaluating anticonvulsants. Lancet. 1982;10:71–3. doi: 10.1016/s0140-6736(82)91691-9. [DOI] [PubMed] [Google Scholar]
- 113.Yonkers KA, Pearlstein T, Fayyad R, Gillespie JA. Luteal phase treatment of premenstrual dysphoric disorder improves symptoms that continue into the postmenstrual phase. J Affect Disord. 2005;85:3171–2. doi: 10.1016/j.jad.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 114.Richman A, Heinrichs SC. Seizure prophylaxis in an animal model of epilepsy by dietary fluoxetine supplementation. Epilepsy Res. 2007;74:19–27. doi: 10.1016/j.eplepsyres.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 115.Jobe PC, Browning RA. The serotonergic and noradrenergic effects of antidepressant drugs are anticonvulsant, not proconvulsant. Epilepsy Behav. 2005;7:602–19. doi: 10.1016/j.yebeh.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 116.Reddy DS, Kulkarni SK. Proconvulsant effects of neurosteroids pregnenolone sulfate and dehydroepiandrosterone sulfate in mice. Eur J Pharmacol. 1998;345:55–9. doi: 10.1016/s0014-2999(98)00034-x. [DOI] [PubMed] [Google Scholar]
- 117.Bergerson R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dihydroepiandosterone and its suppression by progesterone: effects medicated via sigma receptors. J Neurosci. 1996;16:1193–202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]