Abstract
Mutant Chinese hamster ovary cell lines temperature-sensitive (TS) for growth and containing TS mutations in RNA polymerase II (nucleosidetriphosphate:RNA nucleotidyltransferase, EC 2.7.7.6) have been isolated. Wild-type cells were treated with the mutagen N-methyl-N′-nitro-N-nitrosoguanidine and a population of cells possessing mutations in RNA polymerase II was initially selected by isolating α-amanitin-resistant clones at 34°. Of 168 such α-amanitin-resistant isolates screened for temperature sensitivity, nine were TS for growth at 39.5°. By examining the behavior of the α-amanitin resistance of these TS cell lines in somatic cell hybrids, the TS mutation in a number of them was shown to be in RNA polymerase II. Hybrid cells obtained by the fusion of the TS and α-amanitin-resistant cells with cells possessing α-amanitin-sensitive polymerase II grew at both 34° and 39.5°; the TS mutations were recessive. At 34° all the hybrids were α-amanitin-resistant and possessed a mixture of α-amanitin-resistant and sensitive polymerase II. At 39.5° the α-amanitin-resistant polymerase II activities in hybrids of four of the TS cell lines were lost; these four lines were α-amanitin-sensitive and possessed only α-amanitin-sensitive polymerase II. Temperature-insensitive revertants of two of these mutants were isolated. Reversion of the TS phenotype for mutants TsAmaR-1 and TsAmaR-8 was accompanied by an alteration in the level of α-amanitin resistance of the RNA polymerase II activities in the revertant cells. Together these data provide convincing evidence that TS mutations in RNA polymerase II can be coselected with α-amanitin resistance.
Keywords: α-amanitin resistance, nitrosoguanidine mutagenesis, cell hybridization, reversion, RNA nucleotidyltransferase
Full text
PDF
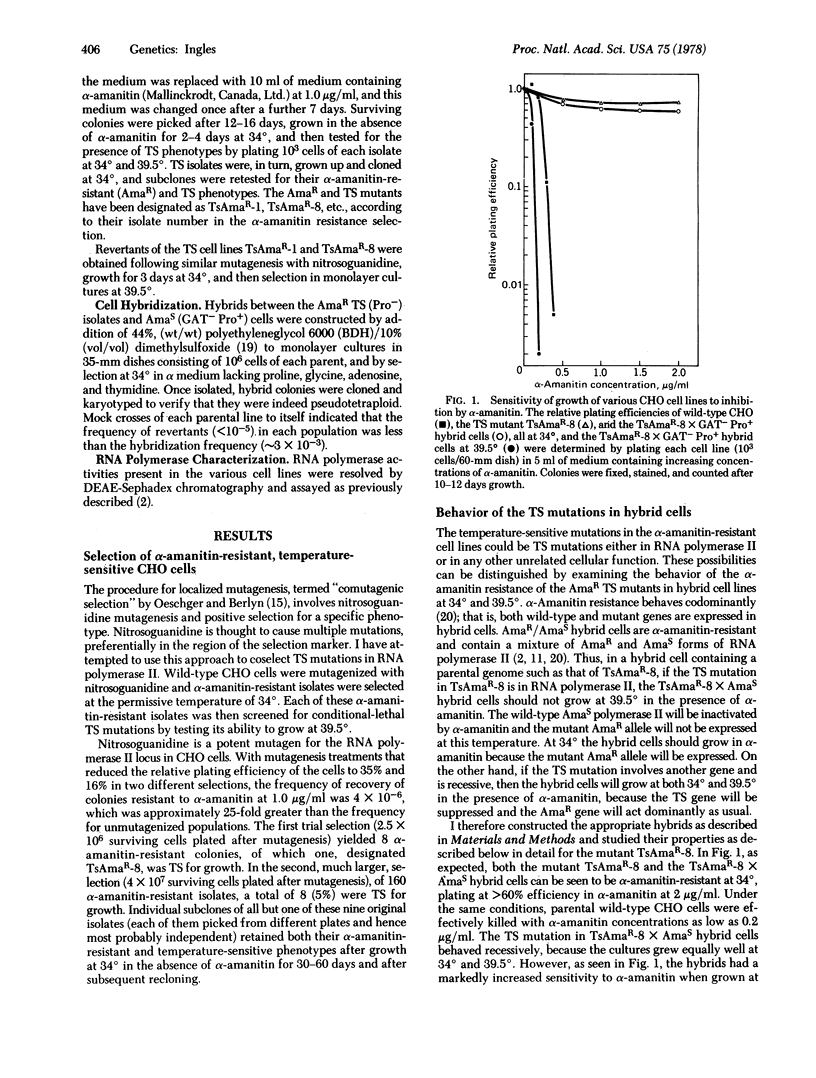
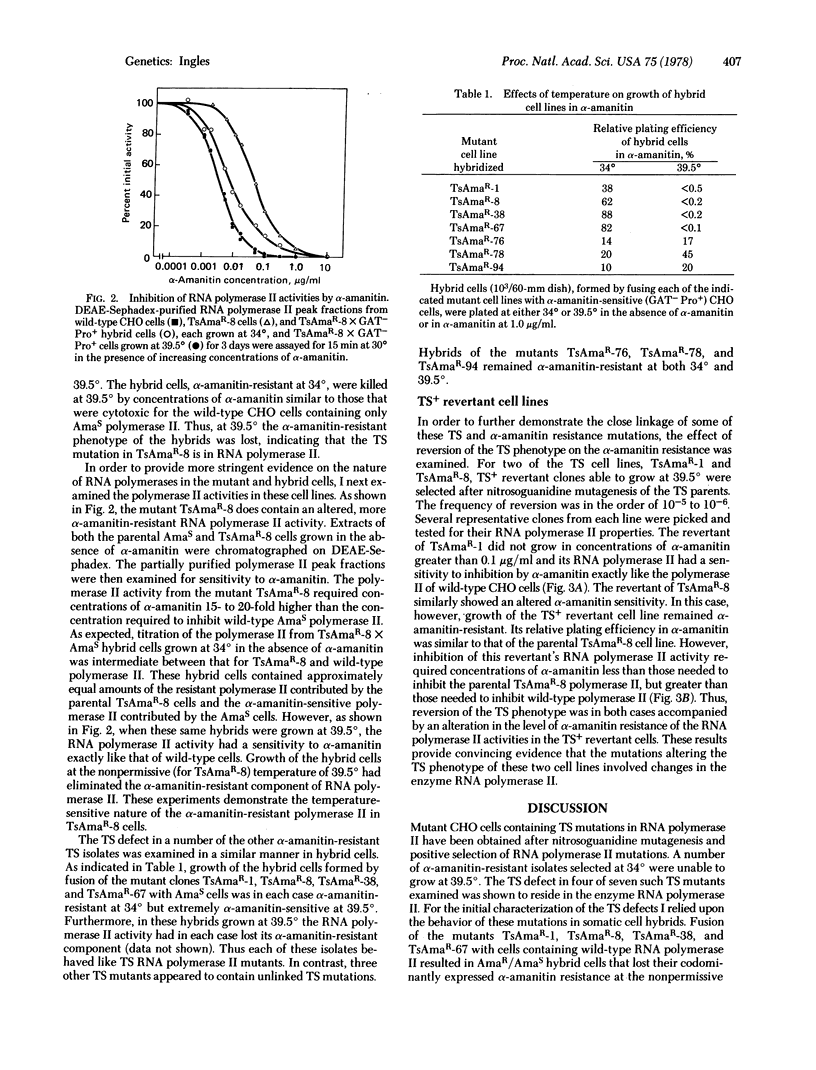
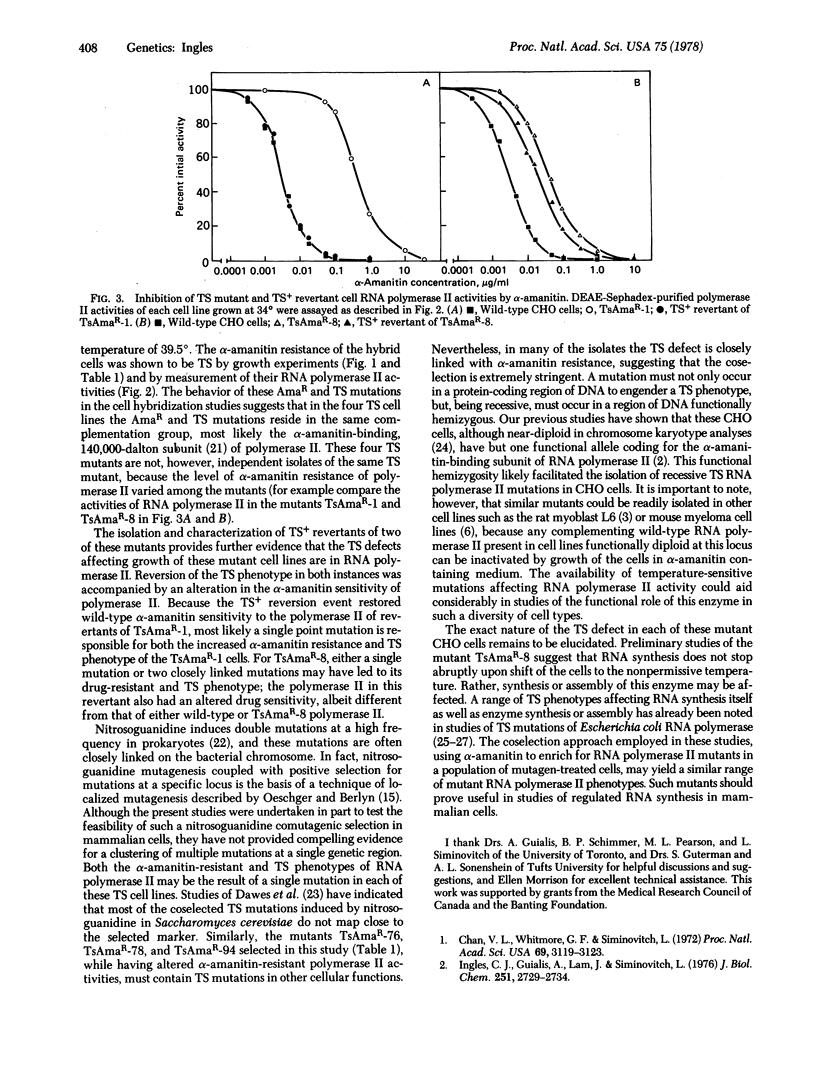

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amati P., Blasi F., Di Porzio U., Riccio A., Traboni C. Hamster alpha-amanitine-resistant RNA polymerase II able to transcribe polyoma virus genome in somatic cell hybrids. Proc Natl Acad Sci U S A. 1975 Feb;72(2):753–757. doi: 10.1073/pnas.72.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodner O. G., Wieland T. Identification of the amatoxin-binding subunit of RNA polymerase B by affinity labeling experiments. Subunit B3-the true amatoxin receptor protein of multiple RNA polymerase B. Biochemistry. 1976 Aug 10;15(16):3480–3484. doi: 10.1021/bi00661a013. [DOI] [PubMed] [Google Scholar]
- Buchwald M., Ingles C. J. Human diploid fibroblast mutants with altered RNA polymerase II. Somatic Cell Genet. 1976 May;2(3):225–233. doi: 10.1007/BF01538961. [DOI] [PubMed] [Google Scholar]
- Chan V. L., Whitmore G. F., Siminovitch L. Mammalian cells with altered forms of RNA polymerase II. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3119–3123. doi: 10.1073/pnas.69.11.3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crerar M. M., Andrews S. J., David E. S., Somers D. G., Mandel J. L., Pearson M. L. Amanitin binding to RNA polymerase II in alpha-amanitin-resistant rat myoblast mutants. J Mol Biol. 1977 May 15;112(2):317–329. doi: 10.1016/s0022-2836(77)80147-2. [DOI] [PubMed] [Google Scholar]
- Dawes I. W., Mackinnon D. A., Ball D. E., Hardie I. D., Sweet D. M., Ross F. M., Macdonald F. Identifying sites of simultaneous DNA replication in eukaryotes by N-methyl-N'-nitro-N-nitrosoguanidine multiple mutagenesis. Mol Gen Genet. 1977 Mar 28;152(1):53–57. doi: 10.1007/BF00264939. [DOI] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Guialis A., Beatty B. G., Ingles C. J., Crerar M. M. Regulation of RNA polymerase II activity in alpha-amanitin-resistant CHO hybrid cells. Cell. 1977 Jan;10(1):53–60. doi: 10.1016/0092-8674(77)90139-8. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Ames B. N. Localized mutagenesis of any specific small region of the bacterial chromosome. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3158–3162. doi: 10.1073/pnas.68.12.3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingles C. J., Guialis A., Lam J., Siminovitch L. Alpha-Amanitin resistance of RNA polymerase II in mutant Chinese hamster ovary cell lines. J Biol Chem. 1976 May 10;251(9):2729–2734. [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells. IV. Properties of Chinese hamster cell mutants with respect to the requirement for proline. Genetics. 1967 Mar;55(3):513–524. doi: 10.1093/genetics/55.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum J. B., Claeys I. V., Nasi S., Molholt B., Miller J. H. Temperature-sensitive RNA polymerase mutants with altered subunit synthesis and degradation. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2375–2379. doi: 10.1073/pnas.72.6.2375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobban P. E., Siminovitch L. Alpha-amanitin resistance: a dominant mutation in CHO cells. Cell. 1975 Feb;4(2):167–172. doi: 10.1016/0092-8674(75)90123-3. [DOI] [PubMed] [Google Scholar]
- McBurney M. W., Whitmore G. F. Isolation and biochemical characterization of folate deficient mutants of Chinese hamster cells. Cell. 1974 Jul;2(3):173–182. doi: 10.1016/0092-8674(74)90091-9. [DOI] [PubMed] [Google Scholar]
- Milman G., Lee E., Ghangas G. S., McLaughlin J. R., George M., Jr Analysis of HeLa cell hypoxanthine phosphoribosyltransferase mutants and revertants by two-dimensional polyacrylamide gel electrophoresis: evidence for silent gene activation. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4589–4593. doi: 10.1073/pnas.73.12.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norwood T. H., Zeigler C. J., Martin G. M. Dimethyl sulfoxide enhances polyethylene glycol-mediated somatic cell fusion. Somatic Cell Genet. 1976 May;2(3):263–270. doi: 10.1007/BF01538964. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Berlyn M. K. A simple procedure for localized mutagenesis using nitrosoguanidine. Mol Gen Genet. 1974;134(1):77–83. doi: 10.1007/BF00332814. [DOI] [PubMed] [Google Scholar]
- Oeschger M. P., Berlyn M. K. Regulation of RNA polymerase synthesis in Escherichia coli: a mutant unable to synthesize the enzyme at 43 degrees. Proc Natl Acad Sci U S A. 1975 Mar;72(3):911–915. doi: 10.1073/pnas.72.3.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siminovitch L. On the nature of hereditable variation in cultured somatic cells. Cell. 1976 Jan;7(1):1–11. doi: 10.1016/0092-8674(76)90249-x. [DOI] [PubMed] [Google Scholar]
- Somers D. G., Pearson M. L., Ingles C. J. Isolation and characterization of an alpha-amanitin-resistant rat myoblast mutant cell line possessing alpha-amanitin-resistant RNA polymerase II. J Biol Chem. 1975 Jul 10;250(13):4825–4831. [PubMed] [Google Scholar]
- Somers D. G., Pearson M. L., Ingles C. J. Regulation of RNA polymerase II activity in a mutant rat myoblast cell line resistant to alpha-amanitin. Nature. 1975 Jan 31;253(5490):372–374. doi: 10.1038/253372a0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., O'Farrell P. H., Friedrich U., Coffino P. Mutations causing charge alterations in regulatory subunits of the cAMP-dependent protein kinase of cultured S49 lymphoma cells. Cell. 1977 Mar;10(3):381–391. doi: 10.1016/0092-8674(77)90025-3. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Ho C. C., Duff C. Chromosome stability in CHO cells. Somatic Cell Genet. 1977 Jan;3(1):27–45. doi: 10.1007/BF01550985. [DOI] [PubMed] [Google Scholar]
- Wulf E., Bautz L. RNA polymerase B from an alpha-amanitin resistant mouse myeloma cell line. FEBS Lett. 1976 Oct 15;69(1):6–10. doi: 10.1016/0014-5793(76)80641-2. [DOI] [PubMed] [Google Scholar]


