Abstract
This study traces the development of spatial memory abilities in monkeys and reports the effects of selective neonatal hippocampal lesions on performance across development. Two different versions of the visual paired-comparison (VPC) task were used. The VPC-Spatial-Location task tested memory for object-locations that could be solved using an egocentric spatial frame of reference and the VPC-Object-In-Place task taxed memory for spatial relations using an allocentric reference frame. Eleven rhesus macaques (6 neonatal sham-operated controls and 5 with neonatal neurotoxic hippocampal lesions) were tested on both tasks as infants (8 months), juveniles (18 months), and adults (5–6 years). Memory for spatial locations was present by 18 months of age, whereas memory for object-place relations was present only in adulthood. Also, neonatal hippocampal lesions delayed the emergence of memory for spatial locations and abolished memory for object-place associations, particularly in animals that had sustained extensive and bilateral hippocampal lesions. The differential developmental time course of spatial memory functions and of the effects of neonatal hippocampal lesions on these functions are discussed in relation to morphological maturation of the medial temporal lobe structures in monkeys. Implications of the findings for the neural basis of spatial memory development in humans are also considered.
Keywords: Macaca mulatta, Egocentric space, Allocentric space, Parahippocampal cortex, Visual-paired comparison task, Developmental amnesia
Introduction
The hippocampus consistently has been linked to spatial memory processes in several species (Burgess, Maguire, & O'Keefe, 2002; Glavis-Bloom, Alvarado, & Bachevalier, 2013; Lavenex, Amaral, & Lavenex, 2006; O'Keefe & Nadel, 1978). Furthermore, in rodents and humans, spatial memory abilities have a protracted development associated with a prolonged postnatal development of the hippocampus (see for reviews Alvarado & Bachevalier, 2000; Bachevalier & Beauregard, 1993; Overman, Pate, Moore, & Peuster, 1996; Ribordy, Jabès, Lavenex, & Lavenex, 2012).
The rodent hippocampus is poorly developed at birth and reaches morphologic adult-pattern at approximately the end of the third week (21–30 days of age), an age that coincides remarkably well with the appearance of adult levels of performance in a variety of spatial memory tasks. Moreover, early damage to the hippocampus, well before its complete maturation, disrupts the third-week emergence of adult-performance levels of spatial memory (see for review Alvarado & Bachevalier, 2000). A similar association between hippocampal maturation and the development of spatial memory processes has been suggested in humans as well. As compared to rodents, the human hippocampus is more fully developed at birth, although important morphological changes in its circuitry continue until around 5–7 years of age (Giedd et al., 1996; Lenroot & Giedd, 2006; Seress, 2001, 2007; Seress & Abraham, 2008). Of interest, children demonstrate poorer abilities in several spatial memory tasks until around the same age (see for review Ribordy et al., 2012) and patients with early hippocampal pathology demonstrate impaired spatial memory skills (Burgess et al., 2002; King, Burgess, Hartley, Vargha-Khadem, & O'Keefe, 2002; Spiers, Burgess, Hartley, Vargha-Khadem, & O'Keefe, 2001), although the age at which the spatial memory deficits occur is at the present time unknown.
Likewise, recent studies in monkeys have shown a pattern of morphologic development of the hippocampus similar to that of humans, albeit with a shorter timeline, reaching adult level of maturity around the end of the second year of life (Jabès, Lavenex, Amaral, & Lavenex, 2010, 2011; Payne, Machado, Bliwise, & Bachevalier, 2010; Seress, 1992, 2007; Seress & Ribak, 1995a, b). Accordingly, this protracted maturation of the hippocampus suggests that proficient use of spatial relational memory in monkeys would appear by the end of the second year of life. Yet, there has been no systematic longitudinal investigation to support this proposal. In addition, damage to the hippocampus in the first few weeks of life results in no spatial memory impairment at 9 months of age (Lavenex, Lavenex, & Amaral, 2007), but severe deficits are reported when animals were tested as adults (Alvarado & Rudy, 1995; Alvarado, Wright, & Bachevalier, 2002; Mahut & Moss, 1986; Málková, Mishkin, & Bachevalier, 1995; Pascalis & Bachevalier, 1999). Given the paucity of information regarding the development of spatial memory abilities in monkeys and of their dependence on an intact hippocampus, the goal of this study was three-fold: (1) to longitudinally trace the development of spatial memory abilities in monkeys, using two versions of the visual paired-comparison (VPC) task and assess the age at which these abilities reached adult levels of proficiency, (2) to investigate whether selective neonatal hippocampal lesions hinder or delay the emergence of spatial memory, and if yes (3) whether the magnitude of the deficits observed was equivalent to that of adult animals that have received the same hippocampal damage in adulthood and were tested using the same tasks (Bachevalier & Nemanic, 2008). Given the current knowledge supporting a role of the hippocampus in spatial relational memory, but not in memory for spatial locations (Bachevalier & Nemanic, 2008; Lavenex & Lavenex, 2009; Morris, Garrud, Rawlins, & O'Keefe 1982; Nadel & Hardt, 2004; O'Keefe & Nadel, 1978), spatial memory performance in the present study was measured using two different versions of the VPC task. A VPC-Spatial-Location task tested memory for object-locations in which egocentric spatial frame of reference (left, right, up, down in relation to the observer viewpoint) could be used to solve the task. A VPC-Object-in-Place was used to assess memory for spatial relations among several objects (allocentric spatial representation). A preliminary report of these data has been presented in abstract form (Blue, Kazama, & Bachevalier, 2009; Kazama, Lay, & Bachevalier, 2003).
Methods
Subjects
Subjects were 11 rhesus macaque monkeys (Macaca mulatta) that received either neonatal neurotoxic lesions of the hippocampus (Group Neo-Hibo, N = 5, 2 females) or sham operations (Group Neo-C, N = 6, 3 females) between 10 and 15 days of age. All animals were surrogate nursery-reared and received daily social interactions, intensive human contact, and cognitive testing that began within the first few weeks of life (see Goursaud & Bachevalier, 2007; Zeamer, Heuer, & Bachevalier, 2010; for more information on rearing conditions). All experimental rearing, surgeries, and experimental testing of animals were approved by the Institutional Animal Care and Use Committees at the University of Texas at Houston and Emory University.
Neuroimaging and Surgical Procedures
All neuroimaging and surgical procedures as well as lesion extent of the operated animals have been described previously in detail (Goursaud & Bachevalier, 2007; Zeamer et al., 2010). Briefly, these procedures were performed under deep anesthesia (Isoflurane gas, 1.0–2.0%, v/v, to effect) and vital signs (body temperature, heart rate, blood pressure, rate of respiration, and expired gas CO2 levels) were monitored and recorded.
Monkeys received three magnetic resonance imaging (GE Signa 1.5 Tesla, GE Medical Systems, Milwaukee, WI) sessions of the brain, that is, the day of surgery, 6–8 days after the surgical procedure and finally 1–2 years after surgery. The first two sessions included three-dimensional (3D) T1-weighted fast spoiled gradient (FSPGR)-echo scans and 3 fluid attenuated inversion recovery scans (FLAIR, contiguous 3-mm sections offset by 1 mm posteriorly), but only the 3D T1 scan was performed at 1–2 years. The pre-surgical T1 images were used to select and calculate the coordinates of each injection site along the hippocampus, which were then transformed into stereotaxic plans. The FLAIR images taken 6–8 days after surgery were used to locate the extent of hypersignals produced by brain edema following the neurotoxin injections, and estimate the percent of extent of damage. Finally, the T1 images taken 1–2 years post-surgery were used to estimate the percent of hippocampal volume reduction.
After the first imaging session, the animals remained under anesthesia and were immediately transferred to the surgical suite. An intravenous drip containing 5.0% dextrose and 0.45% sodium chloride maintained normal hydration, and a blanket placed around the animals and attached to a Bair Hugger® Therapy warming unit provided warm air to maintain body temperature. Under aseptic conditions, the tissues were retracted to expose the skull and small bilateral craniotomies were made above the hippocampus. Small slits were made in the dura to allow penetration of the injection needles. For sham-operated controls (Neo-C), the surgical procedures stopped at this point and no injections were made.
For neurotoxin injections into the hippocampus, two 10-ml Hamilton syringes held in Kopf manipulators (David Kopf Instrument, Tujunga, CA) were used to inject ibotenic acid (Biosearch Technologies, Novato, CA, 10 mg/mL in phosphate buffered saline, pH = 7.4) in seven to eight sites along the hippocampus bilaterally (0.6–0.8 μL injected at each site at a rate of 0.2 μL/30 s). The injections were made in the two hemispheres simultaneously and were intended to damage the dentate gyrus, the Cornu Ammonis fields, and the subicular complex.
Following surgical procedures, all tissues were closed in anatomical layer, first the dura (5.0 Vicryl with a Taper needle; Ethicon, Somerville, NJ), then the galea (4.0 Dexon with a Taper needle; Ethicon), and finally the skin (4.0 Dexon with a cutting needle; Ethicon). The animals were treated with dexamethasone sodium phosphate (0.4 mg/kg, im) and cephazolin (25 mg/kg, im) beginning 12 h before surgery and lasted until 7 days after surgery to reduce brain swelling and to prevent infection, respectively. In addition, acetaminophen (10 mg/kg, po) was given four times a day for 3 days after surgery for relief of pain.
Behavioral Task
All animals had already been tested in the VPC task to assess the development of object recognition memory over increasing delays at the ages of 1.5, 6, and 18 months and as adults (Zeamer et al., 2010; Zeamer & Bachevalier, 2013). For the present experiment, they were given the VPC spatial memory tasks (VPC-location, VPC-Object-In-Place, and VPC-control, see Figure 1) identical to those described in Bachevalier & Nemanic (2008) at the age of 8 months (infants), 18 months (juveniles), and 5–6 years (adults).
Fig. 1.
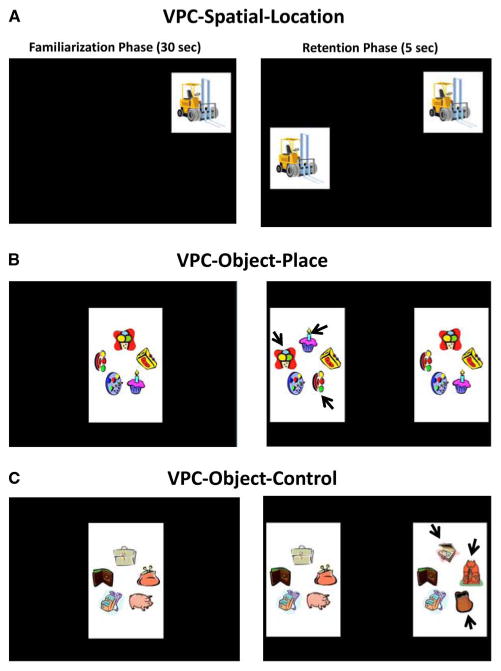
Examples of trials for the visual paired-comparison (VPC) -Spatial-Location (A), VPC-Object-In-Place (B), and VPC-Object-Control (C) tasks. Note that for the Spatial-Location task, the novel image is the same as the familiar image but is placed in a different position on the screen. For the Object-In-Place tasks, the novel image differed from the familiar image only in the location of three of the five objects forming the images (black arrows). In the Object-Control task, the novel image consisted of replacing three objects of the familiar image with three new objects (black arrows).
Apparatus
At 8 months, monkeys were held by an experimenter approximately 30 cm in front of a computer monitor (Dell Ultrasharp 2407WFP-HC 24-inch widescreen LCD) enclosed inside a testing box located in a darkened room. As juveniles and adults, they were seated in a size-appropriate-Plexiglas primate chair (University of Texas Machine Shop and Crist Instruments, Damascus, MD) and positioned 30 cm in front of the computer monitor.
VPC-spatial-location
The VPC-Spatial-Location task was used to assess memory for the location of an object. During the familiarization phase of each trial, a single image (10 cm × 10 cm) was presented in a random location on the screen for a 30-s cumulative time, followed by a short delay of 5 s. During test phase of 5 s, the familiar image was presented again, in the same location as before, along with an identical image presented in a random novel location on the screen. Preferential looking in the test phase was measured by longer viewing of the familiar object occupying the novel location (Figure 1A).
VPC-object-in-place
The VPC-Object-In-Place was used to measure memory for object-place associations. During familiarization of 30 s, a single image consisting of an array of five objects was presented in the center of the screen. During the two 5-s test phases, the familiar image was presented together with the novel image for which the location of three objects were re-arranged and the left/right position of the novel images was changed between the two tests (Figure 1B). Preferential looking in the test phases was measured by longer viewing at the novel re-arranged image.
VPC-object-control
The VPC-Object-Control task was used to ensure that any impairment in the VPC Object-In-Place could not be due to difficulty in perceiving complex visual images. The task parameters were identical to those in the Object-In-Place task. The only difference was that three of the five objects were replaced with new ones in the novel image (see Figure 1C). Preferential looking in the test phases was measured by longer viewing at the image with the novel objects.
Data Analyses
Task parameters
A frame-by-frame examination of the corneal reflection of the stimuli recorded on the videotapes (see for details Pascalis & Bachevalier, 1999) was conducted to quantify three viewing parameters: (1) the time necessary to reach the 30 s cumulative looking at the stimulus during familiarization (familiarization time), (2) the amount of time spent fixating the stimuli during the two test phases (total looking time), and (3) the percent looking time at the novel stimulus (preferential looking) as a measure of recognition memory. Any trial for which the total looking time during the two test phases did not exceed 1s was excluded from the analyses. Inter-observer reliability (Pearson r = 0.94).
Statistical analyses
Scores of Group Neo-C were analyzed first to establish normal developmental pattern of performance on each task. One-way analyses of variance (ANOVAs) were used with Age as a repeated-measures factor, followed by pairwise comparisons. Group differences were assessed with two-way ANOVAs (Group × Age) and Huynh-Feldt correction was used when appropriate. Post hoc (Tukey) and a priori (Holm-Bonferroni) were used for pairwise comparisons. Significant interactions were further explored using GLM (generalized linear model) multivariate analyses, simple effects, or planned comparisons (Pedhazur, 1982). One-sample t tests were used to evaluate group differences from chance.
Finally, to assess the effects of early-onset versus late-onset hippocampal lesions, ANOVAs (Group × Age at lesions) were used to compare performance from animals with Neo-Hibo lesions and their controls obtained at 5–6 years of age with that of adult monkeys (3–12 years) that had received similar sham lesions (Adult-C, N = 6) or neurotoxic hippocampal lesions (Adult-Hibo, N = 4) in adulthood (data from Bachevalier & Nemanic, 2008).
Effect sizes (eta-squared values) were provided for all data analyses reported and correlations of behavioral parameters with extent of hippocampal or adjacent damage were investigated using one-tailed Pearson correlations.
Results
Hippocampal Lesion Evaluation
Table 1 provides the extent of bilateral hypersignals seen on 1-week post-surgery FLAIR images in the hippocampus, amygdala, and parahippocampal cortical areas TH and TF. Extent of hippocampal lesion estimated from the FLAIR images was used here since this procedure has proven to be an adequate estimate of the cell loss later found on histological sections (Málková, Lex, Mishkin, & Saunders, 2001; Nemanic, Alvarado, Price, Jackson, & Bachevalier, 2002). Figure 2 illustrates that the 67.6% bilateral damage observed in Case Neo-Hibo-2 from FLAIR images resulted in a significant and bilateral volume reduction (see Goursaud & Bachevalier, 2007; Heuer & Bachevalier, 2010; Zeamer et al., 2010, for illustration of other cases).
Table 1. Intended and unintended damage for monkeys with neonatal hippocampal lesions.
| Case | Intended damage | Unintended damage | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Hippocampus | Amygdala | TH/TF | ||||||||||
|
|
|
|
||||||||||
| L% | R% | X% | W% | L% | R% | X% | W% | L% | R% | X% | W% | |
| Neo-Hibo-1 | 63.8 | 2.9 | 33.2 | 1.9 | 14.0 | 0.0 | 7.0 | 0.0 | 3.1 | 0.5 | 1.8 | 0.0 |
| Neo-Hibo-2 | 54.4 | 80.9 | 67.6 | 44.0 | 0.0 | 0.0 | 0.0 | 0.0 | 21.4 | 2.7 | 12.1 | 0.6 |
| Neo-Hibo-3 | 78.5 | 96.3 | 87.4 | 75.6 | 1.7 | 0.0 | 0.8 | 0.0 | 6.1 | 5.5 | 5.8 | 0.3 |
| Neo-Hibo-4 | 20.3 | 67.3 | 43.8 | 13.7 | 0.0 | 4.7 | 2.4 | 0.0 | 15.3 | 0.0 | 7.6 | 0.0 |
| Neo-Hibo-5 | 20.7 | 84.0 | 52.6 | 17.4 | 0.0 | 4.9 | 2.4 | 0.0 | 6.1 | 4.0 | 5.1 | 0.2 |
| Mean | 47.5 | 66.3 | 56.9 | 30.5 | 3.1 | 1.9 | 2.5 | 0.0 | 10.4 | 2.5 | 6.5 | 0.2 |
Note. Percentage of hippocampal damage as estimated from hypersignals seen on pre-surgical and post-surgical FLAIR images.
L%=percent damage in the left hemisphere; R%=percent damage in the right hemisphere; X%5averaged percent damage to both hemispheres;
W%=weighted average damage to both hemispheres (W%= [L% × R%]/100; Hodos & Bobko, 1984) as an index of laterality of the lesion (<40% reflecting more unilateral lesions); TH/TF=cytoarchitectonic fields of the parahippocampal gyrus as defined by von Bonin & Bailey (1947).
Fig. 2.
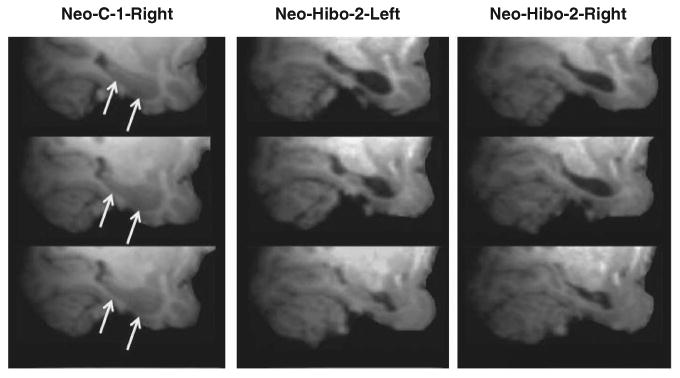
High-resolution T1 images at three levels of the hippocampal formation in a sham-operated animal (case Neo-C-1, right hemisphere) and in an animal with neonatal hippocampal lesions (case Neo-Hibo-2, left and right hemispheres) taken when the animals were 1–2 years of age. Note the almost complete loss of hippocampal tissue in this case.
Two cases (Neo-Hibo-2 and Neo-Hibo-3) had extensive symmetrical bilateral lesions of the hippocampus (X% > 50% bilaterally). The other three cases (Neo-Hibo-1, Neo-Hibo-4, and Neo-Hibo-5) had mostly asymmetrical lesions (% Left vs. % Right: 63.8% vs. 2.9%; 20.3% vs. 67.3%; 20.7% vs. 84%, respectively). Unintended damage was mild in all cases, averaging 2.5% to the posterior amygdala and 6.5% to parahippocampal area TH/TF.
Viewing Behaviors during the VPC tasks
Viewing parameters were analyzed for both groups to ensure that spatial memory performance was not affected by differences in the time animals allocated viewing the stimuli during the three tasks and across the three ages (see Table 2). The familiarization time in animals of both Groups increased with age in all three tasks [Age: FHuyhn-Feldt (2, 18) = 12.45; p=.000; η2 =0.58; FHuyhn-Feldt = 8.882; p<.005; η2 =0.51; and FHuyhn-Feldt (2, 18) =18.53; p=.000; η2 = 0.66; for the Spatial Location, Object-In-Place, and Object-Control tasks, respectively] with no main effect of Group and no Group × Age interaction. The total time looking at the two images during the two test phases decreased with age for both groups in the three tasks [Age: FHuyhn-Feldt (2, 18) =8.568; p=.004, η2 =0.49; FHuyhn-Feldt (2, 18) =25.455; p=.000; η2 =0.74; FHuyhn-Feldt (2, 18) =25.365; p=.000; η2 =0.74; for the Spatial Location, Object-In-Place, and Object-Control tasks, respectively] with no effect of Group and no Group ×Age interaction. The data indicate that both groups explored the stimuli in the same way.
Table 2. Viewing behavior for each subject across VPC tasks and age.
| Group/case | Spatial location | Object-in-Place | Object-Control | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| 8 Mo | 18 Mo | 5–6 Yr | 8 Mo | 18 Mo | 5–6 Yr | 8 Mo | 18 Mo | 5–6 Yr | |
| Familiarization time (s) | |||||||||
| Neo-C | |||||||||
| Neo-C-1 | 76 | 308 | 249 | – | 172 | 163 | 95 | 70 | 308 |
| Neo-C-2 | 59 | 54 | 224 | 79 | 93 | 165 | 55 | 63 | 139 |
| Neo-C-3 | 123 | 87 | 344 | 126 | 109 | 162 | 71 | 99 | 207 |
| Neo-C-4 | 63 | 133 | 383 | 54 | 129 | 427 | 56 | 141 | 373 |
| Neo-C-5 | 65 | 244 | 70 | 89 | 253 | 69 | 79 | 58 | 65 |
| Neo-C-6 | 55 | 50 | 233 | 66 | 94 | 489 | 49 | 41 | 162 |
| Mean | 74 | 146 | 251 | 83 | 142 | 246 | 68 | 79 | 209 |
| Group Neo-Hibo | |||||||||
| Neo-Hibo-1 | 47 | 84 | 90 | 52 | 175 | 98 | 55 | 54 | 140 |
| Neo-Hibo-2 | 52 | 175 | 415 | 54 | 148 | 314 | 50 | 72 | 308 |
| Neo-Hibo-3 | 70 | 104 | 247 | 97 | 127 | 232 | 59 | 129 | 235 |
| Neo-Hibo-4 | 93 | 128 | 203 | 85 | 144 | 297 | 55 | 209 | 189 |
| Neo-Hibo-5 | 81 | 80 | 223 | 59 | 174 | 300 | 71 | 55 | 131 |
| Mean | 69 | 114 | 236 | 69 | 154 | 248 | 58 | 104 | 201 |
| Total time (s) | |||||||||
| Group Neo-C | |||||||||
| Neo-C-1 | 2.4 | 1.7 | 1.2 | 4.8 | 1.9 | 1.7 | 6.4 | 2.6 | 1.6 |
| Neo-C-2 | 3.0 | 1.5 | 2.7 | 3.8 | 2.7 | 3.2 | 7.9 | 4.7 | 3.3 |
| Neo-C-3 | 3.2 | 1.9 | 2.4 | 5.3 | 2.7 | 2.9 | 4.7 | 2.9 | 4.0 |
| Neo-C-4 | 3.7 | 2.1 | 1.2 | 5.1 | 3.1 | 1.5 | 7.2 | 2.6 | 2.1 |
| Neo-C-5 | 2.7 | 2.3 | 3.5 | 4.4 | 2.5 | 4.3 | 6.2 | 6.8 | 4.0 |
| Neo-C-6 | 3.9 | 2.6 | 1.7 | 5.2 | 5.6 | 2.2 | 5.7 | 5.3 | 3.1 |
| Mean | 3.2 | 2.0 | 2.1 | 4.8 | 3.1 | 2.6 | 6.4 | 4.2 | 3.0 |
| Group Neo-Hibo | |||||||||
| Neo-Hibo-1 | 3.4 | 2.9 | 2.2 | 5.1 | 2.2 | 1.9 | 6.2 | 3.4 | 2.7 |
| Neo-Hibo-2 | 3.8 | 2.4 | 1.7 | 6.8 | 3.8 | 1.9 | 7.8 | 5.5 | 1.6 |
| Neo-Hibo-3 | 2.8 | 1.7 | 2.7 | 4.4 | 2.0 | 3.4 | 4.5 | 2.8 | 2.8 |
| Neo-Hibo-4 | 2.3 | 2.2 | 1.7 | 5.8 | 2.3 | 1.6 | 5.2 | 3.1 | 2.4 |
| Neo-Hibo-5 | 2.3 | 2.1 | 2.0 | 5.8 | 2.5 | 2.9 | 6.2 | 5.4 | 2.7 |
| Mean | 2.9 | 2.3 | 2.1 | 5.6 | 2.6 | 2.3 | 6.0 | 4.0 | 2.4 |
Note. Time (s) to accumulate 30-sec viewing in the familiarization phase (Familiarization Time) and Total Time (s) viewing the two stimuli during the test phases. Group Neo-C represents animals with neonatal sham lesions, and Neo-H-ibo represents animals with neonatal hippocampal lesions.
Novelty Preference in the VPC tasks
Memory performance was measured by the amount of time the subject spent looking at the novel stimulus (see Table 3; Figure 3), and data were analyzed for each task separately below.
Table 3. Novelty preference scores across development.
| Spatial location | Object-in-Place | Object-Control | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| 8 Mo | 18 Mo | 5–6 Yr | 8 Mo | 18 Mo | 5–6 Yr | 8 Mo | 18 Mo | 5–6 Yr | |
| Group Neo-C | |||||||||
| Neo-C-1 | 47 | 62 | 72 | 46 | 57 | 63 | 69 | 52 | 63 |
| Neo-C-2 | 59 | 57 | 66 | 52 | 50 | 61 | 71 | 67 | 66 |
| Neo-C-3 | 55 | 57 | 70 | 49 | 46 | 56 | 62 | 67 | 65 |
| Neo-C-4 | 58 | 55 | 52 | 48 | 59 | 71 | 73 | 55 | 74 |
| Neo-C-5 | 69 | 83 | 63 | 52 | 49 | 52 | 58 | 69 | 65 |
| Neo-C-6 | 42 | 65 | 73 | 54 | 55 | 58 | 71 | 57 | 62 |
| Mean | 55 | 63 | 66 | 50 | 52 | 60 | 67 | 61 | 66 |
| Group Neo-Hibo | |||||||||
| Neo-H-ibo1 | 42 | 62 | 39 | 54 | 40 | 65 | 72 | 54 | 58 |
| Neo-H-ibo2 | 53 | 43 | 73 | 42 | 36 | 42 | 64 | 64 | 68 |
| Neo-H-ibo3 | 36 | 46 | 66 | 44 | 55 | 51 | 62 | 62 | 80 |
| Neo-H-ibo4 | 48 | 60 | 72 | 60 | 49 | 59 | 60 | 53 | 62 |
| Neo-H-ibo5 | 47 | 55 | 75 | 53 | 60 | 76 | 71 | 57 | 86 |
| Mean | 45 | 53 | 65 | 51 | 48 | 59 | 66 | 58 | 71 |
Note. Individual scores and group means for percentage of time animals looked at the novel image in the two retention tests across three visual paired-comparison tasks (Spatial-Location, Object-In-Place, and Object-Control) for infants (8-months), juveniles (18-months), and adults (5–6 years). Neo-C represents sham-operated controls, and Neo-Hibo represents animals with neonatal lesions to the hippocampus.
Fig. 3.
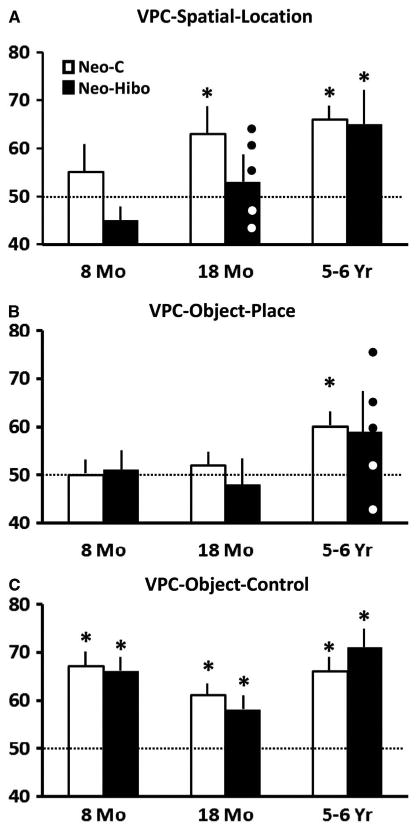
Mean percent of time (± SEM) looking at the familiar object appearing in the novel location in the visual paired-comparison (VPC) -Spatial-Location task (A) and at the novel image in the VPC-Object-In-Place (B) and VPC-Object-Control (C) tasks for animals with neonatal sham operations (Group Neo-C, white bars) and animals with neonatal hippocampal lesions (Group Neo-Hibo, black bars). Animals were tested at 8 months, 18 months, and 5–6 years of age for the 3 tasks. Note that for the VPC-Spatial-Location at 18 months and the VPC-Object-In-Place at 5–6 years when novelty preference did not differ from chance, white circles illustrate scores of two animals with bilateral hippocampal lesions, whereas black circles illustrate scores of the 3 animals with mostly unilateral hippocampal lesions (see Table 1). The horizontal dashed line represents chance performance and asterisk indicates significant difference from chance (p < .05).
VPC-spatial-location
For sham-operated controls, novelty preference increased with age; although this change did not reach significance, the effect size was large [FHuyhn-Feldt (2,=10) = 2.31; p = .15; η2 5 0.316]. Thus, in the Spatial Location task (Figure 3A), control animals did not show novelty preference as infants (M = 55; t = .94, ns), but showed robust memory for location as juveniles (M = 63.05; t = 3.140; p = .026) and adults [M=66.02; t=5.133; p=.004].
A similar increase in novelty preference with age was also found for animals of Group Neo-Hibo. Again, although the age factor did not reach significance, the effect size was large [FHuyhn-Feldt (2, 8) = 4.15; p = .058; η2 = .509; Figure 3A]. However, as compared to controls for which strong novelty preference was present as juveniles and adults, for Group Neo-Hibo, it did not differ from chance in the juvenile period (M = 53; t = .80, ns) and showed only a trend in adulthood (M = 65; t = 2.26; p = .08). This group difference showed only a trend toward significance [Group effect: F(1,9) = 3.89; p = .08; η2 = 0.32].
Of interest, at the juvenile age only, novelty preference correlated negatively with bilateral lesions of the hippocampus as well as bilateral unintended damage to area TH/TF (r = −.89; p< .02 and r = −.95; p < .004, respectively), indicating that novelty preference scores decreased with greater bilateral damage to the hippocampus and TH/TF. As shown in Figure 3A, at this age, poor performance was observed in the two animals with bilateral damage to the hippocampus (white circles) but not in those with most unilateral damage (black circles).
VPC-object-in-place
Novelty preference in sham-operated controls increased significantly with age [FHuyhn-Feldt (2, 10) = 7.24; p = .02; η2 = .592; Figure 3B]. As infants and juveniles, controls had lower novelty scores than as adults (p < .035 and .006, respectively), and only as adults did novelty preference scores differ from chance (M = 60; t = 3.75; p = .01).
The increase in novelty preference in animals with Neo-Hibo lesions (Figure 3) did not reach significance [FHuyhn-Feldt (2, 8) = 2.73, ns; η2 = 0.41], and the group difference did not reach significance [Group: F(1,9) = 0.27, ns; Group × Age: FHuyhn-Feldt (2, 18)=0.48, ns; η2 = .051]. However, unlike controls, novelty preference scores in Group Neo-Hibo did not differ from chance at any age.
There was only a weak negative correlation between novelty scores as adults and extent of bilateral damage to the hippocampus estimated on FLAIR images (r=−.64). However, as for the VPC-Spatial-Location task, the two animals with the most extensive and bilateral hippocampal lesions measured as the weighted average damage to both hemisphere (e.g., W%: Neo-Hibo-2=44.0%; Neo-Hibo-3=75.6%) performed at chance (42% and 51%, respectively; see Figure 3B white circles), whereas the three animals with mostly unilateral lesions (e.g., W%: Neo-Hibo-1=1.9%; Neo-Hibo-4=13.7% and Neo-Hibo-5= 17.4%) obtained novelty scores greater than chance (65%, 59%, and 76%, respectively; see Figure 3B black circles).
VPC-object-control
The control task was used to ensure that any impairment of Group Neo-Hibo in the VPC-Object-In-Place could not be due to any difficulty in visual appraisal to the complex visual display instead of difficulty in Object-Place memory. As shown in Figure 3C, the two groups showed novelty preference above chance at each age (all ps < .05) and did not differ from each other [Group: F(1,9) = 0.001, ns;η2 = 0.00; Age: FHuyhn-Feldt (2, 18) = 4.98; p = .023; η2 = .342; Group × Age: FHuyhn-Feldt (2, 18)=1.013, ns; η2 = .101].
Age at Lesions
To assess any functional sparing in spatial memory after neonatal hippocampal lesions, we compared novelty performance scores of animals with Neo-H lesions obtained in adulthood with those of adult animals that had received their hippocampal lesions in adulthood and were tested in the same way in the VPC tasks (Bachevalier & Nemanic, 2008). There were important differences in lesion extent between the neonatal and adult hippocampal lesions, given that all six animals with adult-onset lesions had extensive bilateral lesions (>50%), whereas only two of the cases with infant-onset lesions had comparable bilateral lesions (Cases Neo-Hibo-2, Neo-Hibo-3). Thus, to evaluate functional sparing, the three cases of this study with mostly unilateral lesions to the hippocampus (<50%) were excluded for this analysis.
For VPC-Spatial-Location (Figure 4A), adult animals with both infant-onset and adult-onset lesions preferred looking at a familiar object in a novel location and both groups obtained scores above chance (all ps < .05). There was no main effect of group [F(1,15) = 0.02, ns; η2 = 0.00] and of time of lesion [F(1,15) = 2.28, ns; η2 = 0.13] and no interaction [F(1,15) = .71, ns; η2 = 0.05]. Thus, adults with infant-onset lesions showed strong memory for spatial location as did adult animals with adult-onset hippocampal lesions.
Fig. 4.
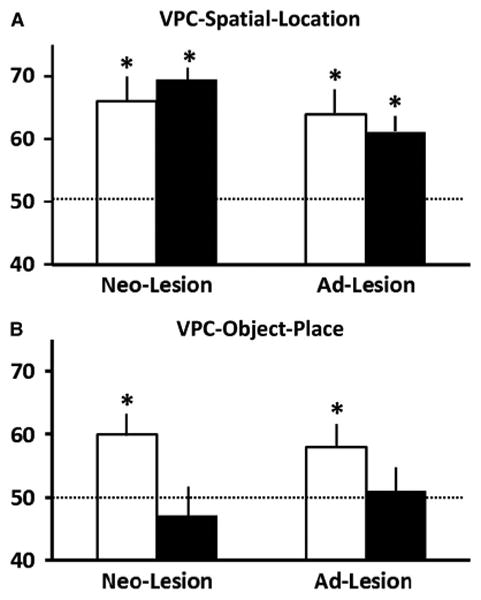
Mean percent of time (± SEM) looking at the novel image in the visual paired-comparison (VPC) -Spatial-Location task (A) and VPC-Object-In-Place task (B) for adult animals with sham operations (white bars) and animals with hippocampal lesions (black bars). Neo-lesion represents adult animals with infant-onset lesions and Ad-lesion represents adult animals with adult-onset lesions. Conventions as in Figure 3.
For VPC-Object-In-Place (Figure 4B), animals with both infant-onset and adult-onset hippocampal lesions were impaired on the Object-In-Place task (M = 46.39 and M = 51.30, respectively), as compared to their sham-operated controls [t (5) =3.749; p=.013 and t (5) =4.854 p=.005, respectively]. There was a main effect of group [F(1,15) = 7.10; p = .02; η2 = 0.32], but no main effect of time of lesion [F(1,15)=0.09, ns; η2 = 0.01] and no interaction [F(1,15)= 0.70, ns; η2 = 0.05]. Thus, regardless of the timing of the damage, animals with bilateral hippocampal lesions showed impaired memory for object-place associations.
Discussion
Development of Spatial Memory Abilities
The data on the sham-operated controls demonstrate that spatial memory has a protracted development in monkeys as has been shown already in rodents (Green & Stanton, 1989; Rudy, Stadler-Morris, & Albert, 1987) and humans (see for review Ribordy et al., 2012). However, this protracted development measured with the VPC tasks could be due to changes in perceptual visual abilities with age (see Table 2 for the three tasks). Thus, infant monkeys may not only require more time to inspect objects and extract visual information from them, but they also may be unable to distinguish minute changes between the familiarized and manipulated images regardless of whether the change be in location or in the perceptual attributes of the image. However, this seems unlikely given that, in the VPC-Object-Control that required similar perceptual abilities than in the two spatial VPC tasks, infant monkeys as young as 8 months of age demonstrated novelty preference as robust as that obtained at 18 months and 5–6 years (see Table 3). Thus, changes in novelty preference in the VPC-Spatial-Location and VPC-Object-In-Place tasks across age likely represent maturational changes in spatial memory processes.
Moreover, the two forms of spatial memory processes measured by the spatial VPC tasks in the current study have a different developmental time course. Memory for spatial locations, which relies on encoding and formation of spatial representation relative to an egocentric reference frame, was present in the juvenile period. By contrast, memory for object-place relations, which relies more in encoding and formation of spatial representation relative to an allocentric reference frame, emerges later.
For the VPC-Spatial-Location, novelty preference scores were at chance at the youngest age of 8 months but became stronger and significantly above chance by 18-months of age. This developmental time course indicates that memory for spatial locations in monkeys emerges during the juvenile period, although the exact age at which this type of memory becomes available is still unknown. Given that Lavenex and Lavenex (2006) demonstrated that by 9 months infant monkeys can forage in new locations and avoid locations in which they had already visited, it is likely that memory for a familiar object in a new location may be present by the end of the first year in monkeys.
As compared to the VPC-Spatial-Location, novelty preference in the VPC-Object-In-Place was absent in infancy and in the juvenile period, but was evident when the animals were re-tested as adults. Thus, although the data do not allow determining the exact age during which memory for spatial relations emerge in monkeys, it is clear that this memory process has a more protracted appearance and emerges only after the juvenile period. This protracted development of relational spatial memory is in sharp contrast with the early development of object recognition memory as measured by the VPC-Object-Control task. In this later task, robust novelty preference was present as early as 8 months of age and is consistent with earlier studies (see for review Bachevalier, & Vargha-Khadem, 2005; Zeamer et al., 2010). Thus, the difference in performance across ages in the VPC-Object-In-Place and VPC-Object-Control relates likely to differences in cognitive processes between the two tasks rather than differences in perceptual abilities, attentional processes, or novelty preference.
Spatial Memory Development after Neonatal Hippocampal Damage
Animals with Neo-H lesions, like sham-operated controls, demonstrated similar changes in looking behaviors across age (Table 2) as well as intact object recognition memory at short delays of 5 s in the VPC-Object-Control task (Figure 3C). This functional sparing is consistent with the normal performance of the same monkeys with an easier version of the VPC task using discriminable color objects (Zeamer et al, 2010), at least for delays (5 s) as short as those used in the VPC spatial tasks. By contrast, the Neo-H lesions delayed the emergence of memory for spatial location and abolished memory for object-place associations in early adulthood and these changes seem to be related to the extent of hippocampal damage. Thus, deficits in the two spatial VPC tasks were more severe in the two animals with the most extensive and bilateral lesions than in those that had more unilateral hippocampal lesions.
Neo-H lesions did impact memory for spatial location at an age (juvenile) when this type of memory was apparent in the sham-operated controls. However, this effect was only transient since as adult monkeys with Neo-H lesions showed novelty scores in the normal range, even in animals that had the most extended hippocampal lesions. The lack of spatial memory impairment at the adult age is totally consistent with the intact memory for locations found in the same Neo-H animals when tested as adults in a spatial memory span task Heuer & Bachevalier, 2010), as well as with the sparing of memory for spatial locations found in adult animals with adult-onset hippocampal lesions when tested in the same VPC-Spatial-Location task (Bachevalier & Nemanic, 2008).
Of interest, the transient deficit in memory for spatial location at 18 months correlated not only with extent of damage to the hippocampus but also with extent of damage to para-hippocampal areas TH/TF, even though damage to these cortical areas was relatively small (e.g., <12% bilaterally, see Table 1). Given that memory for spatial location is impaired in adult monkeys with damage to TH/TF areas, but not in those with damage to the hippocampus (Bachevalier & Nemanic, 2008), the impairment of memory for spatial location in the juvenile period may have resulted from damage to areas TH/TF rather than damage to the hippocampus. If so, then the recovery of memory functions in animals with Neo-H lesions in adulthood could be due to functional recovery within most of TH/TF that was spared after the Neo-H lesions. This conclusion seems likely given that, contrary to the present results, two earlier studies (Alvarado et al., 2002; Málková et al., 1995) reported severe spatial location deficits on a delayed nonmatching-to-location task in adult monkeys with Neo-H lesions, but in these two studies, the lesions extended to include almost all para-hippocampal areas. If this conclusion is correct, neonatal lesions restricted to parahippocampal areas may yield severe and long-lasting deficits in memory for spatial locations; a proposal that will need to be addressed in future studies.
In contrast to the recovery of memory for spatial locations, memory for object-place associations was impaired after Neo-H lesions, at least in animals with substantial bilateral hippocampal damage. In those animals, the object-place memory loss was as severe as that observed in adult animals with adult-onset hippocampal lesions (Bachevalier & Nemanic, 2008). This impairment cannot be associated with difficulty in perceptual decoding of complex visual stimuli because, using similar complex stimuli (VPC-Object-control), the same animals demonstrated normal object recognition memory at the same age (see Figure 3C). Notably, the spatial relational memory (object-place association) deficit is in line with a similar memory impairment the same animals demonstrated when required to form memory for place-food associations in a free-foraging spatial working memory task (Glavis-Bloom et al., 2013). All together these findings demonstrate that the hippocampus is critical for spatial relational memory and that its involvement in object-space association emerges after 18-months of age when the hippocampus circuitry reaches its morphological maturity (Jabès et al., 2010, 2011).
The present data, however, contradict those of recent studies (Lavenex & Lavenex, 2006; Lavenex et al., 2007) showing that spatial relational abilities, as measured with a foraging task known to be affected by adult-onset hippocampal lesions (Lavenex et al., 2006), are present by 9 months of age and are spared following neonatal hippocampal damage (Lavenex et al., 2007). Although both studies intended to measure encoding and remembering of spatial relational information, there were also important procedural differences that could explain the inconsistent results. As already proposed by Lavenex et al. (2007), one important difference is that the VPC-Object-In-Place task uses trial-unique stimuli (new array of 5 items presented on each trial) that may have favored the formation of memory for specific event (a kind of “episodic” spatial memory), whereas the foraging task in which the same spatial locations were experienced every day in the same environment may have favored the formation of memory for “episodic-free” spatial relations (a kind of “semantic” memory). One of the caveats with this interpretation, however, is that it does not fit with our own study on the same group of monkeys (Glavis-Bloom et al., 2013). In the foraging Place/Food association task, animals with Neo-H lesions were impaired even though they returned daily in the same environment to visit the same locations (e.g., a type of “episodic-free” memory as proposed by Lavenex et al., 2007, 2009). Another important difference between the two studies relates to the timing of behavioral assessment, which was performed only at 9 months in Lavenex et al. (2007), a very young age when control animals showed good performance on the task. Thus, it is still unknown whether performance of the animals with Neo-H lesions would deteriorate or not with further brain maturation. Deterioration of memory performance as development proceeds has already been demonstrated in our own study (Zeamer et al., 2010).
The data indicate that the long-term effects of hippocampal damage spared memory for locations (egocentric-like processes), while severely impacting spatial relational memory (allocentric-like processes). Although the present findings will need to be confirmed with a larger sample size, they parallel recent reports of spatial memory performance in developmental amnesic cases with perinatal hippocampal damage. Gadian et al. (2000) reported normal performance on a task measuring visuo-spatial memory that could be solved using an egocentric frame of reference. By contrast, the same patients demonstrated profound memory deficits for scenes and topographical information (Bird, Vargha-Khadem, & Burgess, 2008; King, Trinkler, Hartley, Vargha-Khadem, & Burgess, 2004; Spiers et al., 2001).
The deficits described in monkeys and humans in many of the memory processes supported by the hippocampus suggest that compensatory mechanisms do not operate to ensure recovery of functions after early hippocampal insult. This is in sharp contrast to effects of early cortical injury, which in many cases results in a better prognosis for functional sparing (for review, see Finger & Almli, 1984; Kolb, Halliwell, & Gibb, 2010; Levin & Grafman, 2000; Webster, Ungerleider, & Bachevalier, 1995). However, any attempt to predict the outcomes of early brain damage is complicated by many factors, such as the specific locus of the damage (cortical vs. subcortical), the maturational stage of the structures at the time of the insult, the age of the subject at the time of testing, the nature of the behavioral test used, as well as environmental factors (rearing conditions, maternal care, diet, etc.).
Relationships to Human Spatial Memory Development
The encoding and remembering of spatial information is realized through different forms of spatial representation, that is, egocentric versus allocentric frame of reference (O'Keefe & Nadel, 1978). In addition, as recently reviewed (Lavenex & Lavenex, 2009), the hippocampus is critical for solving tasks requiring memory of objects relative to their spatial relationship among other objects or features in the environment (i.e., foraging task and VPC-Object-In-Place), but not for solving tasks requiring the memory of spatial locations relative to a subject (i.e., delayed nonmatching-to-locations, spatial memory span, VPC-Spatial-Location).
Based on this knowledge, the present results indicate that these two forms of spatial memory processes have a different developmental time course. Thus, encoding and remembering of object locations, which could be accomplished using an egocentric representation of space, emerge earlier than encoding and remembering of spatial relationships among objects requiring the use of allocentric representations. This developmental pattern of spatial memory abilities has been related to morphological changes within the hippocampus and medial temporal cortical areas (Alvarado & Bachevalier, 2000; Ribordy et al., 2012). Of interest, the development of spatial memory abilities in humans appears to follow a similar developmental time course. The ability to remember the location of an object in an egocentric frame of reference (spatial location memory) is present in infants younger than 3 years (see Newcombe & Huttenlocher, 2005, for review; Sluzenski, Newcombe, & Satlow, 2004), whereas the ability to encode and remember object/place relationships emerges after 3 years of age with substantial improvement still present until 7 years (Bullens et al., 2010; Cestari, Lucidi, Pieroni, & Rossi-Arnaud, 2007; Foreman, Warry, & Savage, 1990; Lehnung et al., 1998; Leplow et al., 2003; Mandolesi, Petrosini, Mewnghini, Addona, & Vicari, 2009; Nardini, Burgess, Breckenridge, & Atkinson, 2006; Oveman et al., 1996; Pentland, Anderson, Dye, & Wood, 2003; Ribordy et al., 2012; Smith et al., 2008). As recently discussed by others (Ribordy et al., 2012), the substantial improvement of spatial relational memory between 3 and 7 years of age may parallel the progressive maturation of the dentate gyrus and trisynaptic hippocampal pathway.
The remarkable parallel between the development of spatial memory processes and the morphologic hippocampal maturation in monkeys and humans clearly demonstrate the significant benefits of using a comparative neuropsychological approach to further our understanding of the neural circuits underlying spatial memory processes in humans. Additional morphological and functional (electrophysiological recording and neuroimaging) studies in non-human primates will be critical for defining the neural substrate available to support spatial memory functions at different time points in development. Finally, longitudinal studies that take advantage of tasks and paradigms that can be used across species will be most important to fulfill this endeavor.
Acknowledgments
This work was supported by the National Institute of Mental Health (MH-58846), National Center for Research Resources P51RR165, currently supported by the Office of Research Infrastructure Programs/OD P51OD11132, and by Center for Behavioral Neuroscience grant NSF IBN-9876754. We thank the veterinary and animal husbandry staff at the University of Texas Health Science Center at Houston and the Yerkes National Primate Research Center for expert animal care, Roger E. Price, Belinda Rivera for the care and handling of animals during the MR imaging procedures, Edward F. Jackson for assistance in neuroimaging techniques, and Diana Lay for assistance with the testing of the animals. We also thank Dr. Maria Alvarado for helpful comments on an earlier version of this manuscript. Data in this manuscript contributed to the Master's Thesis of Shala Blue.
Footnotes
We acknowledge that none of the authors have a conflict of interest with the publication of this manuscript.
References
- Alvarado MC, Bachevalier J. Revisiting the development of medial temporal lobe memory functions in primates. Learning & Memory. 2000;7:244–256. doi: 10.1101/lm.35100. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Rudy JW. Rats with damage to the hippocampal-formation are impaired on the transverse-patterning problem but not on elemental discriminations. Behavioral Neuroscience. 1995;109(2):204–211. doi: 10.1037//0735-7044.109.2.204. [DOI] [PubMed] [Google Scholar]
- Alvarado MC, Wright AA, Bachevalier J. Object and spatial relational memory in adult rhesus monkeys is impaired by neonatal lesions of the hippocampal formation but not the amygdaloid complex. Hippocampus. 2002;12(4):421–433. doi: 10.1002/hipo.1115. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Beauregard M. Maturation of medial temporal lobe memory functions in rodents, monkeys, and humans. Hippocampus. 1993;3 Spec No:191–201. [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place associations are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18(1):64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Vargha-Khadem F. The primate hippocampus: Ontogeny, early insult and memory. Current Opinion in Neurobiology. 2005;15(2):168–174. doi: 10.1016/j.conb.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bird CM, Vargha-Khadem F, Burgess N. Impaired memory for scenes but not faces in developmental hippocampal amnesia: A case study. Neuropsychologia. 2008;46(4):1050–1059. doi: 10.1016/j.neuropsychologia.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Blue S, Kazama AM, Bachevalier J. The normal development of object-place association memory is altered by neonatal hippocampal lesions in rhesus monkeys. Washington, DC: Society for Neuroscience; 2009. Online. [Google Scholar]
- Bullens J, Nardini M, Doeller CF, Braddick O, Postma A, Burgess N. The role of landmarks and boundaries in the development of spatial memory. Developmental Science. 2010;13(1):170–180. doi: 10.1111/j.1467-7687.2009.00870.x. [DOI] [PubMed] [Google Scholar]
- Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35(4):625–641. doi: 10.1016/s0896-6273(02)00830-9. [DOI] [PubMed] [Google Scholar]
- Cestari V, Lucidi A, Pieroni L, Rossi-Arnaud C. Memory for object location: A span study in children. Canadian Journal of Experimental Psychology. 2007;61(1):13–20. doi: 10.1037/cjep2007002. [DOI] [PubMed] [Google Scholar]
- Finger S, Almli CR. Early brain damage: Neurobiology and behavior (vol 2) Orlando, FL: Academic Press; 1984. [Google Scholar]
- Foreman N, Warry R, Murray P. Development of reference and working spatial memory in preschool children. Journal of General Psychology. 1990;117(3):267–276. [PubMed] [Google Scholar]
- Gadian DG, Aicardi J, Watkins KE, Porter DA, Mishkin M, Vargha-Khadem F. Developmental amnesia associated with early hypoxic-ischaemic injury. Brain. 2000;123(Pt 3):499–507. doi: 10.1093/brain/123.3.499. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Snell JW, Lange N, Rajapakse JC, Casey BJ, Kozuch PL, Rapoport JL. Quantitative magnetic resonance imaging of human brain development: Ages 4–18. Cerebral Cortex. 1996;6(4):551–560. doi: 10.1093/cercor/6.4.551. [DOI] [PubMed] [Google Scholar]
- Glavis-Bloom C, Alvarado M, Bachevalier J. Neonatal hippocampal damage impairs specific food/place associations in adult macaques. Behavioral Neuroscience. 2013;127:9–22. doi: 10.1037/a0031498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goursaud AP, Bachevalier J. Social attachment in juvenile monkeys with neonatal lesion of the hippocampus, amygdala and orbital frontal cortex. Behavioral Brain Research. 2007;176(1):75–93. doi: 10.1016/j.bbr.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Green RJ, Stanton ME. Differential ontogeny of working memory and reference memory in the rat. Behavioral Neuroscience. 1989;103(1):98–105. doi: 10.1037//0735-7044.103.1.98. [DOI] [PubMed] [Google Scholar]
- Heuer E, Bachevalier J. Effects of selective neonatal hippocampal lesions on tests of object and spatial recognition memory in monkeys. Behavioral Neuroscience. 2010;125:137–149. doi: 10.1037/a0022539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodos W, Bobko P. A weighted index of bilateral brain lesions. Journal of Neuroscience Methods. 1984;12(1):43–47. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Jabès A, Lavenex PB, Amaral DG, Lavenex P. Postnatal development of the hippocampal formation: A stereo-logical study in macaque monkeys. Journal of Comparative Neurology. 2010;519(6):1051–1070. doi: 10.1002/cne.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jabès A, Lavenex PB, Amaral DG, Lavenex P. Quantitative analysis of postnatal neurogenesis and neuron number in the macaque monkey dentate gyrus. European Journal of Neuroscience. 2011;31(2):273–285. doi: 10.1111/j.1460-9568.2009.07061.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama AM, Lay D, Bachevalier J. Delayed maturation of spatial memory in infant monkeys as assessed by a visual paired-comparison task. New Orleans, LA: Society for Neuroscience; 2003. [Google Scholar]
- King JA, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Human hippocampus and viewpoint dependence in spatial memory. Hippocampus. 2002;12(6):811–820. doi: 10.1002/hipo.10070. [DOI] [PubMed] [Google Scholar]
- King JA, Trinkler I, Hartley T, Vargha-Khadem F, Burgess N. The hippocampal role in spatial memory and the familiarity-recollection distinction: A case study. Neuropsychology. 2004;18(3):405–417. doi: 10.1037/0894-4105.18.3.405. [DOI] [PubMed] [Google Scholar]
- Kolb B, Halliwell C, Gibb R. Factors influencing neocortical development in the normal and injured brain. In: Blumberg MS, Freeman JH, Robinson SR, editors. Oxford handbook of developmental behavioral neuroscience. New York: Oxford University Press; 2010. [Google Scholar]
- Lavenex P, Lavenex PB. Spatial relational memory in 9-month-old macaque monkeys. Learning & Memory. 2006;13(1):84–96. doi: 10.1101/lm.97606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex P, Lavenex P. Spatial memory and the monkey hippocampus: Not all space is created equal. Hippocampus. 2009;19(1):8–19. doi: 10.1002/hipo.20485. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Lavenex PB, Amaral DG. Spatial relational learning persists following neonatal hippocampal lesions in macaque monkeys. Nature Neuroscience. 2007;10(2):234–239. doi: 10.1038/nn1820. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Lavenex PB, Bennett JL, Amaral DG. Postmortem changes in the neuroanatomical characteristics of the primate brain: Hippocampal formation. Journal of Comparative Neurology. 2009;512(1):27–51. doi: 10.1002/cne.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. Journal of Neuroscience. 2006;26(17):4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnung M, Leplow B, Friege L, Herzog A, Ferstl R, Mehdorn M. Development of spatial memory and spatial orientation in preschoolers and primary school children. British Journal of Psychology. 1998;89(3):463–480. doi: 10.1111/j.2044-8295.1998.tb02697.x. [DOI] [PubMed] [Google Scholar]
- Leplow B, Lehnung M, Pohl J, Hertzog A, Ferstl R, Mehdorn M. Navigational place learning in children and young adults as assessed with a standardized locomotor search task. British Journal of Psychology. 2003;94(Pt3):299–317. doi: 10.1348/000712603767876244. [DOI] [PubMed] [Google Scholar]
- Lenroot RH, Giedd JN. Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience and Biobehavioral Reviews. 2006;30:718–729. doi: 10.1016/j.neubiorev.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Levin HS, Grafman J. Cerebral reorganization of function after brain damage. New York: Oxford University Press; 2000. [Google Scholar]
- Mahut H, Moss M. The monkey and the seahorse. In: Isaacson RL, Pribram KH, editors. The hippocampus. New York: Plenum Press; 1986. [Google Scholar]
- Málková L, Lex CK, Mishkin M, Saunders RC. MRI-based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11(4):361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Málková L, Mishkin M, Bachevalier J. Long-term effects of selective neonatal temporal lobe lesions on learning and memory in monkeys. Behavioral Neuroscience. 1995;109(2):212–226. doi: 10.1037//0735-7044.109.2.212. [DOI] [PubMed] [Google Scholar]
- Mandolesi L, Petrosini L, Menghini D, Addona F, Vicari S. Children's radial arm maze performance as a function of age and sex. International Journal of Developmental Neuroscience. 2009;27(8):789–797. doi: 10.1016/j.ijdevneu.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nadel L, Hardt O. The spatial brain. Neuropsychology. 2004;18(3):473–476. doi: 10.1037/0894-4105.18.3.473. [DOI] [PubMed] [Google Scholar]
- Nardini M, Burgess N, Breckenridge K, Atkinson J. Differential developmental trajectories for egocentric, environmental and intrinsic frames of reference in spatial memory. Cognition. 2006;101(1):153–172. doi: 10.1016/j.cognition.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: A replication. Journal of Neuroscience Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Newcombe NS, Huttenlocher J. Development of spatial cognition. In: Kuhn D, Siegler RS, editors. Handbook of child psychology. 6th. Hoboken, NJ: John Wiley and Sons; 2005. [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. New York: Oxford University Press; 1978. [Google Scholar]
- Overman WH, Pate BJ, Moore K, Peuster A. Ontogeny of place learning in children as measured in the radial arm maze, Morris search task, and open field task. Behavioral Neuroscience. 1996;110(6):1205–1228. doi: 10.1037//0735-7044.110.6.1205. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Bachevalier J. Neonatal aspiration lesions of the hippocampal formation impair visual recognition memory when assessed by paired-comparison task but not by delayed nonmatching-to-sample task. Hippocampus. 1999;9(6):609–616. doi: 10.1002/(SICI)1098-1063(1999)9:6<609::AID-HIPO1>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Payne C, Machado CJ, Bliwise NG, Bachevalier J. Maturation of the hippocampal formation and amygdala in Macaca mulatta: A volumetric magnetic resonance imaging study. Hippocampus. 2010;20(8):922–935. doi: 10.1002/hipo.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedhazur EJ. Multiple regression in behavioral research: Explanation and prediction. 2nd. New York: Holt, Rinehart and Winston; 1982. [Google Scholar]
- Pentland LM, Anderson VA, Dye S, Wood SJ. The Nine Box Maze Test: A measure of spatial memory development in children. Brain & Cognition. 2003;52(2):144–154. doi: 10.1016/s0278-2626(03)00079-4. [DOI] [PubMed] [Google Scholar]
- Ribordy F, Jabès A, Banta Lavenex P, Lavenex P. Development of allocentric spatial memory abilities in children from 18 months to 5 years of age. Cognitive Psychology. 2012;66(1):1–29. doi: 10.1016/j.cogpsych.2012.08.001. [DOI] [PubMed] [Google Scholar]
- Rudy JW, Stadler-Morris S, Albert P. Ontogeny of spatial navigation behaviors in the rat: Dissociation of “proximal”- and “distal”-cue-based behaviors. Behavioral Neuroscience. 1987;101(1):62–73. doi: 10.1037//0735-7044.101.1.62. [DOI] [PubMed] [Google Scholar]
- Seress L. Morphological variability and developmental aspects of monkey and human granule cells: Differences between the rodent and primate dentate gyrus. Epilepsy Research Supplement. 1992;7:3–28. [PubMed] [Google Scholar]
- Seress L. Morphological changes of the human hippocampal formation from midgestation to early childhood. In: Nelson C, Luciana M, editors. Handbook of developmental cognitive neuroscience. Cambridge: MIT Press; 2001. [Google Scholar]
- Seress L. Comparative anatomy of the hippocampal dentate gyrus in adult and developing rodents, non-human primates and humans. Progress in Brain Research. 2007;163:23–41. doi: 10.1016/S0079-6123(07)63002-7. [DOI] [PubMed] [Google Scholar]
- Seress L, Abraham H. Pre- and postnatal morphological development of the human hippocampal formation. In: Nelson C, Luciana M, editors. Handbook of developmental cognitive neuroscience. 2nd. Cambridge: MIT Press; 2008. [Google Scholar]
- Seress L, Ribak CE. Postnatal development and synaptic connections of hilar mossy cells in the hippocampal dentate gyrus of rhesus monkeys. Journal of Comparative Neurology. 1995a;355(1):93–110. doi: 10.1002/cne.903550111. [DOI] [PubMed] [Google Scholar]
- Seress L, Ribak CE. Postnatal development of CA3 pyramidal neurons and their afferents in the Ammon's horn of rhesus monkeys. Hippocampus. 1995b;5(3):217–231. doi: 10.1002/hipo.450050308. [DOI] [PubMed] [Google Scholar]
- Sluzenski J, Newcombe NS, Satlow E. Knowing where things are in the second year of life: Implications for hippocampal development. Journal of Cognitive Neuroscience. 2004;16(8):1443–1451. doi: 10.1162/0898929042304804. [DOI] [PubMed] [Google Scholar]
- Smith AD, Gilchrist ID, Cater K, Ikram N, Nott K, Hood BM. Reorientation in the real world: The development of landmark use and integration in a natural environment. Cognition. 2008;107(3):1102–1111. doi: 10.1016/j.cognition.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Spiers HJ, Burgess N, Hartley T, Vargha-Khadem F, O'Keefe J. Bilateral hippocampal pathology impairs topographical and episodic memory but not visual pattern matching. Hippocampus. 2001;11(6):715–725. doi: 10.1002/hipo.1087. [DOI] [PubMed] [Google Scholar]
- von Bonin G, Bailey P. The neocortex of Macaca mulatta. Urbana, IL: University of Illinois Press; 1947. [Google Scholar]
- Webster MJ, Ungerleider LG, Bachevalier J. Development and plasticity of the neural circuitry underlying visual recognition memory. Canadian Journal of Physiology and Pharmacology. 1995;73:1364–1371. doi: 10.1139/y95-191. [DOI] [PubMed] [Google Scholar]
- Zeamer A, Bachevalier J. Long-term effects of neonatal hippocampal lesions on novelty preference in monkeys. Hippocampus. 2013 doi: 10.1002/hipo.22139. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeamer A, Heuer E, Bachevalier J. Developmental trajectory of object recognition memory in infant rhesus macaques with and without neonatal hippocampal lesions. Journal of Neuroscience. 2010;30(27):9157–9165. doi: 10.1523/JNEUROSCI.0022-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


